Differences in Lipid Metabolism between the Perirenal Adipose Tissue of Chinese Simmental Cattle and Angus Cattle (Bos taurus) Based on Metabolomics Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Experimental Animals and Sample Collection
2.2. Measurement of Adipocyte Area
2.3. Determination of Triglyceride Content
2.4. LC-MS/MS Detection
2.5. Metabolomics Analysis
2.6. Statistical Analysis
3. Results
3.1. Observation of Organizational Morphology and Determination of Lipid Content
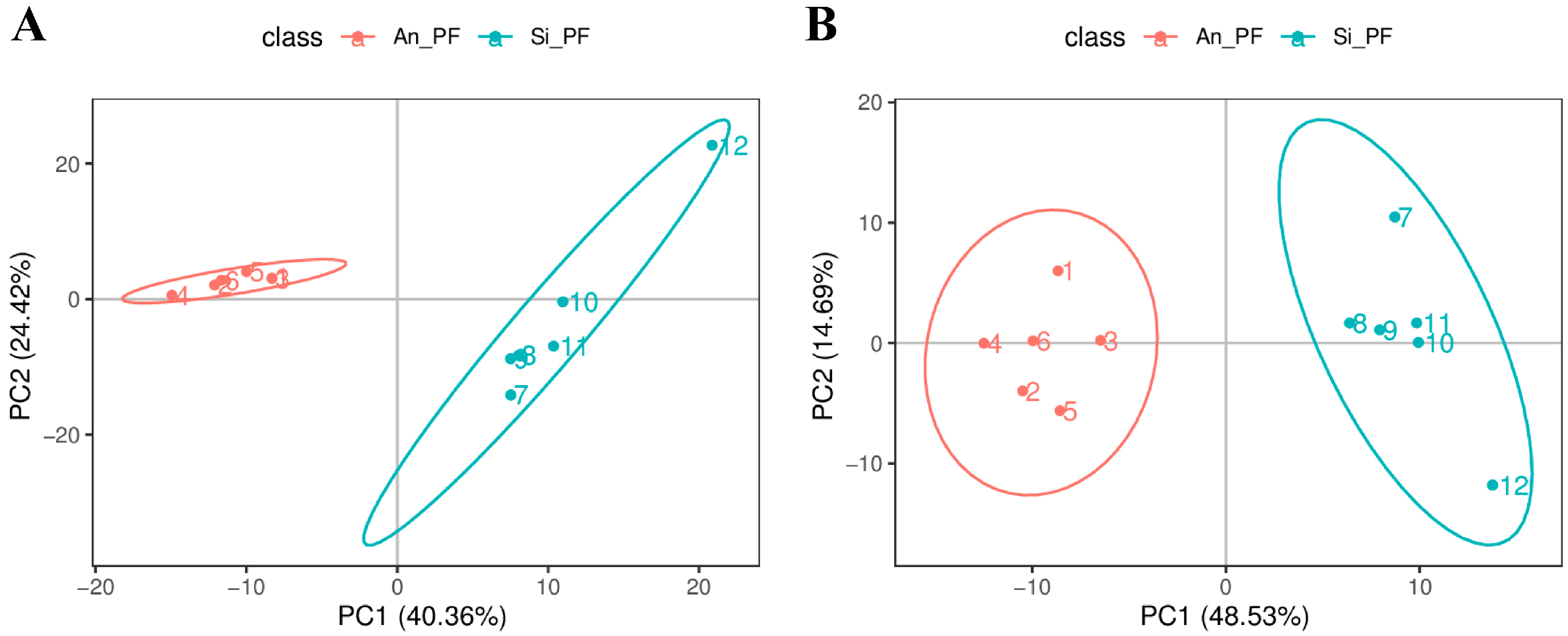
3.2. Metabolite Identification and Principal Component Analysis
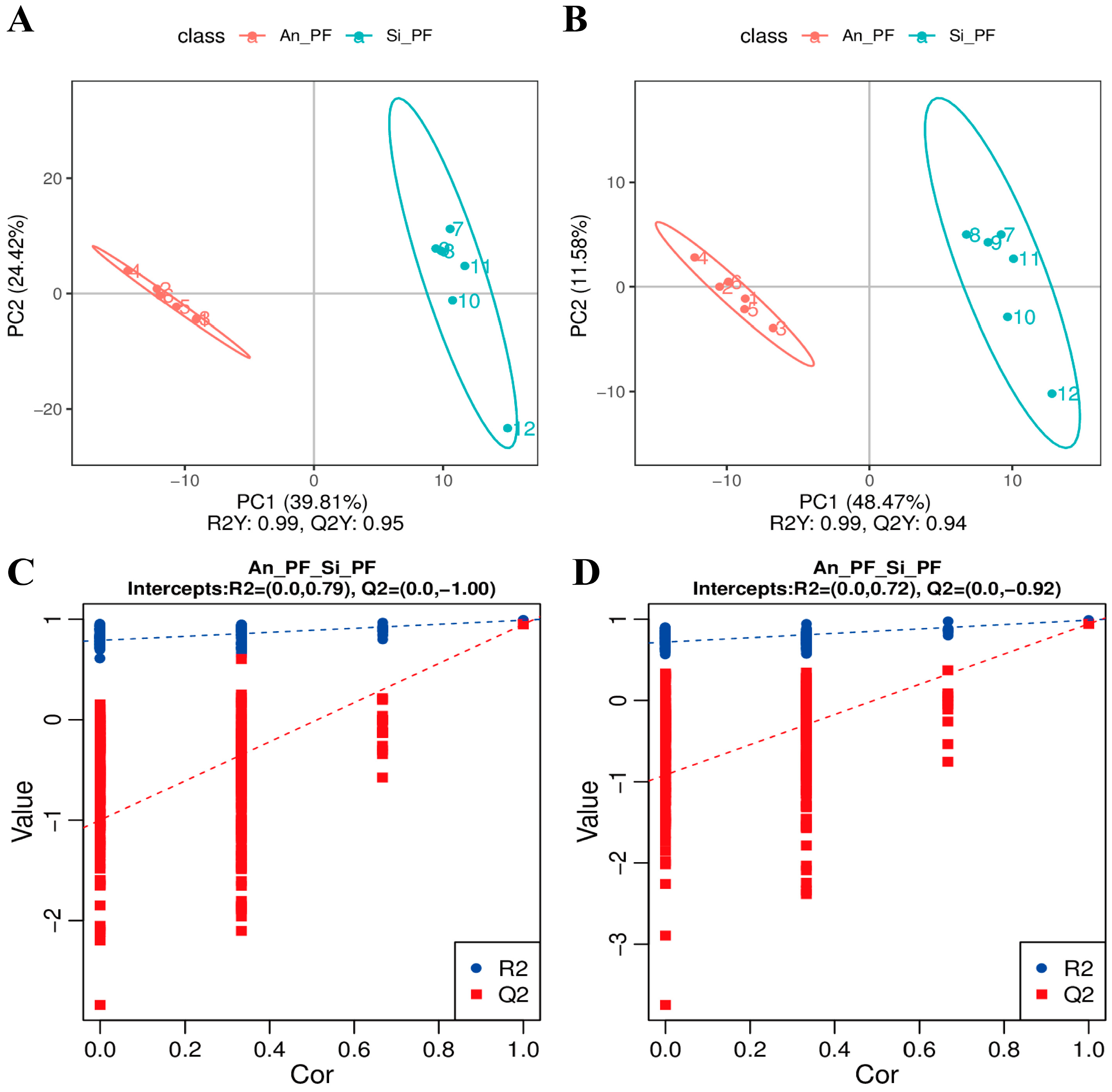
3.3. Partial Least Squares Discrimination Analysis
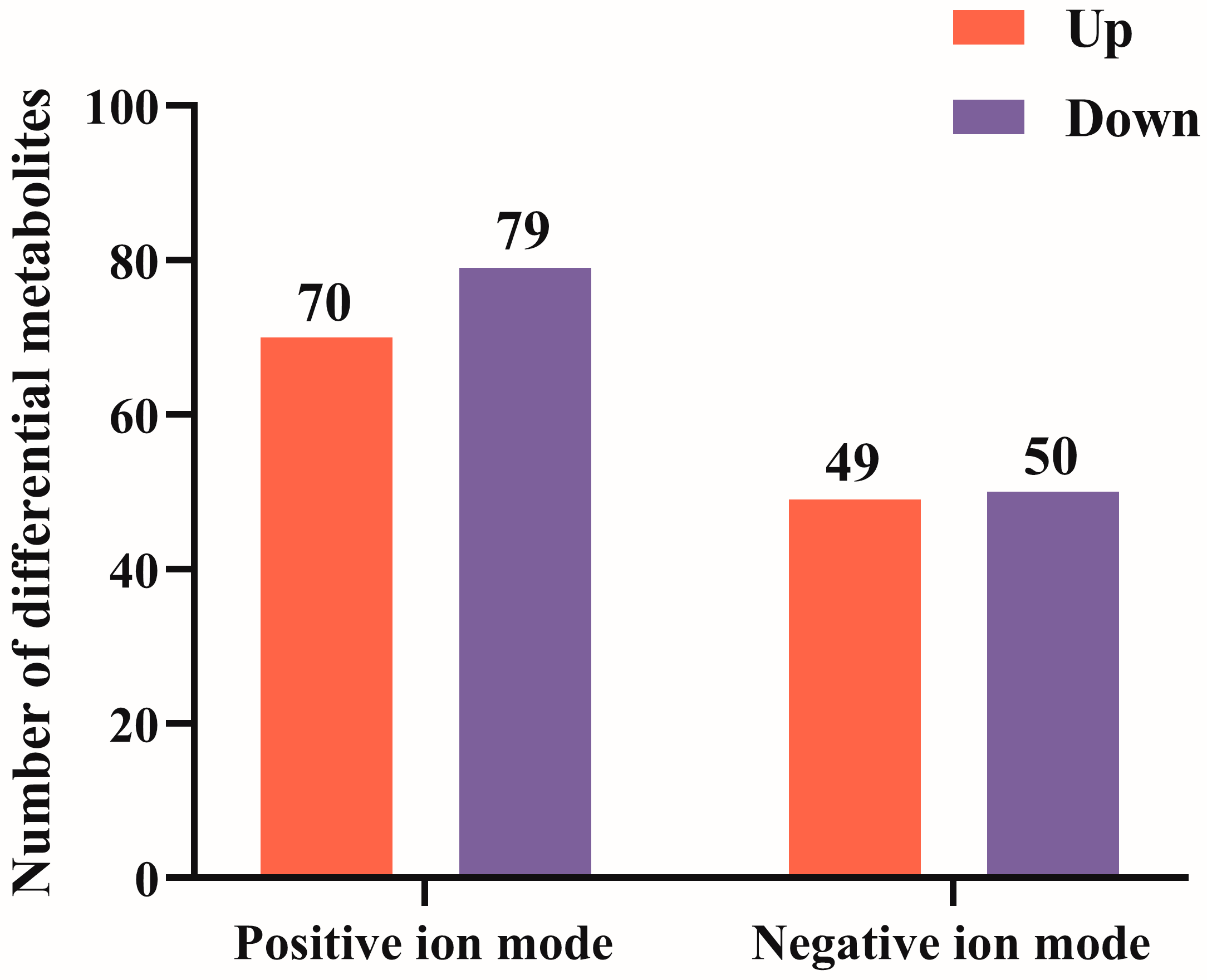
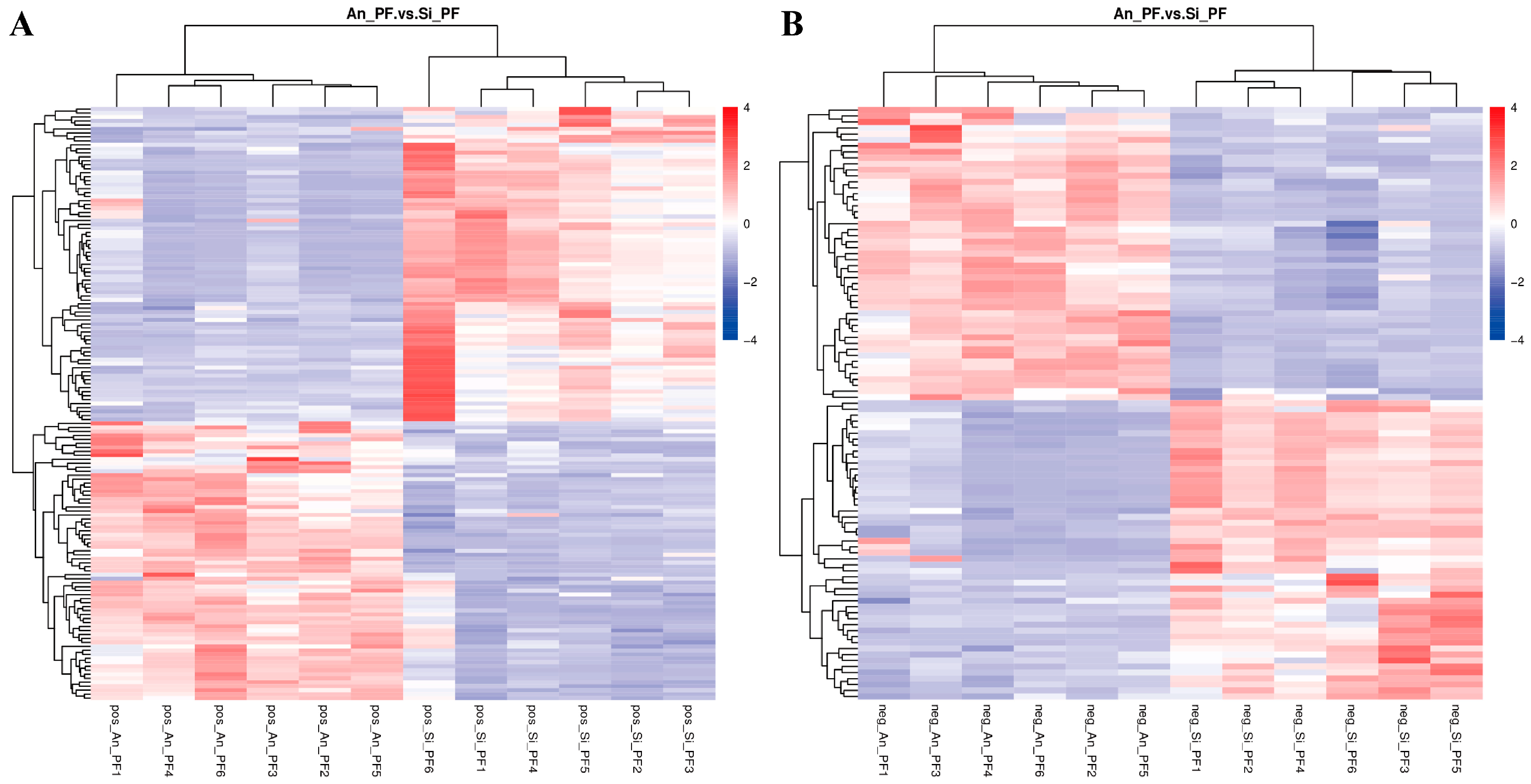
3.4. Screening of Differential Metabolites
3.5. Enrichment Pathway Analysis of Differential Metabolites
4. Discussion
4.1. Effects of Different Metabolites on Perirenal Fat Metabolism
4.2. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Chen, J.; Wang, X.; Han, L.; Yang, Y.; Wang, Q.; Yu, Q. Metagenomic and transcriptomic analyses reveal the differences and associations between the gut microbiome and muscular genes in Angus and Chinese Simmental cattle. Front. Microbiol. 2022, 13, 815915. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, denovo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, G.; Yamada, T.; Liu, J.; Zan, L.; Tong, B. Effect of expressions and SNPs of candidate genes on intramuscular fat content in Qinchuan cattle. Animals 2020, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kamiya, M.; Higuchi, M. Metabolomic analysis of plasma and intramuscular adipose tissue between Wagyu and Holstein cattle. J. Vet. Med. Sci. 2022, 84, 186–192. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, R.; Song, Z.; Shi, D.; Huang, J. Diversity in cell morphology, composition, and function among adipose depots in river buffaloes. Int. J. Mol. Sci. 2023, 24, 8410. [Google Scholar] [CrossRef]
- Alonso-García, M.; Suárez-Vega, A.; Fonseca, P.A.S.; Marina, H.; Pelayo, R.; Mateo, J.; Arranz, J.J.; Gutiérrez-Gil, B. Transcriptome analysis of perirenal fat from Spanish Assaf suckling lamb carcasses showing different levels of kidney knob and channel fat. Front. Vet. Sci. 2023, 10, 1150996. [Google Scholar] [CrossRef]
- Jiang, M.; Li, M.; Liu, C.; Jing, L.; Huang, Q.; Wu, T.; Kong, X.; Liu, J. Perirenal fat volume is positively associated with serum uric acid levels in Chinese adults. Front. Endocrinol 2022, 13, 865009. [Google Scholar] [CrossRef]
- Xia, S.; Shao, J.; Elzo, M.A.; Tang, T.; Li, Y.; Lai, T.; Gan, M.; Ma, Y.; Jia, X.; Lai, S.; et al. Untargeted metabolomics analysis revealed lipometabolic disorders in perirenal adipose tissue of rabbits subject to a high-fat diet. Animals 2021, 11, 2289. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Luo, M.; Chen, N.; Deng, X.; He, J.; Zhang, L.; Luo, P.; Wu, J. Inhibition of PAI-1 attenuates perirenal fat inflammation and the associated nephropathy in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E260–E267. [Google Scholar] [CrossRef]
- Foster, M.T.; Pagliassotti, M.J. Metabolic alterations following visceral fat removal and expansion: Beyond anatomic location. Adipocyte 2012, 1, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Depreester, E.; De Koster, J.; Van Poucke, M.; Hostens, M.; Van den Broeck, W.; Peelman, L.; Contreras, G.A.; Opsomer, G. Influence of adipocyte size and adipose depot on the number of adipose tissue macrophages and the expression of adipokines in dairy cows at the end of pregnancy. J. Dairy Sci. 2018, 101, 6542–6555. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Z.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, H.Y.; Guan le, L.; Liu, J.X. Metabolomics of four biofluids from dairy cows: Potential biomarkers for milk production and quality. J. Proteome. Res. 2015, 14, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- ISO SAC/TC 281; Guidelines for Ethical Review of Laboratory Animal Welfare. GB/T 35892-2018; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 6 February 2018.
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Yanting, C.; Yang, Q.Y.; Ma, G.L.; Du, M.; Harrison, J.H.; Block, E. Dose- and type-dependent effects of long-chain fatty acids on adipogenesis and lipogenesis of bovine adipocytes. J. Dairy Sci. 2018, 101, 1601–1615. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of oleic acid in the gut-liver axis: From diet to the regulation of its synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Nagaya, H.; Kanno, T.; Nishizaki, T. Oleic acid stimulates glucose uptake into adipocytes by enhancing insulin receptor signaling. J. Pharmacol. Sci. 2014, 126, 337–343. [Google Scholar] [CrossRef]
- Regassa, A.; Kim, W.K. Effects of oleic acid and chicken serum on the expression of adipogenic transcription factors and adipogenic differentiation in hen preadipocytes. Cell. Biol. Int. 2013, 37, 961–971. [Google Scholar] [CrossRef]
- Malodobra-Mazur, M.; Cierzniak, A.; Dobosz, T. Oleic acid influences the adipogenesis of 3T3-L1 cells via DNA Methylation and may predispose to obesity and obesity-related disorders. Lipids. Health Dis. 2019, 18, 230. [Google Scholar] [CrossRef]
- Komiya, Y.; Iseki, S.; Ochiai, M.; Takahashi, Y.; Yokoyama, I.; Suzuki, T.; Tatsumi, R.; Sawano, S.; Mizunoya, W.; Arihara, K. Dietary oleic acid intake increases the proportion of type 1 and 2X muscle fibers in mice. Sci. Rep. 2024, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Campos, H.; McGarvey, S.; Wu, Z.; Goldberg, R.; Baylin, A. Adipose tissue palmitoleic acid and obesity in humans: Does it behave as a lipokine? Am. J. Clin. Nutr. 2011, 93, 186–191. [Google Scholar] [CrossRef]
- Paillard, F.; Catheline, D.; Duff, F.L.; Bouriel, M.; Deugnier, Y.; Pouchard, M.; Daubert, J.C.; Legrand, P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.M.; Simão, J.J.; de Sá, R.D.C.C.; Farias, T.S.M.; da Silva, V.S.; Abdala, F.; Antraco, V.J.; Armelin-Correa, L.; Alonso-Vale, M.I.C. Palmitoleic acid decreases non-alcoholic hepatic steatosis and increases lipogenesis and fatty acid oxidation in adipose tissue from obese mice. Front. Endocrinol. 2020, 11, 537061. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid. Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Song, Y.; Zhang, X.; Wang, C. Effect of omega-3 polyunsaturated fatty acids-derived bioactive lipids on metabolic disorders. Front. Physiol. 2021, 12, 646491. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Harwood, J.L. Polyunsaturated fatty acids: Conversion to lipid mediators, roles in inflammatory diseases and dietary sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm (2020) 2023, 4, e363. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, X.; Li, Y.; Geng, X.; Jia, X.; Zhang, L.; Yang, H. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARgamma dependent manner. Front. Immunol. 2021, 12, 618501. [Google Scholar]
- Demmelmair, H.; Koletzko, B. Perinatal polyunsaturated fatty acid status and obesity risk. Nutrients 2021, 13, 3882. [Google Scholar] [CrossRef]
- Lands, B. Omega-3 PUFAs lower the propensity for arachidonic acid cascade overreactions. Biomed. Res. Int. 2015, 2015, 285135. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Huang, Q.; Chen, Y.; Zeng, K.; Yang, W.; Chen, J.; Chen, J. Important Hormones regulating lipid metabolism. Molecules 2022, 27, 7052. [Google Scholar] [CrossRef]
- Omar, B.; Zmuda-Trzebiatowska, E.; Manganiello, V.; Göransson, O.; Degerman, E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: Roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell. Signal. 2009, 21, 760–766. [Google Scholar] [CrossRef]
- Hattori, A.; Mawatari, K.; Tsuzuki, S.; Yoshioka, E.; Toda, S.; Yoshida, M.; Yasui, S.; Furukawa, H.; Morishima, M.; Ono, K.; et al. Beta-adrenergic-AMPK pathway phosphorylates acetyl-CoA carboxylase in a high-epinephrine rat model, SPORTS. Obesity 2010, 18, 48–54. [Google Scholar] [CrossRef]
- Rappou, E.; Jukarainen, S.; Rinnankoski-Tuikka, R.; Kaye, S.; Heinonen, S.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Saunavaara, V.; Rissanen, A.; et al. Weight loss is associated with increased NAD(+)/SIRT1 expression but reduced PARP activity in white adipose tissue. J. Clin. Endocrinol. Metab. 2016, 101, 1263–1273. [Google Scholar] [CrossRef]
- Wei, X.; Jia, R.; Wang, G.; Hong, S.; Song, L.; Sun, B.; Chen, K.; Wang, N.; Wang, Q.; Luo, X.; et al. Depot-specific regulation of NAD(+)/SIRTs metabolism identified in adipose tissue of mice in response to high-fat diet feeding or calorie restriction. J. Nutr. Biochem. 2020, 80, 108377. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell. Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, X.; Zhou, X.; Wang, Z.; Li, S.; Sun, Q.; Jiang, Y.; Ji, C.; Ling, W.; An, X.; et al. Spermidine alleviating oxidative stress and apoptosis by inducing autophagy of granulosa cells in Sichuan white geese. Poult. Sci. 2023, 102, 102879. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.Q.; Liu, Y.S. New insights into the roles and mechanisms of spermidine in aging and age-related diseases. Aging. Dis. 2021, 12, 1948–1963. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Content/% | Nutrient Composition | Content/% |
|---|---|---|---|
| Straw | 25.00 | NEmf (MJ/kg) | 7.01 |
| Corn silage | 25.00 | Crude protein | 13.11 |
| Corn | 25.00 | Neutral detergent fiber | 34.91 |
| Bran | 9.00 | Acid detergent fiber | 19.88 |
| Soybean meal | 7.00 | Hemicellulose | 16.59 |
| Cottonseed meal | 6.00 | Cellulose | 16.42 |
| Urea | 0.60 | Crude ash | 8.44 |
| CaCO3 | 0.75 | Acid detergent lignin | 2.39 |
| NaCl | 0.55 | ||
| NaHCO3 | 0.35 | ||
| CaHPO4 | 0.25 | ||
| Premix 1 | 0.50 | ||
| Total | 100.00 |
| Differential Metabolites | FC | VIP | p-Value | Up–Down Regulation 1 |
|---|---|---|---|---|
| Oleic acid | 4.91 | 1.58 | p < 0.01 | Up |
| Palmitoleic acid | 3.25 | 1.49 | p < 0.01 | Up |
| Palmitic acid | 2.46 | 1.05 | p = 0.02 | Up |
| Arachidonic acid | 2.98 | 1.15 | p = 0.01 | Up |
| Prostaglandin H2 | 2.46 | 1.39 | p < 0.01 | Up |
| Docosahexaenoic acid | 4.13 | 1.19 | p = 0.01 | Up |
| Docosapentaenoic acid | 6.99 | 1.43 | p < 0.01 | Up |
| Eicosapentaenoic acid | 1.70 | 1.28 | p < 0.01 | Up |
| Adrenaline | 0.13 | 1.31 | p < 0.01 | Down |
| NAD+ | 0.28 | 1.35 | p < 0.01 | Down |
| NADH | 0.32 | 1.06 | p = 0.01 | Down |
| Spermidine | 0.19 | 1.37 | p < 0.01 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Pang, Y.; Wang, L.; Wang, Q.; Chen, Z.; Li, C.; Li, F.; Zhang, G.; Wang, X.; Gao, S.; et al. Differences in Lipid Metabolism between the Perirenal Adipose Tissue of Chinese Simmental Cattle and Angus Cattle (Bos taurus) Based on Metabolomics Analysis. Animals 2024, 14, 2536. https://doi.org/10.3390/ani14172536
Wang S, Pang Y, Wang L, Wang Q, Chen Z, Li C, Li F, Zhang G, Wang X, Gao S, et al. Differences in Lipid Metabolism between the Perirenal Adipose Tissue of Chinese Simmental Cattle and Angus Cattle (Bos taurus) Based on Metabolomics Analysis. Animals. 2024; 14(17):2536. https://doi.org/10.3390/ani14172536
Chicago/Turabian StyleWang, Siyuan, Yue Pang, Lixiang Wang, Qi Wang, Zhongling Chen, Chengjiao Li, Fengjiao Li, Guoxi Zhang, Xiaoying Wang, Shuxin Gao, and et al. 2024. "Differences in Lipid Metabolism between the Perirenal Adipose Tissue of Chinese Simmental Cattle and Angus Cattle (Bos taurus) Based on Metabolomics Analysis" Animals 14, no. 17: 2536. https://doi.org/10.3390/ani14172536
APA StyleWang, S., Pang, Y., Wang, L., Wang, Q., Chen, Z., Li, C., Li, F., Zhang, G., Wang, X., Gao, S., & Xu, X. (2024). Differences in Lipid Metabolism between the Perirenal Adipose Tissue of Chinese Simmental Cattle and Angus Cattle (Bos taurus) Based on Metabolomics Analysis. Animals, 14(17), 2536. https://doi.org/10.3390/ani14172536






