Relationship between Plasma and Saliva Urea Nitrogen Concentrations in New Zealand Red Deer Calves (Cervus elaphus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatment
2.2. Care and Use of Animals
2.3. Herbage
2.4. Statistical Analysis
3. Results
3.1. Diet Composition and Nutritional Value
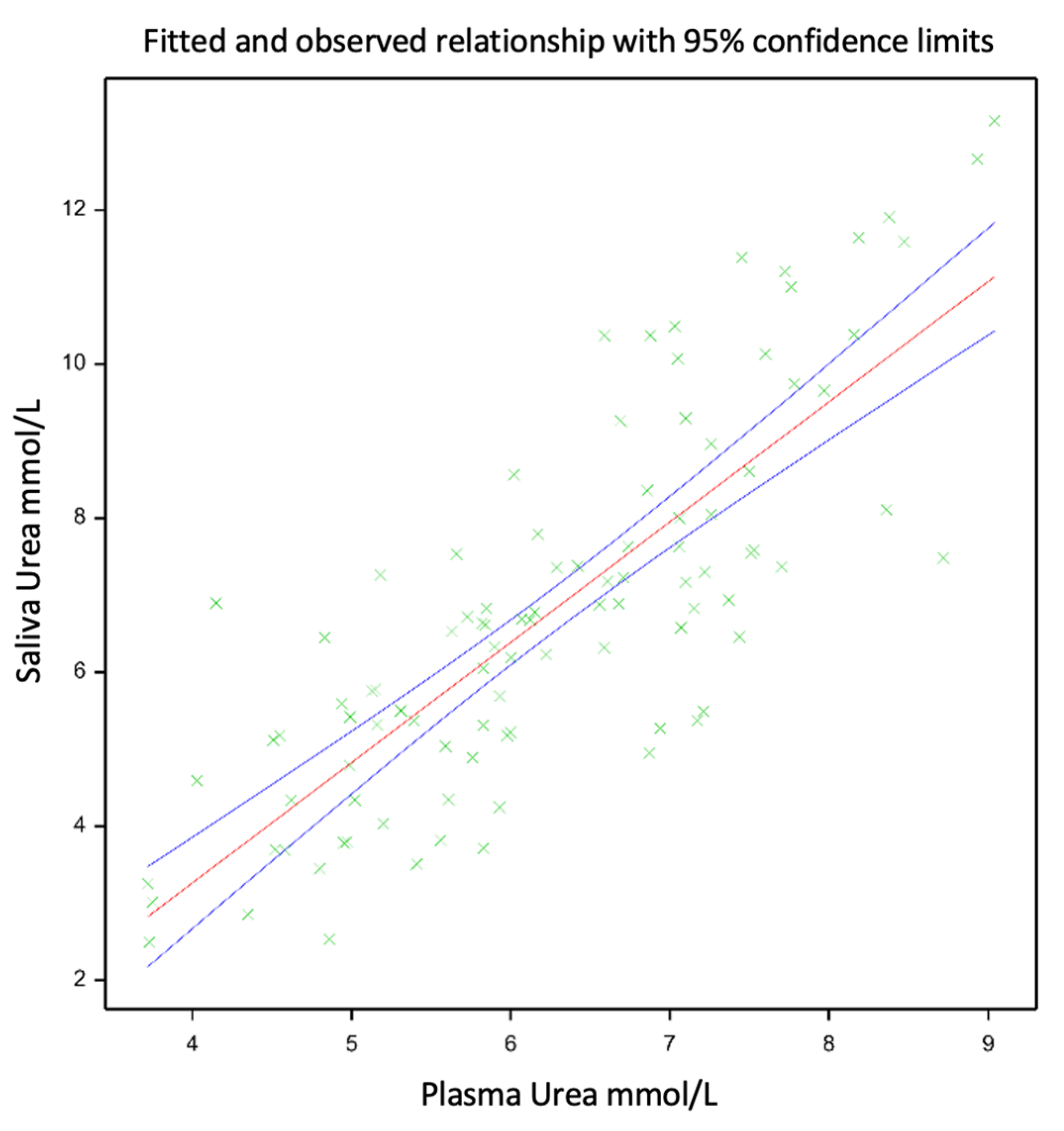
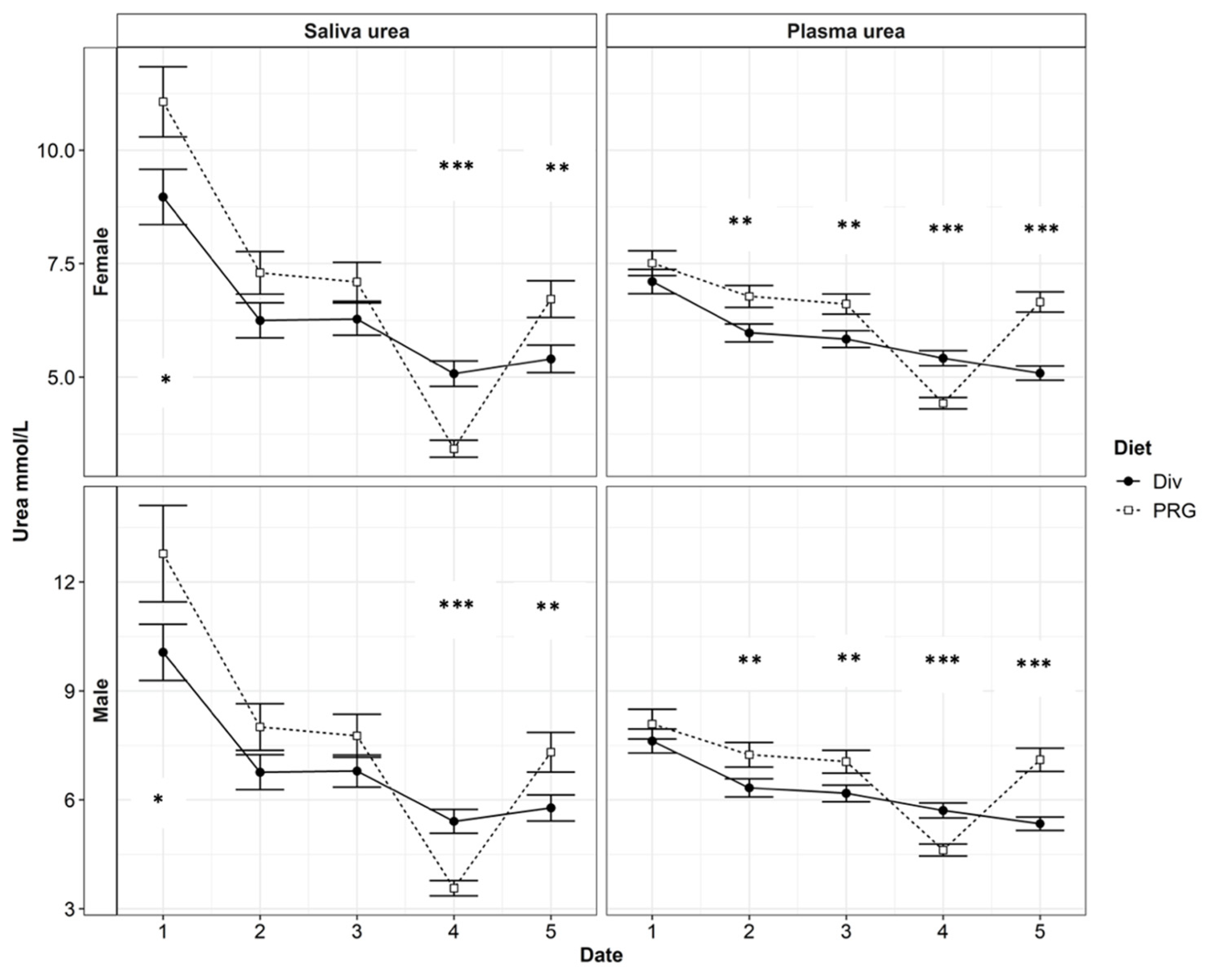
3.2. Relationship between Plasma Urea N and Saliva Urea N
4. Discussion
4.1. The Relationship between Saliva Urea N and Plasma Urea N
4.2. Significant Effects on the Relationship between Saliva Urea N and Plasma Urea N
4.3. Estimation of Plasma Urea Nitrogen from Saliva Urea Nitrogen
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncan, R. Regulating Agricultural Land Use to Manage Water Quality: The Challenges for Science and Policy in Enforcing Limits on Non-Point Source Pollution in New Zealand. Land Use Policy 2014, 41, 378–387. [Google Scholar] [CrossRef]
- Ministry-for-the-Environment. Pressures on Our Water Environment. (ME 612); GP Publications: Wellington, New Zealand, 2021. Available online: https://environment.govt.nz/assets/Publications/Files/ser-1997.pdf (accessed on 15 September 2022).
- Scarsbrook, M.R.; Melland, A.R. Dairying and Water-Quality Issues in Australia and New Zealand. Anim. Prod. Sci. 2015, 55, 856–868. [Google Scholar] [CrossRef]
- Castillo, A.; Kebreab, E.; Beever, D.; Barbi, J.; Sutton, J.; Kirby, H.; Ja, F. The Effect of Protein Supplementation on Nitrogen Utilization in Lactating Dairy Cows fed Grass Silage Diets. J. Anim. Sci. 2001, 79, 247–253. [Google Scholar] [CrossRef]
- Gregorini, P.; Beukes, P.; Romera, A.; Clark, C.; Clark, D. A Preliminary Investigation of Individual Variation in N Excretion by Lactating Dairy Cows. J. Dairy Sci. 2010, 93, 409–410. [Google Scholar]
- Moir, J.; Cameron, K.; Di, H. Potential Pasture Nitrogen Concentrations and Uptake from Autumn or Spring Applied Cow Urine and DCD under Field Conditions. Plants 2016, 5, 26. [Google Scholar] [CrossRef]
- Higgins, C. Urea and the Clinical Value of Measuring Blood Urea Concentration. 2016. Available online: https://acutecaretesting.org/en/articles/urea-and-the-clinical-value-of-measuring-blood-urea-concentration (accessed on 1 October 2022).
- Orsonneau, J.L.; Massoubre, C.; Cabanes, M.; Lustenberger, P. Simple and Sensitive Determination of Urea in Serum and Urine. Clin. Chem. 1992, 38, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Carragher, J.F.; Ingram, J.R.; Matthews, L.R. Effects of Yarding and Handling Procedures on Stress Responses of Red Deer Stags (Cervus elaphus). Appl. Anim. Behav. Sci. 1997, 51, 143–158. [Google Scholar] [CrossRef]
- Bhave, G.; Neilson, E.G. Body Fluid Dynamics: Back to the Future. J. Am. Soc. Nephrol. 2011, 22, 2166–2181. [Google Scholar] [CrossRef]
- Dall Orsoletta, A.; Almeida, J.G.; Oziemblowski, M.; Ribeiro-Filho, H. Corn Supplementation on Milk Urea Nitrogen Content of Dairy Cows Grazing on Temperate Annual Pasture. Ciênc. Rural 2020, 50, e20190077. [Google Scholar] [CrossRef]
- Marshall, C.J.; Beck, M.R.; Garrett, K.; Barrell, G.K.; Al-Marashdeh, O.; Gregorini, P. Grazing Dairy Cows with Low Milk Urea Nitrogen Breeding Values Excrete Less Urinary Urea Nitrogen. Sci. Total Environ. 2020, 739, 139994. [Google Scholar] [CrossRef]
- Bartle, S.J.; Preston, R.L. Plasma, Rumen and Urine Pools in Urea Dilution Determination of Body Composition in Cattle. J. Anim. Sci. 1986, 63, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Spek, J.W.; Dijkstra, J.; Van Duinkerken, G.; Bannink, A. A Review of Factors Influencing Milk Urea Concentration and its Relationship with Urinary Urea Excretion in Lactating Dairy Cattle. J. Agric. Sci. 2013, 151, 407–423. [Google Scholar] [CrossRef]
- Bilancio, G.; Cavallo, P.; Lombardi, C.; Guarino, E.; Cozza, V.; Giordano, F.; Cirillo, M. Salivary Levels of Phosphorus and Urea as Indices of their Plasma Levels in Nephropathic Patients. J. Clin. Lab. Anal. 2018, 32, e22449. [Google Scholar] [CrossRef] [PubMed]
- Kovalčíková, A.; Janšáková, K.; Gyurászová, M.; Podracká, Ľ.; Šebeková, K.; Celec, P.; Tóthová, Ľ. Salivary Creatinine and Urea are Higher in an Experimental Model of Acute but not Chronic Renal Disease. PLoS ONE 2018, 13, e0200391. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Raji, Y.R.; Salako, B.L. Salivary Creatinine and Urea Analysis in Patients with Chronic Kidney Disease: A Case Control Study. BMC Nephrol. 2016, 17, 10. [Google Scholar] [CrossRef]
- Raimann, J.G.; Calice-Silva, V.; Thijssen, S.; Nerbass, F.B.; Vieira, M.A.; Dabel, P.; Pecoits-Filho, R. Saliva Urea Nitrogen Continuously Reflects Blood Urea Nitrogen after Acute Kidney Injury Diagnosis and Management: Longitudinal Observational Data from a Collaborative, International, Prospective, Multicenter Study. Blood Purif. 2016, 42, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Renda, R. Can Salivary Creatinine and Urea Levels be Used to Diagnose Chronic Kidney Disease in Children as Accurately as Serum Creatinine and Urea Levels? A Case–Control Study. Ren. Fail. 2017, 39, 452–457. [Google Scholar] [CrossRef]
- Shannon, I.L.; Feller, R.P.; Eknoyan, G.; Suddick, R.P. Human Parotid Saliva Urea in Renal Failure and During Dialysis. Arch. Oral Biol. 1977, 22, 83–86. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Madsen, P.A.; Lund, P.; Brask-Pedersen, D.N.; Johansen, M. Effect of Dietary Protein Level on Nitrogen Excretion in Dry Cows. Livest. Sci. 2022, 262, 104972. [Google Scholar] [CrossRef]
- Malau-Aduli, A. Energy and Protein Contents in Pastures at Different Times of the Year and Feeding to Meet Animal Nutrient Requirements. In Proceedings of the 16th Annual Conference of the Grasslands Society of Southern Australia, Tasmania Branch, Launceston, Australia, 13 July 2007; Available online: https://www.researchgate.net/publication/228684468_Energy_and_protein_contents_in_pastures_at_different_times_of_the_year_and_feeding_to_meet_animal_nutrient_requirements (accessed on 23 October 2022).
- Mehansho, H.; Butler, L.G.; Carlson, D.M. Dietary Tannins and Salivary Proline-Rich Proteins: Interactions, Induction, and Defense Mechanisms. Annu. Rev. Nutr. 1987, 7, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, J.; Meng, Q.; Fang, S.; Kang, J.; Guo, Q. Differences in Carbon and Nitrogen Metabolism Between Male and Female Populus Cathayana in Response to Deficient Nitrogen. Tree Physiol. 2021, 41, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, E.A.; Fan, Y.; Morgan, B.J.T.; Clutton-Brock, T.H.; Coulson, T. Sexual Dimorphism, Survival and Dispersal in Red Deer. J. Agric. Biol. Environ. Stat. 2004, 9, 1–26. [Google Scholar] [CrossRef]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.S.; Hofmann, R.R. Comparative anatomical and topographic studies of the salivary glands of red deer (Cervus elaphus), fallow deer (Cervus dama), and mouflon (Ovis ammon musimon)--ruminantia: Cervidae, bovidae. Gegenbaurs Morphol. Jahrb. 1984, 130, 273–286. [Google Scholar] [PubMed]
- Kay, R.N.B. Weights of Salivary Glands in Some Ruminant Animals. J. Zool. 1987, 211, 431–436. [Google Scholar] [CrossRef]
- Fickel, J.; Göritz, F.; Joest, B.A.; Hildebrandt, T.; Hofmann, R.R.; Breves, G. Analysis of Parotid and Mixed Saliva in Roe Deer (Capreolus capreolus L.). J. Comp. Physiol. B 1998, 168, 257–264. [Google Scholar] [CrossRef]
- Lamy, E.; Mau, M. Saliva Proteomics as an Emerging, Non-Invasive Tool to Study Livestock Physiology, Nutrition and Diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Montgomery, G.G. Nocturnal Movements and Activity Rhythms of White-Tailed Deer. J. Wildl. Manag. 1963, 27, 422–427. [Google Scholar] [CrossRef]
| Constituent | N | Mean | SD | Established Minimum | Established Maximum | SEC | RSQ | SECV | 1-VR |
|---|---|---|---|---|---|---|---|---|---|
| Protein | 250.00 | 16.09 | 7.01 | 0.00 | 37.11 | 0.86 | 0.99 | 1.04 | 0.98 |
| DOMD | 247.00 | 68.47 | 13.59 | 27.69 | 109.25 | 2.36 | 0.97 | 2.83 | 0.96 |
| OM | 250.00 | 89.40 | 3.54 | 78.79 | 100.02 | 1.19 | 0.89 | 1.39 | 0.85 |
| DMD | 250.00 | 72.81 | 13.23 | 33.11 | 112.51 | 2.15 | 0.97 | 2.44 | 0.97 |
| Treatment | Grasses | Legumes | Forbs | Brassicas | Weeds | Dead Material |
|---|---|---|---|---|---|---|
| Diverse | 25.9% | 20.7% | 8.2% | 13.4% | 2.3% | 29.5% |
| PRG | 24.3% | 22.1% | 0.0% | 0.0% | 17.8% | 35.7% |
| Variable | May–June | June–July | July–August | August–September | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutritive Value | Diverse | SE | PRG | SE | Diverse | SE | Diverse Baleage | SE | PRG | SE | PRG Baleage | SE | Diverse | SE | Diverse Baleage | SE | PRG | SE | PRG Baleage | SE | Diverse Baleage | SE | PRG Baleage | SE |
| Protein g/kg DM | 13.41 | 0.30 | 14.06 | 0.54 | 16.26 | 0.25 | 15.28 | 1.37 | 13.50 | <0.01 | 8.49 | 0.82 | 21.18 | 0.03 | 15.69 | 22.13 | 0.50 | 15.21 | 0.61 | 16.62 | 0.37 | 8.39 | 0.41 | |
| Digestibility (DOMD) g/kg DM | 60.80 | 0.48 | 56.08 | 0.83 | 65.23 | 0.53 | 45.52 | 3.05 | 61.30 | 0.24 | 57.74 | 4.33 | 71.96 | 0.77 | 56.82 | 69.54 | 0.54 | 66.41 | 1.42 | 61.16 | 6.51 | 51.14 | 1.26 | |
| MJ ME/kg DM | 9.63 | 0.12 | 9.17 | 0.09 | 10.12 | 0.02 | 9.71 | 0.01 | 66.41 | 11.02 | 0.02 | 10.79 | 0.22 | |||||||||||
| OM g/kg DM | 93.04 | 0.56 | 93.46 | 0.18 | 91.67 | 0.13 | 92.41 | 0.02 | 93.01 | 0.18 | 94.42 | 0.07 | 90.69 | 0.06 | 91.83 | 90.92 | 0.53 | 90.46 | 0.39 | 90.73 | 0.44 | 93.59 | 0.35 | |
| DM g/kg DM | 25.00 | 6.79 | 29.10 | 8.03 | 24.30 | 7.13 | 29.20 | 0.44 | 40 | 6.65 | 66.40 | 0.71 | 20.30 | 68.31 | 41.40 | 0.03 | 17.80 | 3.55 | 56.90 | 0.44 | 47.80 | 1.04 | 65.9 | 0.37 |
| Average % DM Consumed | 43% | 45% | 53% | 15% | 73% | |||||||||||||||||||
| p-Value * | ||||||||
|---|---|---|---|---|---|---|---|---|
| PRG | Diverse | Diet | Month | Gender | Seasonality | D × M | G × S | |
| SUN (mmol/L) | 6.42 ± 0.231 | 6.39 ± 0.187 | 0.02 | *** | 0.23 | 0.75 | *** | 0.09 |
| PUN (mmol/L) | 6.37 ± 0.155 | 5.97 ± 0.114 | ** | *** | 0.21 | ** | *** | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, E.; Fleming, A.; Vollebregt, M.; Gregorini, P. Relationship between Plasma and Saliva Urea Nitrogen Concentrations in New Zealand Red Deer Calves (Cervus elaphus). Animals 2024, 14, 2565. https://doi.org/10.3390/ani14172565
Wilson E, Fleming A, Vollebregt M, Gregorini P. Relationship between Plasma and Saliva Urea Nitrogen Concentrations in New Zealand Red Deer Calves (Cervus elaphus). Animals. 2024; 14(17):2565. https://doi.org/10.3390/ani14172565
Chicago/Turabian StyleWilson, E., A. Fleming, M. Vollebregt, and P. Gregorini. 2024. "Relationship between Plasma and Saliva Urea Nitrogen Concentrations in New Zealand Red Deer Calves (Cervus elaphus)" Animals 14, no. 17: 2565. https://doi.org/10.3390/ani14172565






