Genome-Wide Identification, Evolution, and miRNA-22 Regulation of Kruppel-Like Factor (KLF) Gene Family in Chicken (Gallus gallus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Bioinformatics Analysis of Chicken KLF Gene Family Members
2.2. Chromosome Location Analysis
2.3. Construction of Phylogenetic Tree
2.4. KLF Gene Structure and Motif Analysis
2.5. Gene Collinearity Analysis
2.6. Prediction of Protein Tertiary Structure and Transmembrane Region
2.7. Preparation of miR-22 Lentiviral Vectors, Chicken Treatment Protocols, and Analysis of KLF Gene Family Expression
2.8. Statement of Ethics
3. Result
3.1. Identification and Physicochemical Properties of KLF Gene Family Members in Chicken
3.2. Chromosomal Location of KLF Gene Family
3.3. Phylogenetic Analysis of the KLF Gene Family
3.4. Gene Structure and Motif Sequence Analysis of KLF Gene Family
3.5. Motif Sequence Analysis of KLF Gene Family
3.6. Gene Collinearity Analysis of Chicken KLF Family
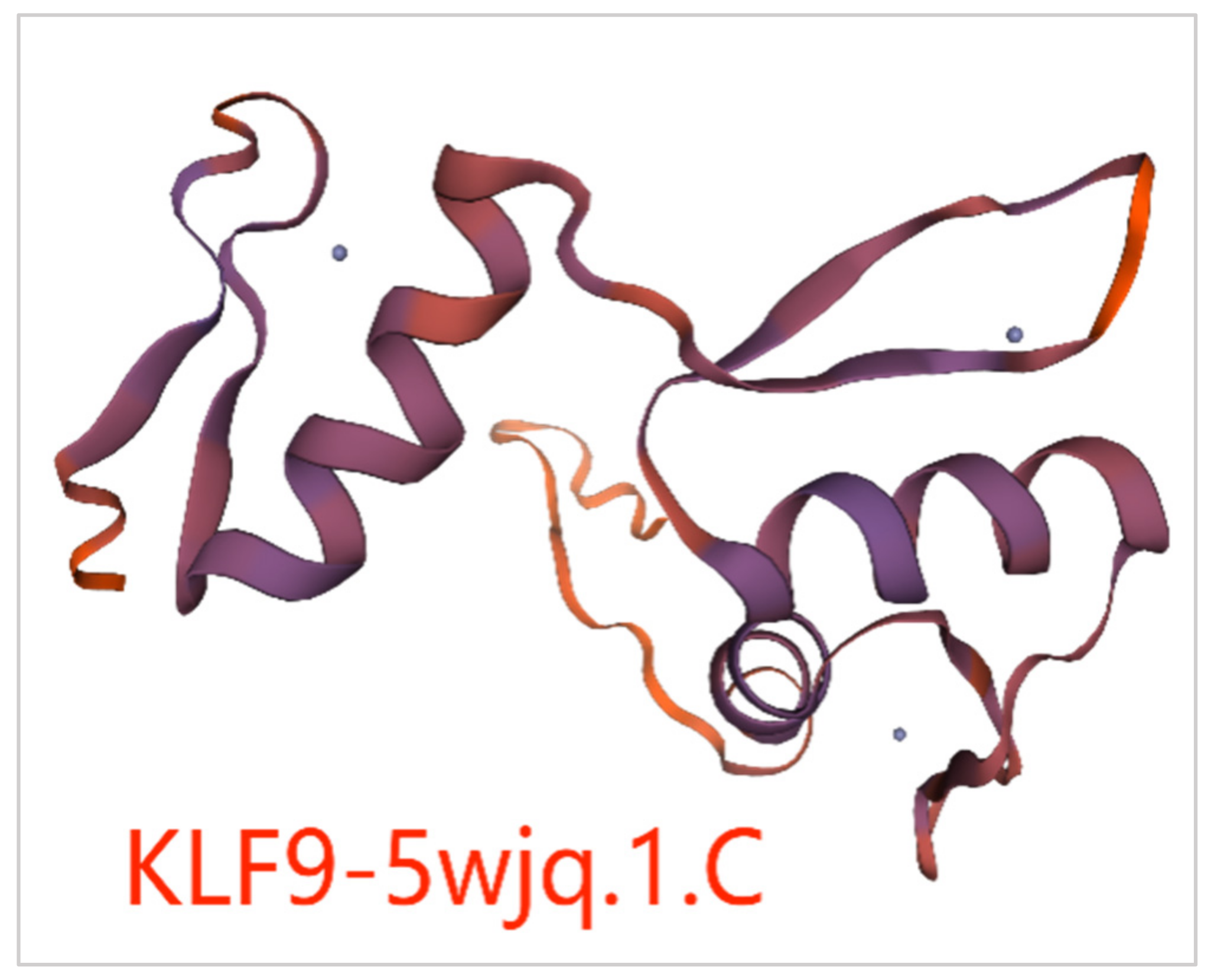
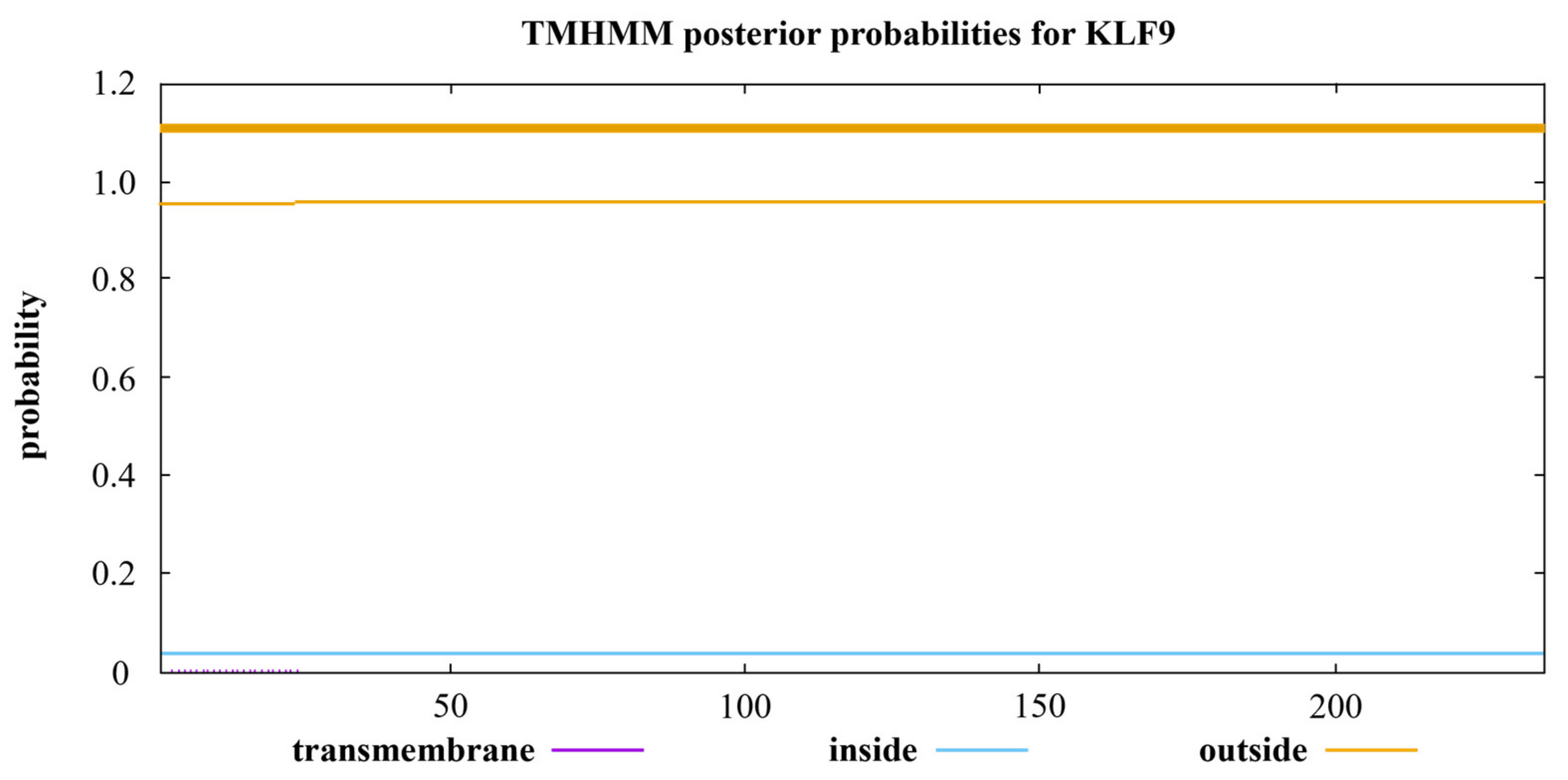
3.7. Prediction of the Tertiary Structure and Transmembrane Region of KLF Family Proteins
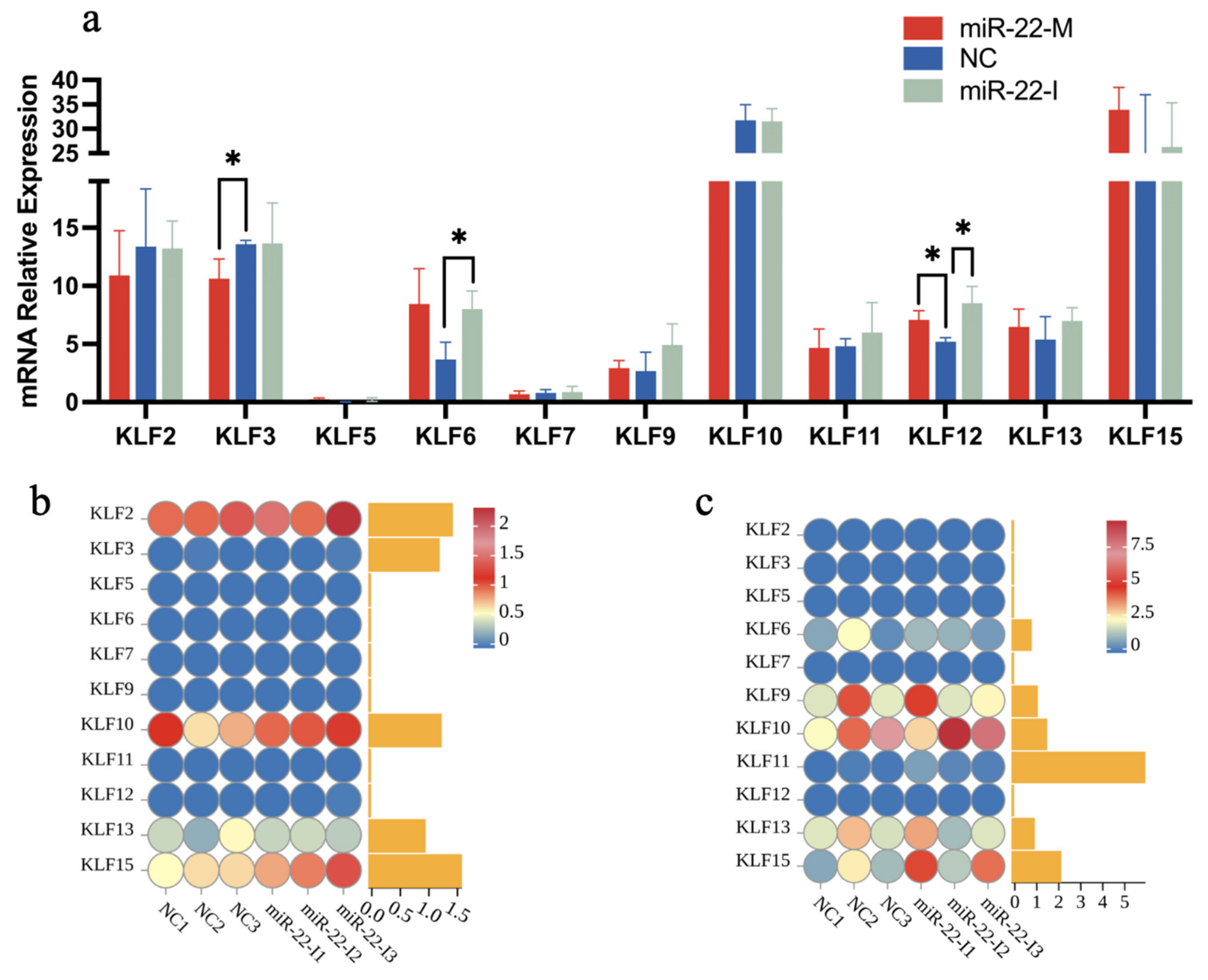
3.8. Effect of miRNA-22 on the Expression of KLF Gene Family in Different Tissues of Qingyuan Partridge Chickens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuh, R.; Aicher, W.; Gaul, U.; Côte, S.; Preiss, A.; Maier, D.; Seifert, E.; Nauber, U.; Schröder, C.; Kemler, R.; et al. A Conserved Family of Nuclear Proteins Containing Structural Elements of the Finger Protein Encoded by Krüppel, a Drosophila Segmentation Gene. Cell 1986, 47, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Fleetwood, J.; Eaton, S.; Crossley, M.; Bao, S. Krüppel-like Transcription Factors: A Functional Family. Int. J. Biochem. Cell Biol. 2008, 40, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Brey, C.W.; Nelder, M.P.; Hailemariam, T.; Gaugler, R.; Hashmi, S. Krüppel-like Family of Transcription Factors: An Emerging New Frontier in Fat Biology. Int. J. Biol. Sci. 2009, 5, 622–636. [Google Scholar] [CrossRef]
- Antin, P.B.; Pier, M.; Sesepasara, T.; Yatskievych, T.A.; Darnell, D.K. Embryonic Expression of the Chicken Krüppel-like (KLF) Transcription Factor Gene Family. Dev. Dyn. 2010, 239, 1879–1887. [Google Scholar] [CrossRef]
- Haldar, S.M.; Ibrahim, O.A.; Jain, M.K. Kruppel-like Factors (KLFs) in Muscle Biology. J. Mol. Cell. Cardiol. 2007, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Chen, Z.; Friedman, J. Transcriptional Regulation of Adipogenesis by KLF4. Cell Metab. 2008, 7, 339–347. [Google Scholar] [CrossRef]
- Park, Y.-K.; Wang, L.; Giampietro, A.; Lai, B.; Lee, J.-E.; Ge, K. Distinct Roles of Transcription Factors KLF4, Krox20, and Peroxisome Proliferator-Activated Receptor γ in Adipogenesis. Mol. Cell. Biol. 2017, 37, e00554-16. [Google Scholar] [CrossRef]
- Xie, G.J.; Du, Y.; Xu, Q.; Ma, J.Q.; Qiu, X.; Lin, Y.Q. Biological Characteristic and Spatio-Temporal Expression Analysis of KLF5 Gene in Goat. Acta Agric. Boreali-Sin. 2020, 35, 225. [Google Scholar] [CrossRef]
- Li, D.; Yea, S.; Li, S.; Chen, Z.; Narla, G.; Banck, M.; Laborda, J.; Tan, S.; Friedman, J.M.; Friedman, S.L.; et al. Krüppel-like Factor-6 Promotes Preadipocyte Differentiation through Histone Deacetylase 3-Dependent Repression of DLK1. J. Biol. Chem. 2005, 280, 26941–26952. [Google Scholar] [CrossRef]
- Guo, H. The Function of KLF3 and KLF15 in Bovine Preadipocyte Differentiation and Lipid Metabolism. Ph.D. Dissertation, Northwest A&F University, Yangling, China, 2019. Available online: https://kns.cnki.net/kcms2/article/abstract?v=WOTiXAdNI6ODePqMo6wCJJzLJm-YvvtA3VA6LSgMHT_JTDnMMgmsSXfyXbmCTp_hAE9aDEN9zos-LrPnixEEcsNSHRA85h9k7RBhZjyV7kYK86IWNsjnXw-aIH0JNiRCJxJ3IOTT8ejtgsaCQbHPULYjdKX6LhJBx8cgRiFIjnrGaxyv_1W9XflflIQEApKAj0zBHAYvD9oXsQjDD_9JqvI9J9dNe7VqxvWZ7kf-s_EEkh6NiXMHYkuKG8Kg2YMQhHkUEY9EZsZdeahBSjtcXMjMz4A87Mf7&uniplatform=NZKPT&language=CHS (accessed on 6 June 2022).
- Banerjee, S.S.; Feinberg, M.W.; Watanabe, M.; Gray, S.; Haspel, R.L.; Denkinger, D.J.; Kawahara, R.; Hauner, H.; Jain, M.K. The Krüppel-like Factor KLF2 Inhibits Peroxisome Proliferator-Activated Receptor-γ Expression and Adipogenesis*. J. Biol. Chem. 2003, 278, 2581–2584. [Google Scholar] [CrossRef]
- Sue, N.; Jack, B.H.A.; Eaton, S.A.; Pearson, R.C.M.; Funnell, A.P.W.; Turner, J.; Czolij, R.; Denyer, G.; Bao, S.; Molero-Navajas, J.C.; et al. Targeted Disruption of the Basic Krüppel-Like Factor Gene (Klf3) Reveals a Role in Adipogenesis. Mol. Cell. Biol. 2008, 28, 3967–3978. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Tanaka, Y.; Kawamori, R.; Maeda, S. Overexpression of Kruppel-Like Factor 7 Regulates Adipocytokine Gene Expressions in Human Adipocytes and Inhibits Glucose-Induced Insulin Secretion in Pancreatic β-Cell Line. Mol. Endocrinol. 2006, 20, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zhang, Z.W.; He, Q.; Wang, N.; Wang, Y.X.; Cao, Z.P. Expression Pattern of Chicken Kruppel-like Factor 3 Gene and lts Effect on Adipocyte Differentiation. Acta Vet. Zootech. Sin. 2015, 46, 26–31. [Google Scholar] [CrossRef]
- Wang, Y.M.; Xu, Y.O.; Wang, Z.M.; Xu, L.Y.; Yang, L.; Lin, Y.Q. Studies on the Cloning of KLF15 Gene, Tissue Expression Profile and the Association between lts Expression and Intramuscular Fat Content in Tibetan Chicken. Acta Vet. Zootech. Sin. 2019, 50, 261–270. [Google Scholar] [CrossRef]
- Jin, Z.; Li, W.; Li, J.; Tan, M.; Sun, Y. KLF7 Promotes Transcription of CDKN3 in Chicken Preadipocytes; College of Life Science and Agriculture Forestry, Qiqihar University: Qiqihar, China, 2019; pp. 109–110. [Google Scholar]
- Wu, Y.; Wang, Z.; Yu, J.; Xu, L.; Gu, Z. Construction of Eukaryotic Expression Vector of Chicken KLF11 Gene and lts Expression in LMH Cells. J. Chang. Inst. Technol. 2020, 34, 68–72+101. [Google Scholar] [CrossRef]
- Tian, W.; Hao, X.; Nie, R.; Ling, Y.; Zhang, B.; Zhang, H.; Wu, C. Integrative Analysis of miRNA and mRNA Profiles Reveals That Gga-miR-106-5p Inhibits Adipogenesis by Targeting the KLF15 Gene in Chickens. J. Anim. Sci. Biotechnol. 2022, 13, 81. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, M.; Qiao, S.; Wang, Y.; Li, H.; Wang, N. Gga-miR-21 Inhibits Chicken Pre-Adipocyte Proliferation in Part by down-Regulating Kruppel-like Factor 5. Poult. Sci. 2017, 96, 200–210. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; He, M.; Wu, P.; Zhou, K.; Zhang, T.; Chu, M.; Zhang, G. miR-7 Regulates the Apoptosis of Chicken Primary Myoblasts through the KLF4 Gene. Br. Poult. Sci. 2022, 63, 39–45. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, F.; Wu, P.; Li, T.; He, M.; Yin, X.; Shi, H.; Duan, Y.; Zhang, T.; Wang, J.; et al. MicroRNA-7 Targets the KLF4 Gene to Regulate the Proliferation and Differentiation of Chicken Primary Myoblasts. Front. Genet. 2020, 11, 842. [Google Scholar] [CrossRef]
- Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef]
- Li, H.; Ma, Z.; Jia, L.; Li, Y.; Xu, C.; Wang, T.; Han, R.; Jiang, R.; Li, Z.; Sun, G.; et al. Systematic Analysis of the Regulatory Functions of microRNAs in Chicken Hepatic Lipid Metabolism. Sci. Rep. 2016, 6, 31766. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, H.; Zheng, H.; Jiang, K.; Yan, F.; Tian, Y.; Kang, X.; Wang, Y.; Liu, X. Hepatic ELOVL6 mRNA Is Regulated by the Gga-miR-22-3p in Egg-Laying Hen. Gene 2017, 623, 72–79. [Google Scholar] [CrossRef]
- Wang, H.; Hu, M.; Shen, Z.; Zhou, X.; Yang, S.; He, K.; Li, X.; Yan, F.; Zhao, A. A Specific microRNA Targets an Elongase of Very Long Chain Fatty Acids to Regulate Fatty Acid Composition and Mitochondrial Morphology of Skeletal Muscle Cells. Animals 2022, 12, 2274. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.M.; Liu, J.; Brandão, B.B.; Lino, C.A.; Silva, C.S.B.; Ribeiro, M.A.C.; Oliveira, T.E.; Real, C.C.; Faria, D.d.P.; Cederquist, C.; et al. miRNA-22 Deletion Limits White Adipose Expansion and Activates Brown Fat to Attenuate High-Fat Diet-Induced Fat Mass Accumulation. Metab.-Clin. Exp. 2021, 117, 154723. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, S.; Bian, C.; Yang, Z.; Zhou, H.; Zeng, Y.; Li, H.; Han, Q.; Zhao, R.C. Upregulation of miR-22 Promotes Osteogenic Differentiation and Inhibits Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells by Repressing HDAC6 Protein Expression. Stem Cells Dev. 2012, 21, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Rong, E.G.; Shi, M.X.; Wu, C.Y.; Sun, B.; Wang, Y.X.; Wang, N.; Li, H. Expression and Functional Analysis of Krüppel-like Factor 2 in Chicken Adipose Tissue1. J. Anim. Sci. 2014, 92, 4797–4805. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Aoki, M.; Endo, T.; Sato, K. Characterization of the Expression Profiles of Adipogenesis-Related Factors, ZNF423, KLFs and FGF10, during Preadipocyte Differentiation and Abdominal Adipose Tissue Development in Chickens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 165, 189–195. [Google Scholar] [CrossRef]
- Chen, Z.L. The Cloning and Molecular Characteristics of Porcine KLF Gene Family. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2013. Available online: https://kns.cnki.net/kcms2/article/abstract?v=XEQRgWHfXDHYjjpsuiFY2FN-HXPTEn39-Gcr_Vfp256yIdZ5fu0tMnNTU-_5Kis7uoysP_zlSDj0QF1NuggOazgOX3GwqW7Mb-IiPMVBk0owcLFjnrG9UpkrVS1yFMx_D8ftwG-ZfOBAT7BBEDhYu5HhTJVML_5l&uniplatform=NZKPT&language=CHS (accessed on 6 June 2022).
- Zhang, Z.-W.; Wu, C.-Y.; Li, H.; Wang, N. Expression and Functional Analyses of Krüppel-like Factor 3 in Chicken Adipose Tissue. Biosci. Biotechnol. Biochem. 2014, 78, 614–623. [Google Scholar] [CrossRef]
- Zhang, D.; Ran, J.; Li, J.; Yu, C.; Cui, Z.; Amevor, F.K.; Wang, Y.; Jiang, X.; Qiu, M.; Du, H.; et al. miR-21-5p Regulates the Proliferation and Differentiation of Skeletal Muscle Satellite Cells by Targeting KLF3 in Chicken. Genes 2021, 12, 814. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Lian, T.; Ran, J.-S.; Li, Z.-Q.; Han, S.-S.; Liu, Y.-P. KLF5 Functions in Proliferation, Differentiation, and Apoptosis of Chicken Satellite Cells. 3 Biotech 2019, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-H.; Yin, H.-D.; Li, J.-J.; Wang, Y.; Yang, C.-W.; Jiang, X.-S.; Du, H.-R.; Liu, Y.-P. KLF5 Regulates Chicken Skeletal Muscle Atrophy via the Canonical Wnt/β-Catenin Signaling Pathway. Exp. Anim. 2020, 69, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Li, Y.; Emu, Q.; Zhu, J.; Lin, Y. miR-214-5p Regulating Differentiation of Intramuscular Preadipocytes in Goats via Targeting KLF12. Front. Genet. 2021, 12, 748629. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Zhang, Y.; Zhu, J.; Lin, Y. RXRα Cooperates with KLF8 to Promote the Differentiation of Intramuscular Preadipocytes in Goat. Anim. Biotechnol. 2021, 32, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, Q.; Zhang, J.; Zhang, G. Genome-Wide Analysis of the KLF Gene Family in Chicken: Characterization and Expression Profile. Animals 2023, 13, 1429. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S. Role of Kruppel-like Factor 8 (KLF8) in Cancer and Cardiomyopathy. Ph.D. Thesis, Burnett School of Biomedical Sciences, Orlando, FL, USA, 2016. [Google Scholar]
- Cheng, S.; Zhang, X.; Xu, Y.; Dai, X.; Li, J.; Zhang, T.; Chen, X. Krüppel-like Factor 8 Regulates VEGFA Expression and Angiogenesis in Hepatocellular Carcinoma. Sci. Rep. 2018, 8, 17415. [Google Scholar] [CrossRef] [PubMed]
- Dobrivojević, M.; Habek, N.; Kapuralin, K.; Ćurlin, M.; Gajović, S. Krüppel-like Transcription Factor 8 (Klf8) Is Expressed and Active in the Neurons of the Mouse Brain. Gene 2015, 570, 132–140. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, Y.; Luo, F.; Lin, Y.; Li, Z. Molecular Cloning, Expression Profiles and Associations of KLF6 Gene with Intramuscular Fat in Tibetan Chicken. Anim. Biotechnol. 2020, 31, 67–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Sun, Y.; Li, H.; Wang, N. Klf7 Modulates the Differentiation and Proliferation of Chicken Preadipocyte. ABBS 2013, 45, 280–288. [Google Scholar] [CrossRef]
- Salinas, Y.D.; Wang, L.; DeWan, A.T. Multiethnic Genome-Wide Association Study Identifies Ethnic-Specific Associations with Body Mass Index in Hispanics and African Americans. BMC Genet. 2016, 17, 78. [Google Scholar] [CrossRef]
- Zobel, D.P.; Andreasen, C.H.; Burgdorf, K.S.; Andersson, E.A.; Sandbæk, A.; Lauritzen, T.; Borch-Johnsen, K.; Jørgensen, T.; Maeda, S.; Nakamura, Y.; et al. Variation in the Gene Encoding Krüppel-like Factor 7 Influences Body Fat: Studies of 14 818 Danes. Eur. J. Endocrinol. 2009, 160, 603–609. [Google Scholar] [CrossRef]
- Parakati, R.; DiMario, J.X. Repression of Myoblast Proliferation and Fibroblast Growth Factor Receptor 1 Promoter Activity by KLF10 Protein. J. Biol. Chem. 2013, 288, 13876–13884. [Google Scholar] [CrossRef]
- Tang, H.; Liu, D.; Zhang, H.; Fan, W.; Hu, J.; Xu, Y.; Guo, Z.; Huang, W.; Hou, S.; Zhou, Z. Genome-Wide Association Studies Demonstrate the Genes Associated with Perimysial Thickness in Ducks. Anim. Genet. 2023, 54, 363–374. [Google Scholar] [CrossRef]
- Zakeri, S.; Aminian, H.; Sadeghi, S.; Esmaeilzadeh-Gharehdaghi, E.; Razmara, E. Krüppel-like Factors in Bone Biology. Cell. Signal. 2022, 93, 110308. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.N.; Fan, L.; Sweet, D.R.; Jain, M.K. The Krüppel-Like Factors and Control of Energy Homeostasis. Endocr. Rev. 2019, 40, 137–152. [Google Scholar] [CrossRef]
- Du, J.; Xu, Y.; Zhang, P.; Zhao, X.; Gan, M.; Li, Q.; Ma, J.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wei, H.; Song, T.; Yang, Y.; Zhang, F.; Zhou, Y.; Peng, J.; Jiang, S. KLF13 Promotes Porcine Adipocyte Differentiation through PPARγ Activation. Cell Biosci. 2015, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Yao, Y.; Yang, Y.; Liao, K.; Wu, J.-R. Krüppel-like Factor KLF9 Regulates PPARγ Transactivation at the Middle Stage of Adipogenesis. Cell Death Differ. 2011, 18, 315–327. [Google Scholar] [CrossRef]
- Shen, P.; Sun, J.; Xu, G.; Zhang, L.; Yang, Z.; Xia, S.; Wang, Y.; Liu, Y.; Shi, G. KLF9, a Transcription Factor Induced in Flutamide-Caused Cell Apoptosis, Inhibits AKT Activation and Suppresses Tumor Growth of Prostate Cancer Cells. Prostate 2014, 74, 946–958. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, R.; Wang, B.; Wang, J.; Yu, W.; Liu, Y.; Shi, G. Transcription Factor KLF13 Inhibits AKT Activation and Suppresses the Growth of Prostate Carcinoma Cells. Cancer Biomark. 2018, 22, 533–541. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Pant, S.D.; Wani, A.K.; Mohamed, H.H.; Khalifa, N.E.; Almohaimeed, H.M.; Alshanwani, A.R.; Assiri, R.; Aggad, W.S.; Noreldin, A.E.; et al. Krüppel-like Factors Family Regulation of Adipogenic Markers Genes in Bovine Cattle Adipogenesis. Mol. Cell. Probes 2022, 65, 101850. [Google Scholar] [CrossRef]
- Lyu, S.J.; Tian, Y.D.; Wang, S.H.; Han, R.L.; Mei, X.X.; Kang, X.T. A Novel 2-Bp Indel within Krüppel-like Factor 15 Gene (KLF15) and Its Associations with Chicken Growth and Carcass Traits. Br. Poult. Sci. 2014, 55, 427–434. [Google Scholar] [CrossRef] [PubMed]
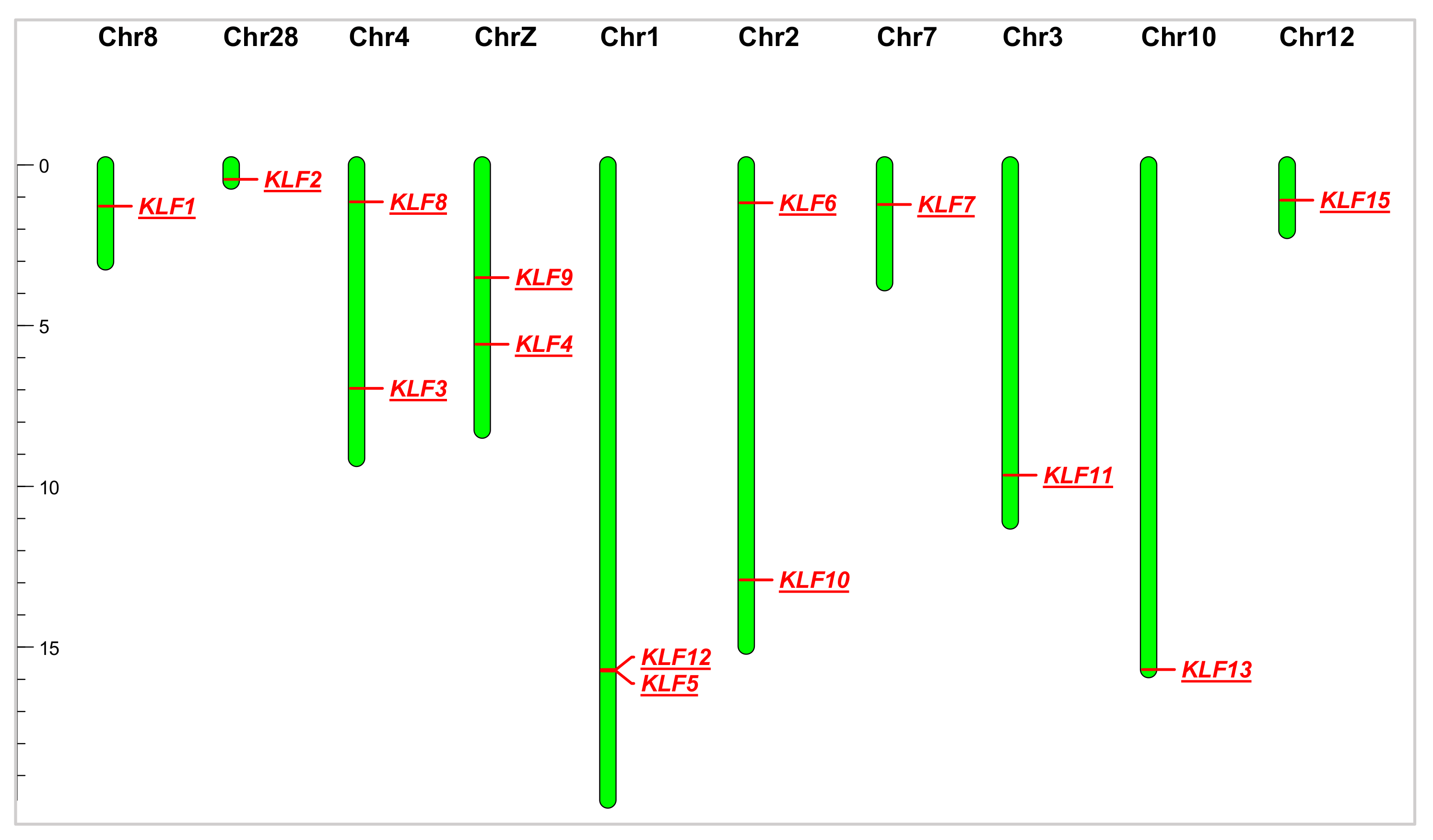
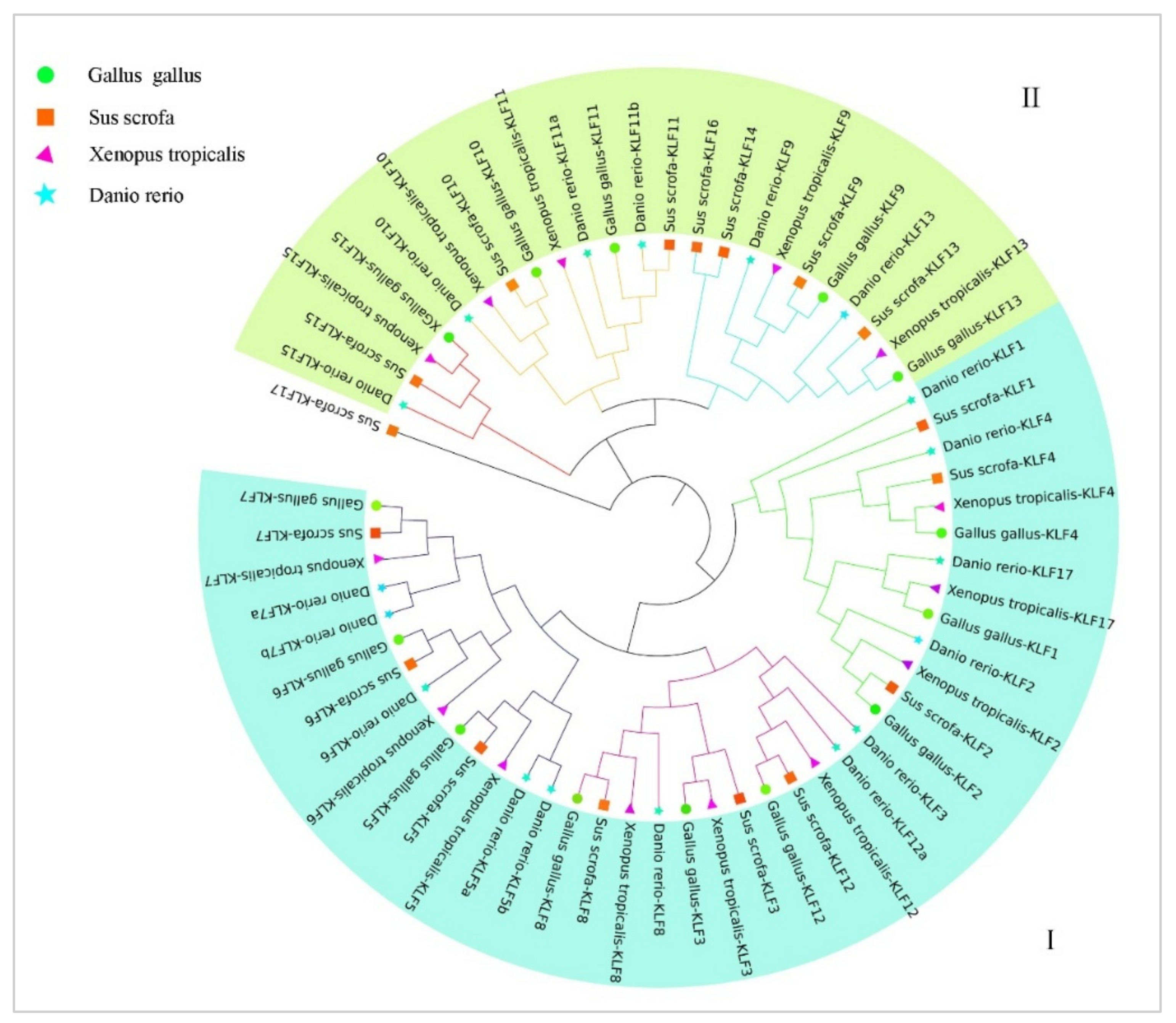
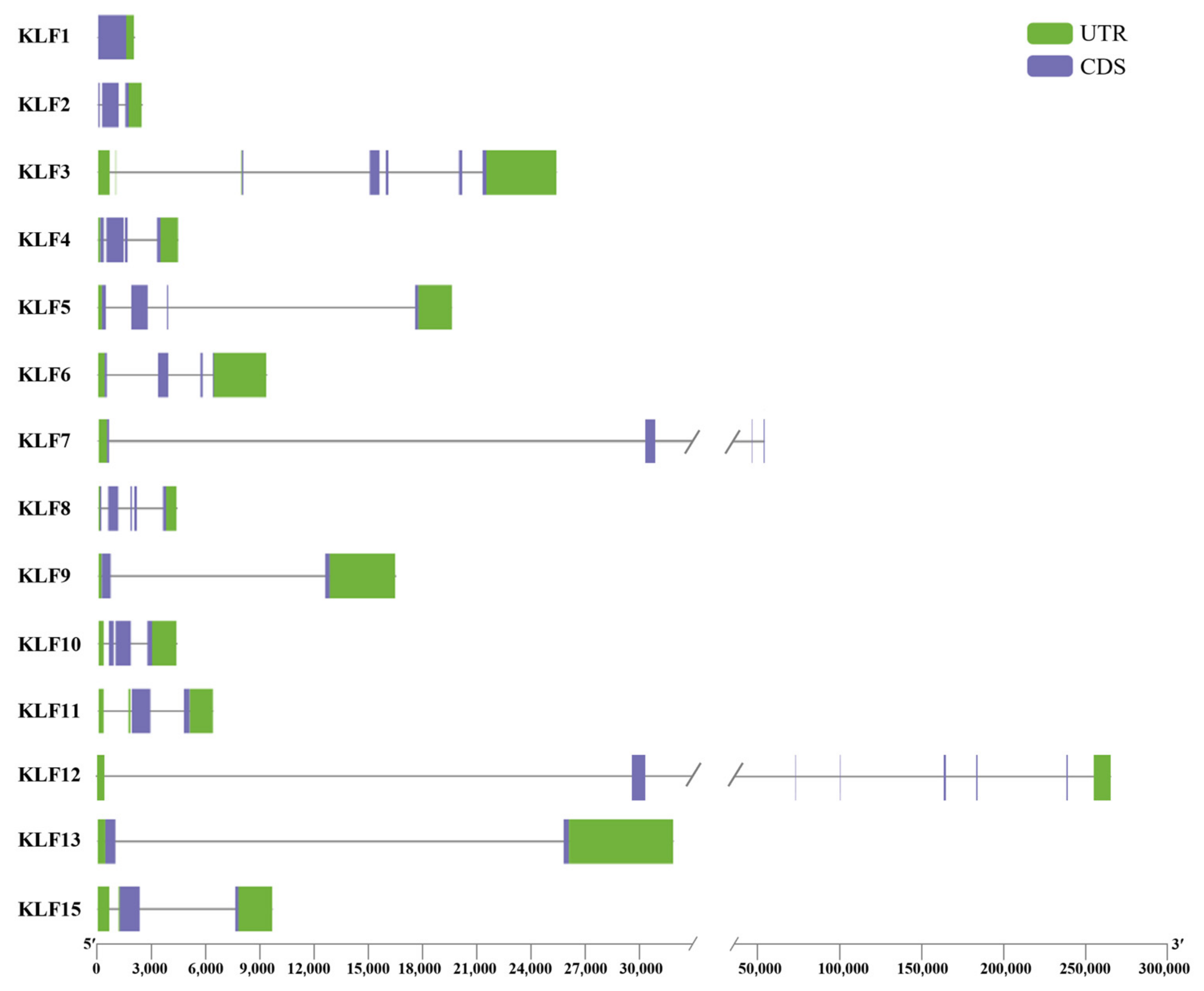
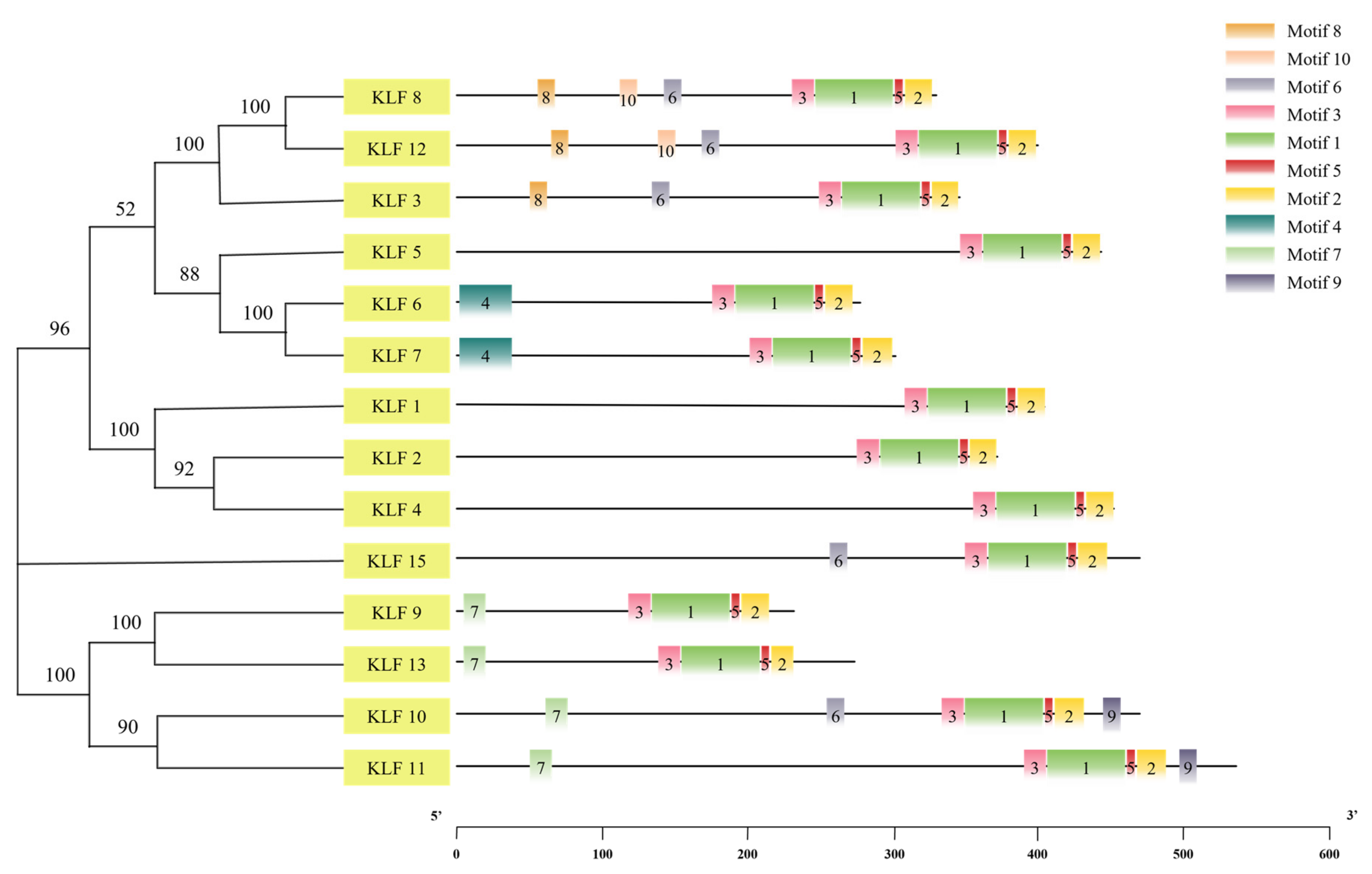

| Gene Name | Gene ID | Chr | Genomic Location | Exon | AA | MW (Da) | PI |
|---|---|---|---|---|---|---|---|
| KLF1 | 424577 | 8 | 20,104,457–20,106,379 | 1 | 501 | 52,966.89 | 7.33 |
| KLF2 | 420148 | 28 | 4,830,902–4,833,231 | 3 | 380 | 42,069.22 | 7.01 |
| KLF3 | 429811 | 4 | 69,048,099–69,075,232 | 11 | 347 | 39,027.04 | 9.53 |
| KLF4 | 770254 | Z | 56,393,942–56,398,448 | 5 | 458 | 48,565.25 | 8.63 |
| KLF5 | 418818 | 1 | 156,007,859–156,026,745 | 4 | 441 | 48,836.51 | 8.63 |
| KLF6 | 420463 | 2 | 11,740,170–11,749,143 | 4 | 283 | 31,965.53 | 6.50 |
| KLF7 | 429011 | 7 | 11,883,355–11,944,567 | 5 | 296 | 32,981.18 | 7.98 |
| KLF8 | 101749773 | 4 | 11,420,793–11,426,412 | 7 | 330 | 35,577.55 | 8.75 |
| KLF9 | 770238 | Z | 35,630,341–35,646,189 | 2 | 235 | 25,661.19 | 9.44 |
| KLF10 | 420255 | 2 | 128,801,525–128,806,420 | 5 | 466 | 50,178.02 | 9.41 |
| KLF11 | 421934 | 3 | 96,177,645–96,185,317 | 5 | 530 | 56,922.34 | 9.11 |
| KLF12 | 418817 | 1 | 155,542,183–155,804,856 | 12 | 396 | 43,470.94 | 9.49 |
| KLF13 | 427493 | 10 | 5,587,589–5,618,322 | 2 | 277 | 30,776.50 | 9.54 |
| KLF15 | 427588 | 12 | 10,806,952–10,841,285 | 14 | 463 | 51,113.08 | 8.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Chu, H.; Li, F.; Han, G.; Cai, Y.; Yi, J.; Lu, M.; Xiang, H.; Kang, H.; Ye, F.; et al. Genome-Wide Identification, Evolution, and miRNA-22 Regulation of Kruppel-Like Factor (KLF) Gene Family in Chicken (Gallus gallus). Animals 2024, 14, 2594. https://doi.org/10.3390/ani14172594
Ma Z, Chu H, Li F, Han G, Cai Y, Yi J, Lu M, Xiang H, Kang H, Ye F, et al. Genome-Wide Identification, Evolution, and miRNA-22 Regulation of Kruppel-Like Factor (KLF) Gene Family in Chicken (Gallus gallus). Animals. 2024; 14(17):2594. https://doi.org/10.3390/ani14172594
Chicago/Turabian StyleMa, Zheng, Huangbin Chu, Fapei Li, Guochao Han, Yingqiu Cai, Jianing Yi, Mingrou Lu, Hai Xiang, Huimin Kang, Fei Ye, and et al. 2024. "Genome-Wide Identification, Evolution, and miRNA-22 Regulation of Kruppel-Like Factor (KLF) Gene Family in Chicken (Gallus gallus)" Animals 14, no. 17: 2594. https://doi.org/10.3390/ani14172594





