Effects of Short-Term Differences in Concentrate Feeding on the Recovery of In Vivo Embryos in Hanwoo Donor Cows through Superovulation Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Estrous Synchronization and Artificial Insemination (AI)
2.3. Blood Sampling and Analysis
2.4. Statistical Methods
3. Results
3.1. Descriptive Statistics and Results of Embryos According to Feeding Differences
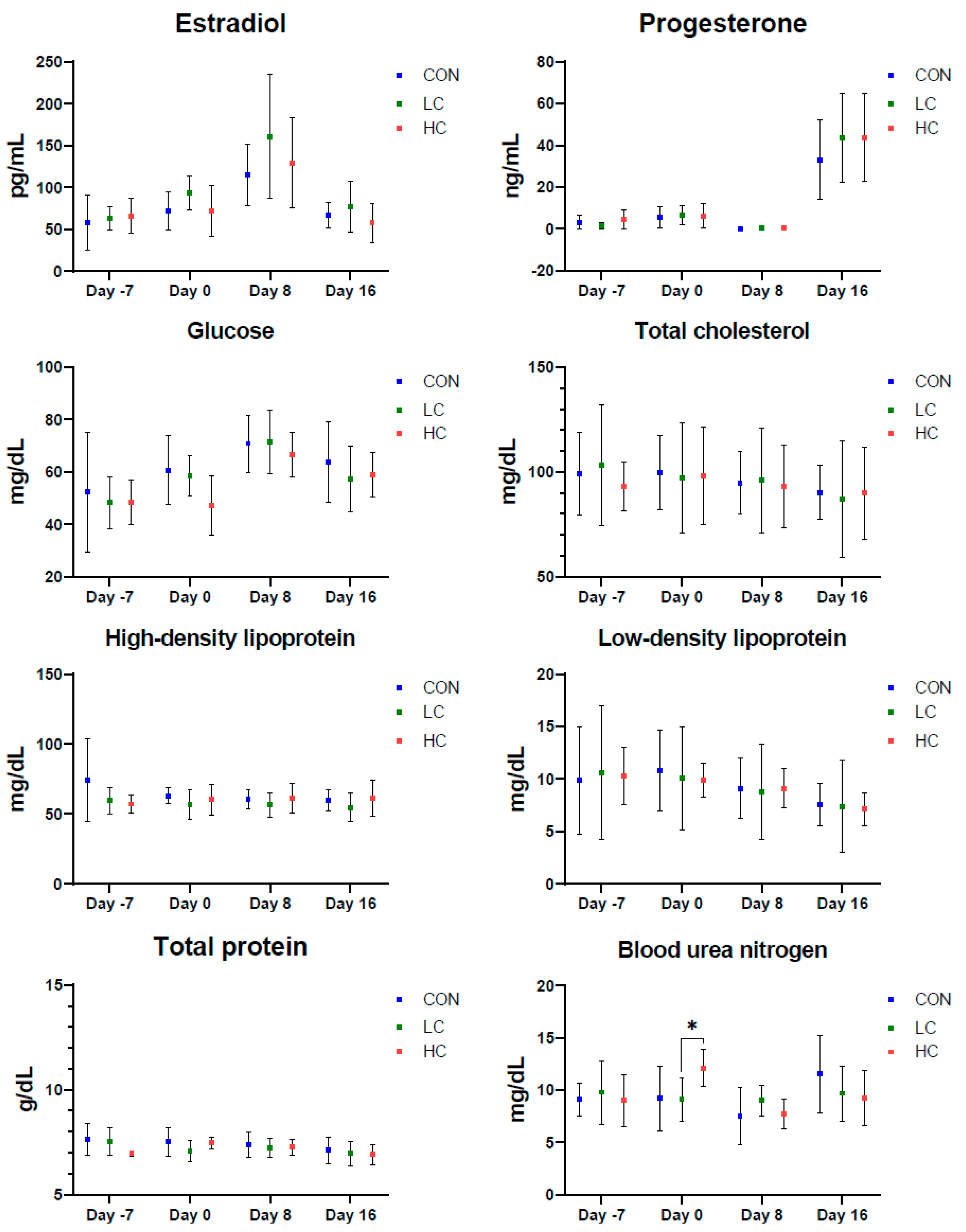
3.2. Changes in Sexual Hormones and Serum Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bó, G.A.; Mapletoft, R.J. Embryo transfer technology in cattle. Anim. Biotechnol. 1 Reprod. Biotechnol. 2018, 107–133. [Google Scholar] [CrossRef]
- Mikkola, M.; Hasler, J.F.; Taponen, J. Factors affecting embryo production in superovulated Bos taurus cattle. Reprod. Fertil. Dev. 2020, 32, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Viana, J. 2018 Statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2019, 36, 17. [Google Scholar]
- Mapletoft, R.J.; Bó, G.A. Superovulation in cattle. Bov. Reprod. 2014, 696–702. [Google Scholar] [CrossRef]
- Butler, W.R. Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livest. Prod. Sci. 2003, 83, 211–218. [Google Scholar] [CrossRef]
- Ciccioli, N.; Wettemann, R.; Spicer, L.; Lents, C.; White, F.; Keisler, D. Influence of body condition at calving and postpartum nutrition on endocrine function and reproductive performance of primiparous beef cows. J. Anim. Sci. 2003, 81, 3107–3120. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.; O’callaghan, D.; Duby, R.; Lonergan, P.; Boland, M. The influence of short–term nutrient changes on follicle growth and embryo production following superovulation in beef heifers. Theriogenology 1998, 50, 1263–1274. [Google Scholar] [CrossRef]
- Gong, J.; Armstrong, D.; Baxter, G.; Hogg, C.; Garnsworthy, P.; Webb, R. The effect of increased dietary intake on superovulatory response to FSH in heifers. Theriogenology 2002, 57, 1591–1602. [Google Scholar] [CrossRef]
- Mollo, M.R.; Monteiro, P.L., Jr.; Surjus, R.S.; Martins, A.C.; Ramos, A.F.; Mourão, G.B.; Carrijo, L.H.; Lopes, G., Jr.; Rumpf, R.; Wiltbank, M.C. Embryo production in heifers with low or high dry matter intake submitted to superovulation. Theriogenology 2017, 92, 30–35. [Google Scholar] [CrossRef]
- Yaakub, H.; O’callaghan, D.; Boland, M. Effect of type and quantity of concentrates on superovulation and embryo yield in beef heifers. Theriogenology 1999, 51, 1259–1266. [Google Scholar] [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Historical perspectives and recent research on superovulation in cattle. Theriogenology 2014, 81, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Facioli, F.L.; De Marchi, F.; Marques, M.G.; Michelon, P.R.; Zanella, E.L.; Caires, K.C.; Reeves, J.J.; Zanella, R. The outcome and economic viability of embryo production using IVF and SOV techniques in the Wagyu breed of cattle. Vet. Sci. 2020, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.; Mackey, D.; Roche, J.; Sreenan, J. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003, 78, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Dunne, L.; Diskin, M.; Boland, M.; O’farrell, K.; Sreenan, J. The effect of pre-and post-insemination plane of nutrition on embryo survival in beef heifers. Anim. Sci. 1999, 69, 411–417. [Google Scholar] [CrossRef]
- Larson, L.; Ball, P. Regulation of estrous cycles in dairy cattle: A review. Theriogenology 1992, 38, 255–267. [Google Scholar] [CrossRef]
- Lee, T.; Kang, S.; Kim, H.; Kang, M.; Yun, Y.; Lee, J.; Kang, T. Changes of hormonal level and blood biochemistry following superovulation treatments of Jeju black cow. J. Embryo Transf. 2006, 21, 225–231. [Google Scholar]
- Henricks, D.; Dickey, J.; Hill, J. Plasma estrogen and progesterone levels in cows prior to and during estrus. Endocrinology 1971, 89, 1350–1355. [Google Scholar] [CrossRef]
- Ciernia, L.; Perry, G.; Smith, M.; Rich, J.; Northrop, E.; Perkins, S.; Green, J.; Zezeski, A.; Geary, T. Effect of estradiol preceding and progesterone subsequent to ovulation on proportion of postpartum beef cows pregnant. Anim. Reprod. Sci. 2021, 227, 106723. [Google Scholar] [CrossRef]
- Lonergan, P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 2011, 76, 1594–1601. [Google Scholar] [CrossRef]
- Walsh, S.W.; Mehta, J.P.; McGettigan, P.A.; Browne, J.A.; Forde, N.; Alibrahim, R.; Mulligan, F.; Loftus, B.; Crowe, M.A.; Matthews, D. Effect of the metabolic environment at key stages of follicle development in cattle: Focus on steroid biosynthesis. Physiol. Genom. 2012, 44, 504–517. [Google Scholar] [CrossRef]
- Ünay, E.; Okuroğlu, A.; Tirpan, M.B.; Coşkun, M.İ.; Sevgi, R.; Yilmaz, M.A.; Ünal, İ.; Erişek, A.; Say, E.; Satilmiş, M. Association between metabolic parameters and embryo production in superovulated dairy cattle. Reprod. Domest. Anim. 2024, 59, e14629. [Google Scholar] [CrossRef] [PubMed]
- Latimer, K.S. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of high-density cholesterol metabolism for oocyte biology and female fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef] [PubMed]

| Variable | CON | LC | HC | p-Value |
|---|---|---|---|---|
| Number | 9 | 10 | 8 | |
| Age, year | 7.20 ± 0.82 | 7.27 ± 1.27 | 7.05 ± 1.13 | 0.684 |
| Parity | 2.11 ± 0.33 | 2.00 ± 0.47 | 1.88 ± 0.35 | 0.464 |
| Days since calving | 333.7 ± 94.3 | 273.8 ± 54.0 | 283.8 ± 50.6 | 0.371 |
| Body Condition Score | ||||
| Beginning of study | 3.28 ± 0.26 | 3.15 ± 0.24 | 3.38 ± 0.44 | 0.428 |
| Harvesting embryo | 3.22 ± 0.26 | 2.95 ± 0.16 | 3.25 ± 0.38 | 0.043 |
| DBBH | −0.06 ± 0.17 | −0.20 ± 0.26 | −0.13 ± 0.23 | 0.370 |
| Body weight, kg | ||||
| Beginning of experiment | 452.9 ± 51.9 | 478.0 ± 46.5 | 502.0 ± 85.9 | 0.370 |
| Harvesting embryo | 439.3 ± 48.8 | 449.8 ± 47.1 | 502.4 ± 76.9 | 0.213 |
| DBBH | −13.6 ± 16.4 | −28.2 ± 15.9 | 0.4 ± 27.3 | 0.029 |
| Number of harvesting embryos | 6.22 ± 4.44 a | 15.00 ± 6.82 b | 13.75 ± 7.85 ab | 0.016 |
| Number of transferable embryos | 4.22 ± 2.82 | 9.90 ± 8.12 | 9.00 ± 6.55 | 0.169 |
| Transferable-to-harvesting embryos | 0.75 ± 0.23 | 0.65 ± 0.36 | 0.59 ± 0.24 | 0.583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.; Kim, N.; Park, M.-R.; Lee, S.; Cho, S.-R.; Song, H.; Jin, D.; Kim, U.-H.; Ko, Y.-G. Effects of Short-Term Differences in Concentrate Feeding on the Recovery of In Vivo Embryos in Hanwoo Donor Cows through Superovulation Treatment. Animals 2024, 14, 2591. https://doi.org/10.3390/ani14172591
Ha S, Kim N, Park M-R, Lee S, Cho S-R, Song H, Jin D, Kim U-H, Ko Y-G. Effects of Short-Term Differences in Concentrate Feeding on the Recovery of In Vivo Embryos in Hanwoo Donor Cows through Superovulation Treatment. Animals. 2024; 14(17):2591. https://doi.org/10.3390/ani14172591
Chicago/Turabian StyleHa, Seungmin, Namtae Kim, Mi-Ryung Park, Seyoung Lee, Sang-Rae Cho, Huimang Song, Daehyeok Jin, Ui-Hyung Kim, and Yeoung-Gyu Ko. 2024. "Effects of Short-Term Differences in Concentrate Feeding on the Recovery of In Vivo Embryos in Hanwoo Donor Cows through Superovulation Treatment" Animals 14, no. 17: 2591. https://doi.org/10.3390/ani14172591
APA StyleHa, S., Kim, N., Park, M.-R., Lee, S., Cho, S.-R., Song, H., Jin, D., Kim, U.-H., & Ko, Y.-G. (2024). Effects of Short-Term Differences in Concentrate Feeding on the Recovery of In Vivo Embryos in Hanwoo Donor Cows through Superovulation Treatment. Animals, 14(17), 2591. https://doi.org/10.3390/ani14172591






