Morpho-Geometric Description of the Skulls and Mandibles of Brown Bears (Ursus arctos) from the Dancing Bear Belitsa Park
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Skulls and Mandibles
2.2. Modeling
2.3. Data
2.4. Statistical Analysis
3. Results
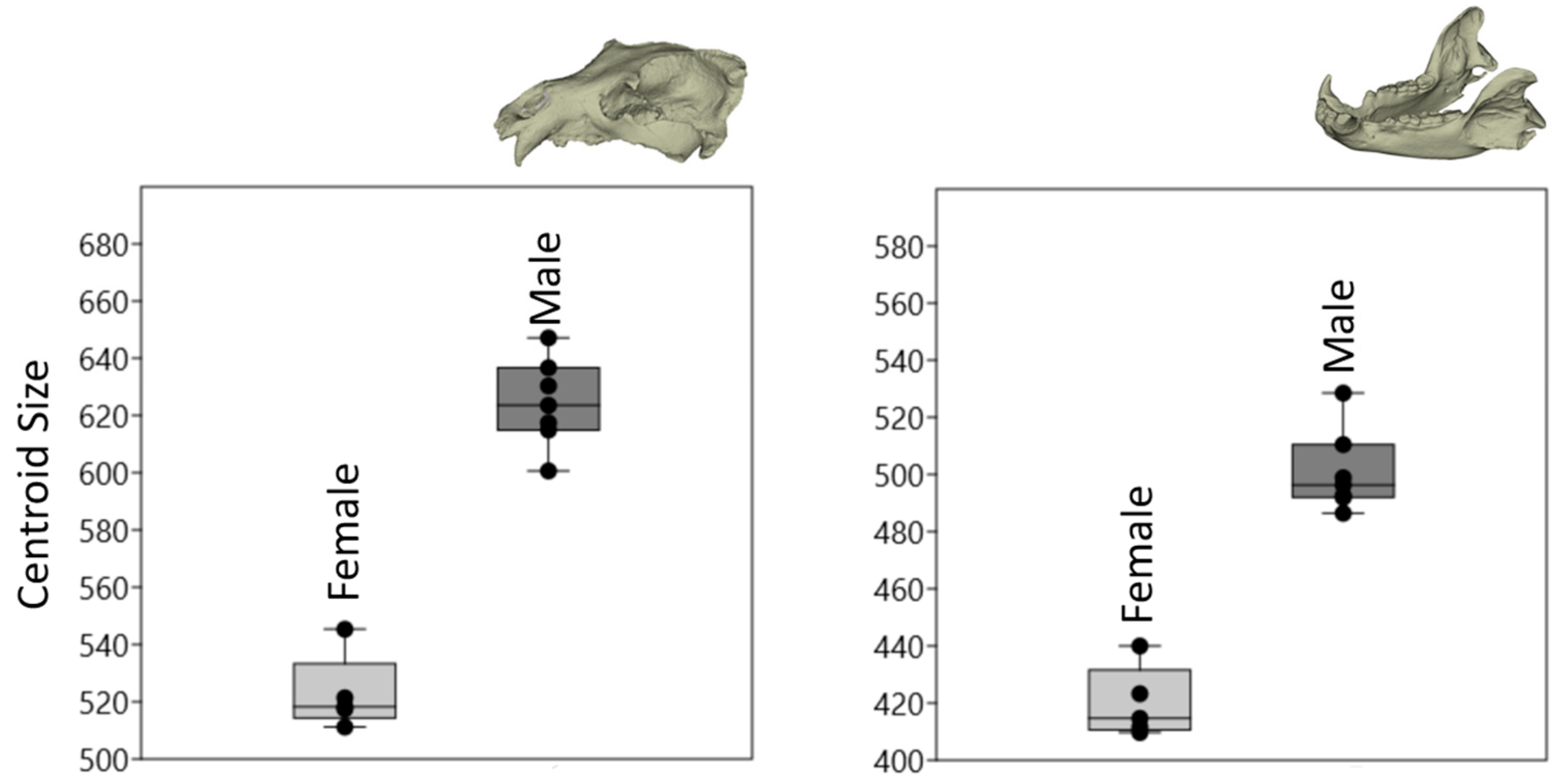
3.1. Size
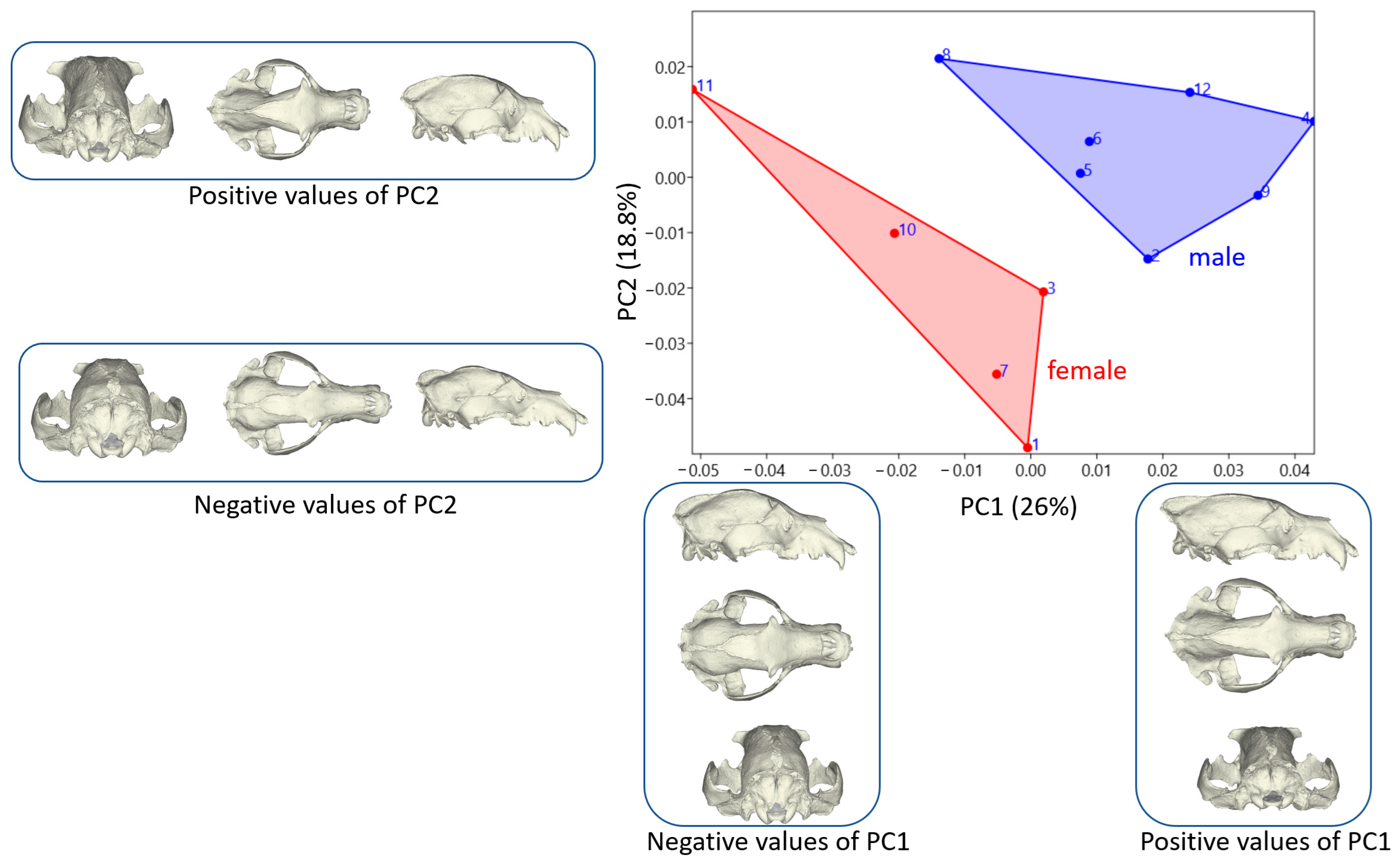
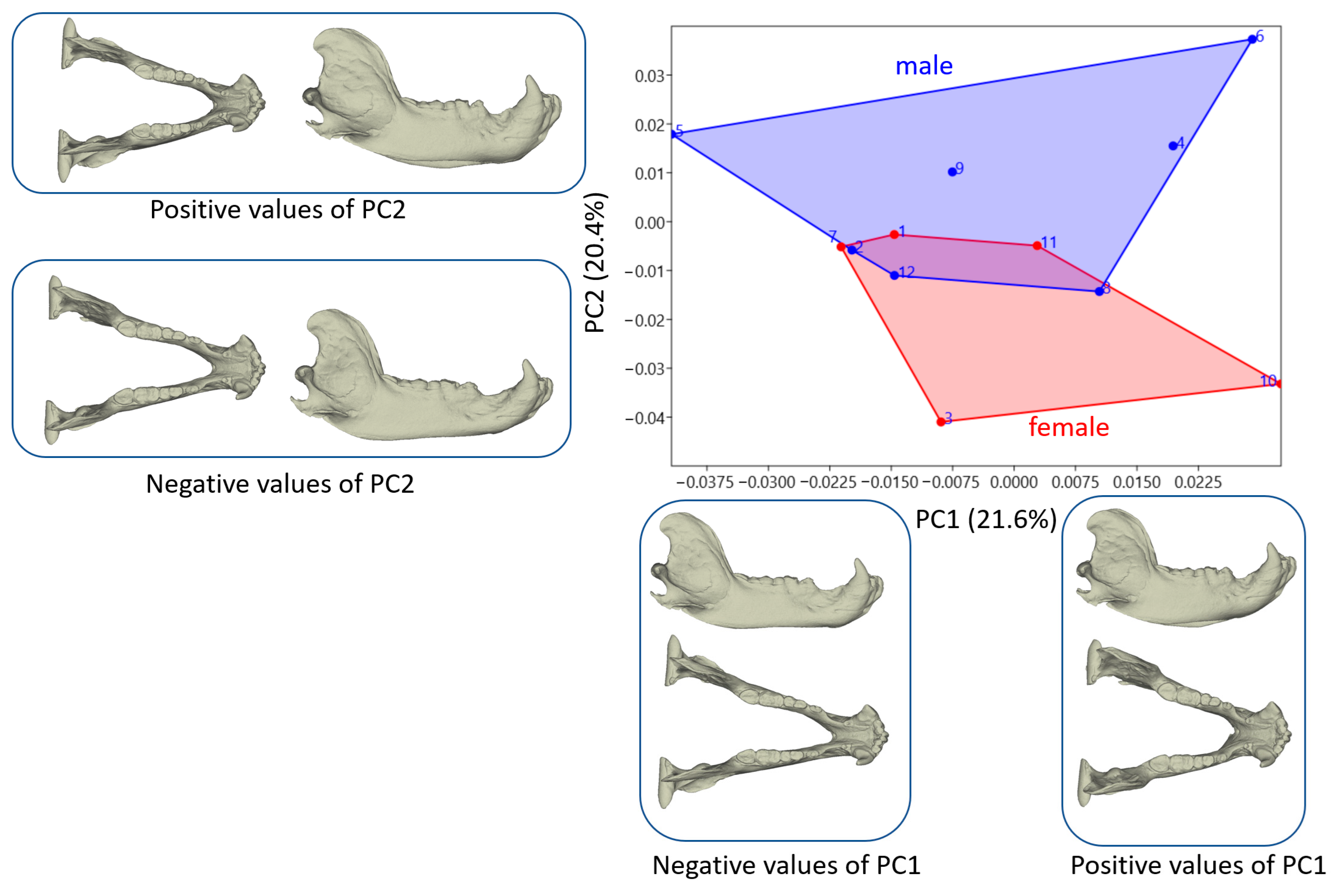
3.2. Shape Variation
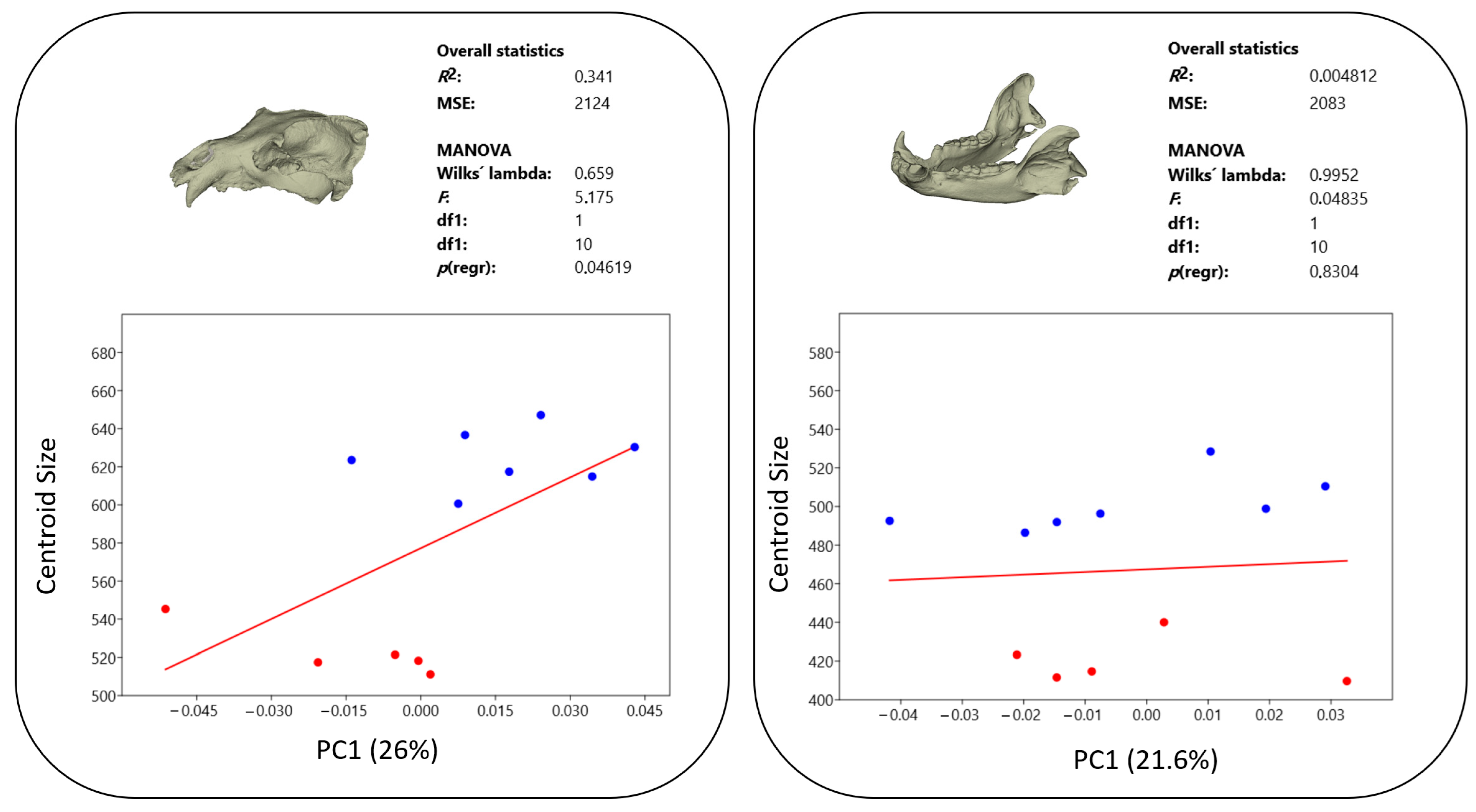
3.3. Allometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLellan, B.; Reiner, D.C. A review of bear evolution. Bears Biol. Manag. 1994, 9, 85–96. [Google Scholar] [CrossRef]
- Wagner, J. Pliocene to early Middle Pleistocene ursine bears in Europe: A taxonomic overview. J. Natl. Mus. (Prague). Nat. Hist. Ser. 2010, 179, 197–215. [Google Scholar]
- Brown, G. Bear Anatomy and Physiology from the Great Bear Almanac; Lyons & Burford: New York, NY, USA, 1993; pp. 46–93. [Google Scholar]
- Slice, D.E. Geometric morphometrics. Annu. Rev. Anthropol. 2007, 36, 261–281. [Google Scholar] [CrossRef]
- Boz, İ.; Altundağ, Y.; Szara, T.; Hadziomerovic, N.; Ince, N.G.; Pazvant, G.; Kahvecioğlu, O.; Özkan, E.; Manuta, N.; Gundemir, O. Geometric morphometry in veterinary anatomy. Veterinaria 2023, 72, 15–27. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Akçasız ZN, Akbaş ZS, Özkan E, Manuta N, Sarıtaş Ö, Szara T, Spataru MC, Aydın Kaya D: Geometric morphometric analysis of scapula at cats and dogs. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 481–487.
- Gjoni Gundemir, M.; Yildiz, A.; Büyükünal, S.; Muratoğlu, K.; Özkan, E.; Demircioglu, A.; Choudhary, O.P.; Guzel, B. Evaluation of Cold Carcasses of Kivircik and Romanov Lambs by Geometric Morphometric Method. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 87–94. [Google Scholar] [CrossRef]
- Demircioğlu, I.; Demiraslan, Y.; Gürbüz, I.; Dayan, M.O. Geometric morphometric analysis of skull and mandible in Awassi ewe and ram. Kafkas Üniv. Vet. Fak. Derg. 2021, 27, 43–49. [Google Scholar] [CrossRef]
- Mcnulty, K.P.; Vinyard, C.J. Morphometry, geometry, function, and the future. Anat. Rec. 2015, 298, 328–333. [Google Scholar] [CrossRef]
- Adams, D.C.; Otárola-Castillo, E. Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P. Advances in geometric morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Kimmerle, E.H.; Ross, A.; Slice, D. Sexual dimorphism in America: Geometric morphometric analysis of the craniofacial region. J. Forensic. Sci. 2008, 53, 54–57. [Google Scholar] [CrossRef]
- Hennessy, R.J.; Stringer, C.B. Geometric morphometric study of the regional variation of modern human craniofacial form. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2002, 117, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wescott, D.J.; Jantz, R.L. Assessing craniofacial secular change in American Blacks and Whites using geometric morphometry. In Modern Morphometrics in Physical Anthropology; Springer: Boston, MA, USA, 2005; pp. 231–245. [Google Scholar]
- Gündemir, O.; Koungoulos, L.; Szara, T.; Duro, S.; Spataru, M.; Michaud, M.; Onar, V. Cranial morphology of Balkan and West Asian livestock guardian dogs. Am. J. Anat. 2023, 243, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Manuta, N.; Çakar, B.; Gündemir, O.; Spataru, M.-C. Shape and size variations of distal phalanges in cattle. Animals 2024, 14, 194. [Google Scholar] [CrossRef]
- López-Cisneros, P.; Linares-Matás, G.; Yravedra, J.; Maté-González, M.; Estaca-Gómez, V.; Mora, R.; Aramendi, J.; Asensio, J.A.R.; Barrera-Logares, J.M.; Aguilera, D.G. Applying new technologies to the taphonomic study of La Lluera (Asturias, Spain). Geometric morphometrics and the study of bone surface modifications (BSM). Quat. Int. 2019, 517, 107–117. [Google Scholar] [CrossRef]
- Ağaç, D.K.; Onuk, B.; Gündemir, O.; Kabak, M.; Manuta, N.; Çakar, B.; Janeczek, M.; Crampton, D.A.; Szara, T. Comparative cranial geometric morphometrics among Wistar albino, Sprague Dawley, and WAG/Rij rat strains. Animals 2024, 14, 1274. [Google Scholar] [CrossRef]
- Koçak, S.; Özaydin, I.; Gündemir, O. Shape analysis of the carpal joint in healthy and septic arthritis in newborn calves. Anat. Histol. Embryol. 2024, 53, e13080. [Google Scholar] [CrossRef] [PubMed]
- Hadžiomerović, N.; Gundemir, O.; Tandir, F.; Avdić, R.; Katica, M. Geometric and morphometric analysis of the auditory ossicles in the red fox (Vulpes vulpes). Animals 2023, 13, 1230. [Google Scholar] [CrossRef]
- Drake, A.G. Dispelling dog dogma: An investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape. Evol. Dev. 2011, 13, 204–213. [Google Scholar] [CrossRef]
- Lawing, A.M.; Polly, P.D. Geometric morphometrics: Recent applications to the study of evolution and development. J. Zool. 2010, 280, 1–7. [Google Scholar] [CrossRef]
- Güzel, B.C.; Manuta, N.; Ünal, B.; Ruzhanova-Gospodinova, I.S.; Duro, S.; Gündemir, O.; Szara, T. Size and shape of the neurocranium of laying chicken breeds. Poult. Sci. 2024, 103, 104008. [Google Scholar] [CrossRef] [PubMed]
- van Heteren, A.H.; MacLarnon, A.; Soligo, C.; Rae, T. 3D geometric morphometrical analyses of intraspecific variation in the mandible of Ursus spelaeus from the Alpine region. Braunschweiger Naturkundliche Schriften 2012, 11, 111–128. [Google Scholar]
- Hartstone-Rose, A.; Dickinson, E.; Deutsch, A.R.; Worden, N.; Hirschkorn, G.A. Masticatory muscle architectural correlates of dietary diversity in Canidae, Ursidae, and across the order Carnivora. Anat. Rec. 2022, 305, 477–497. [Google Scholar] [CrossRef]
- Figueirido, B.; MacLeod, N.; Krieger, J.; De Renzi, M.; Pérez-Claros, J.A.; Palmqvist, P. Constraint and adaptation in the evolution of carnivoran skull shape. Paleobiology 2011, 37, 490–518. [Google Scholar] [CrossRef]
- Sacco, T.; Van Valkenburgh, B. Ecomorphological indicators of feeding behaviour in the bears (Carnivora: Ursidae). J. Zool. 2004, 263, 41–54. [Google Scholar] [CrossRef]
- van Heteren, A.H.; MacLarnon, A.; Soligo, C.; Rae, T.C. Functional morphology of the cave bear (Ursus spelaeus) mandible: A 3D geometric morphometric analysis. Org. Divers. Evol. 2016, 16, 299–314. [Google Scholar] [CrossRef]
- Kupczik, K.; Stynder, D.D. Tooth root morphology as an indicator for dietary specialization in carnivores (Mammalia: Carnivora). Biol. J. Linn. Soc. 2012, 105, 456–471. [Google Scholar] [CrossRef]
- van Heteren, A.H.; MacLarnon, A.; Soligo, C.; Rae, T.C. Functional morphology of the cave bear (Ursus spelaeus) cranium: A three-dimensional geometric morphometric analysis. Quat. Int. 2014, 339, 209–216. [Google Scholar] [CrossRef]
- Van Heteren, A.; MacLarnon, A.; Rae, T.; Soligo, C. Cave bears and their closest living relatives: A 3D geometric morphometrical approach to the functional morphology of the cave bear Ursus spelaeus. Acta Carsologica Slovaka 2009, 47 (Suppl. S1), 33–46. [Google Scholar]
- Bock, W.J.; Von Wahlert, G. Adaptation and the form-function complex. Evolution 1965, 19, 269–299. [Google Scholar] [CrossRef]
- Clauss, M.; Hume, I.D.; Hummel, J. Evolutionary adaptations of ruminants and their potential relevance for modern production systems. Animal 2010, 4, 979–992. [Google Scholar] [CrossRef]
- Rolfe, S.; Pieper, S.; Porto, A.; Diamond, K.; Winchester, J.; Shan, S.; Kirveslahti, H.; Boyer, D.; Summers, A.; Maga, A.M. SlicerMorph: An open and extensible platform to retrieve, visualize, and analyze 3D morphology. Methods Ecol. Evol. 2021, 12, 1816–1825. [Google Scholar] [CrossRef]
- Porto, A.; Rolfe, S.; Maga, A.M. ALPACA: A fast and accurate computer vision approach for automated landmarking of three-dimensional biological structures. Methods Ecol. Evol. 2021, 12, 2129–2144. [Google Scholar] [CrossRef]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1986; Volume 2. [Google Scholar]
- Brown, J.H.; Sibly, R.M. Life-history evolution under a production constraint. Proc. Natl. Acad. Sci. USA 2006, 103, 17595–17599. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Endo, H.; Yamagiwa, D.; Takagi, H.; Arishima, K.; Makita, T.; Hayashi, Y. Adaptation of the muscles of mastication to the flat skull feature in the polar bear (Ursus maritimus). J. Veter. Med. Sci. 2000, 62, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Duro, S.; Gündemir, O.; Sönmez, B.; Jashari, T.; Szara, T.; Pazvant, G.; Kambo, A. A different perspective on sex dimorphism in the adult Hermann’s tortoise: Geometric morphometry. Zool. Stud. 2021, 60, e9. [Google Scholar]
- Gonzalez, P.N.; Bernal, V.; Perez, S.I. Geometric morphometric approach to sex estimation of human pelvis. Forensic. Sci. Int. 2009, 189, 68–74. [Google Scholar] [CrossRef]
- Jashari, T.; Kahvecioğlu, O.; Duro, S.; Gündemir, O. Morphometric analysis for the sex determination of the skull of the Deltari Ilir dog (Canis lupus familiaris) of Kosovo. Anat. Histol. Embryol. 2022, 51, 443–451. [Google Scholar] [CrossRef]
- Ghanbari, F.; Kaboli, M.; Eagderi, S.; Nezami Balouchi, B. Sexual dimorphism in skull morphology of the brown bear (Ursus arctos Linnaeus, 1758) in Iran using geometric morphometric technique. Taxon. Biosyst. 2013, 5, 17–26. [Google Scholar]
- Nezami, B.; Eagdari, S.; Barkhor, M.; Sasanfar, Z. Sexual dimorphism of cranial shape in Iranian brown bear Ursus arctos Linnaeus, 1758 using geometric morphometric approach. BEPLS 2014, 3, 329–335. [Google Scholar]
- Milošević-Zlatanović, S.; Kolarov, N.T.; Vukov, T.; Stamenković, S. Correlation patterns in roe deer cranium: Sexual dimorphism across different habitats. J. Zool. 2016, 300, 291–304. [Google Scholar] [CrossRef]
- Szara, T.; Klich, D.; Wójcik, A.M.; Olech, W. Temporal Trends in Skull Morphology of the European Bison from the 1950s to the Present Day. Diversity 2023, 15, 377. [Google Scholar] [CrossRef]
- Gittleman, J.L.; Van Valkenburgh, B. Sexual dimorphism in the canines and skulls of carnivores: Effects of size, phylogency, and behavioural ecology. J. Zool. 1997, 242, 97–117. [Google Scholar] [CrossRef]
- Forbes-Harper, J.L.; Crawford, H.M.; Dundas, S.J.; Warburton, N.M.; Adams, P.J.; Bateman, P.W.; Calver, M.C.; Fleming, P.A. Diet and bite force in red foxes: Ontogenetic and sex differences in an invasive carnivore. J. Zool. 2017, 303, 54–63. [Google Scholar] [CrossRef]
- Law, C.J.; Mehta, R.S. Carnivory maintains cranial dimorphism between males and females: Evidence for niche divergence in extant Musteloidea. Evolution 2018, 72, 1950–1961. [Google Scholar] [CrossRef]

| Samples | Sex | Age | Weight (kg, Approximately) |
|---|---|---|---|
| 1 | Female | 39 | 100 |
| 2 | Male | 34 | 250 |
| 3 | Female | 30 | 150 |
| 4 | Male | 29 | 300 |
| 5 | Male | 32 | 200 |
| 6 | Male | 31 | 300 |
| 7 | Female | 37 | 120 |
| 8 | Male | 33 | 206 |
| 9 | Male | 36 | 200 |
| 10 | Female | 37 | 100 |
| 11 | Female | 34 | 100 |
| 12 | Male | Unknown | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzhanova-Gospodinova, I.S.; Vladova, S.; Szara, T.; Tandir, F.; Szara, E.; Yalin, E.E.; Gündemir, O. Morpho-Geometric Description of the Skulls and Mandibles of Brown Bears (Ursus arctos) from the Dancing Bear Belitsa Park. Animals 2024, 14, 2541. https://doi.org/10.3390/ani14172541
Ruzhanova-Gospodinova IS, Vladova S, Szara T, Tandir F, Szara E, Yalin EE, Gündemir O. Morpho-Geometric Description of the Skulls and Mandibles of Brown Bears (Ursus arctos) from the Dancing Bear Belitsa Park. Animals. 2024; 14(17):2541. https://doi.org/10.3390/ani14172541
Chicago/Turabian StyleRuzhanova-Gospodinova, Iliana Stefanova, Silvi Vladova, Tomasz Szara, Faruk Tandir, Ewa Szara, Ebru Eravci Yalin, and Ozan Gündemir. 2024. "Morpho-Geometric Description of the Skulls and Mandibles of Brown Bears (Ursus arctos) from the Dancing Bear Belitsa Park" Animals 14, no. 17: 2541. https://doi.org/10.3390/ani14172541
APA StyleRuzhanova-Gospodinova, I. S., Vladova, S., Szara, T., Tandir, F., Szara, E., Yalin, E. E., & Gündemir, O. (2024). Morpho-Geometric Description of the Skulls and Mandibles of Brown Bears (Ursus arctos) from the Dancing Bear Belitsa Park. Animals, 14(17), 2541. https://doi.org/10.3390/ani14172541









