RNA Sequencing Reveals the Involvement of Serum Exosomal miRNAs in Early Pregnancy in Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Extracellular Vesicle Isolation, RNA Library Construction, and RNA-Seq
2.3. miRNA Sequence Alignment and Identification
2.4. Screening and Analysis of DEmiRNAs
2.5. Validation of RNA-Seq Data by qPCR
2.6. Prediction of Target Genes and Functional Enrichment Analysis of DEmiRNAs
2.7. Statistical Analysis
3. Results
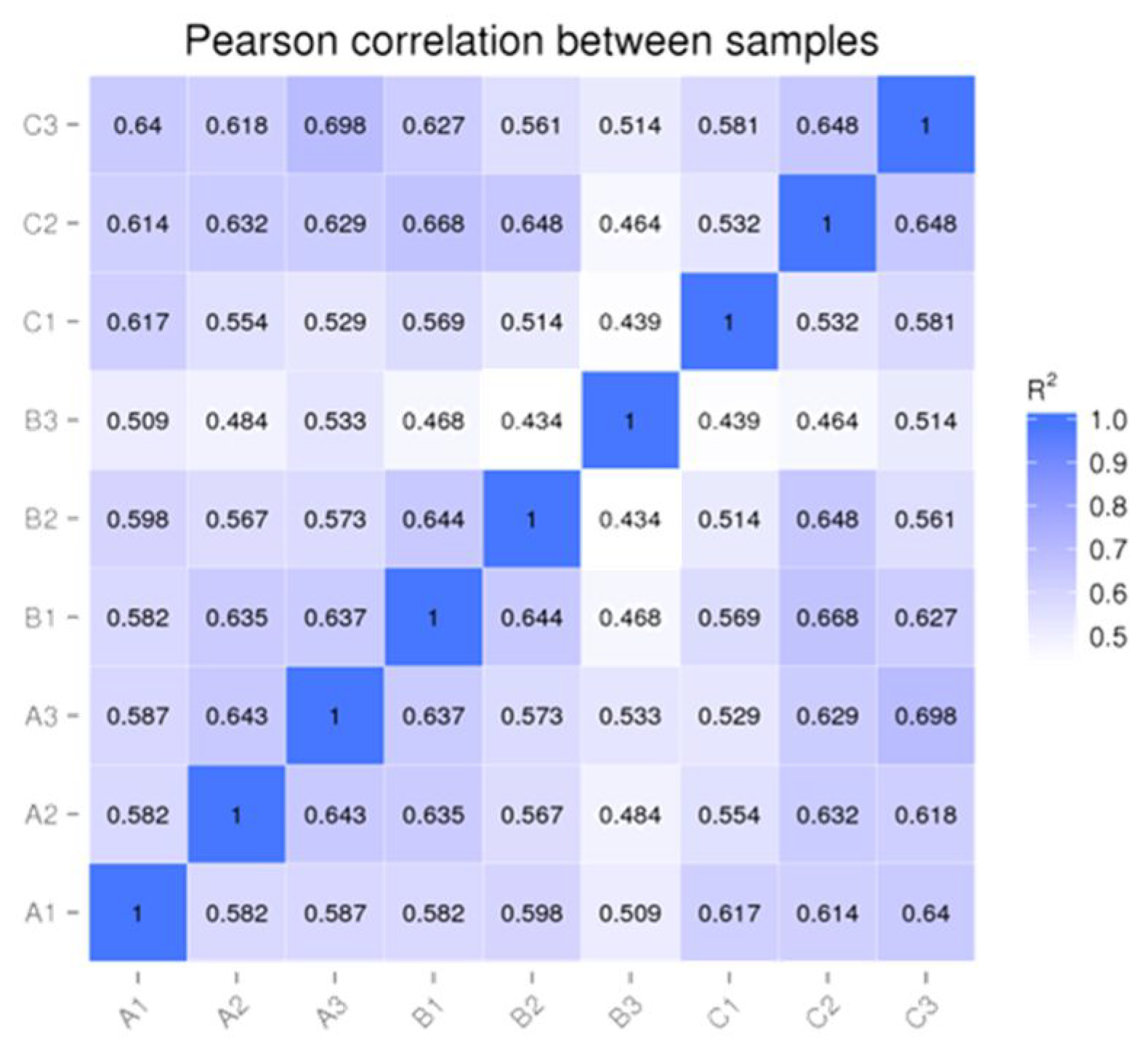
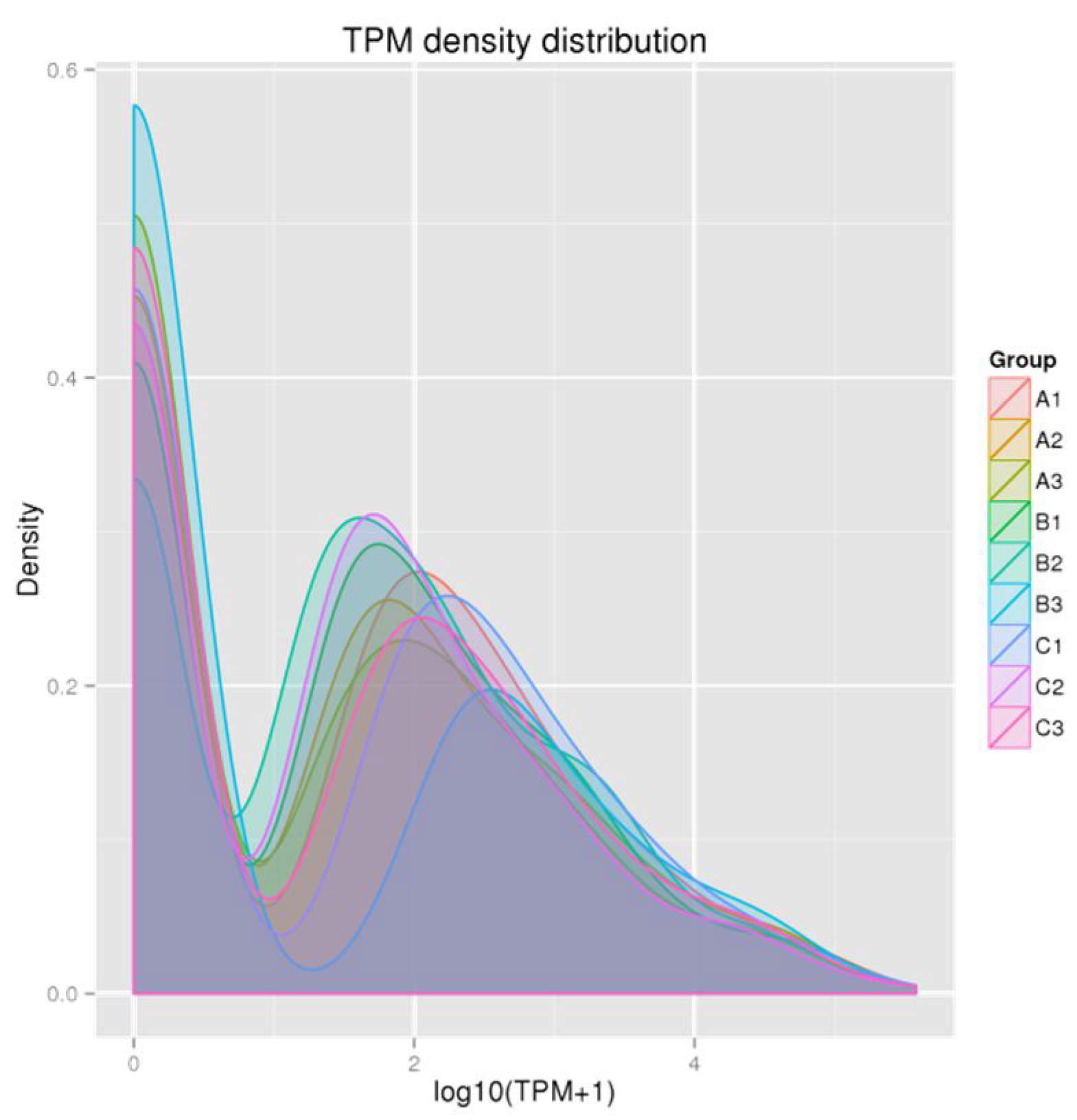
3.1. Quality Analysis of the Sequencing Data
3.2. Identification and Overall Distribution of miRNAs
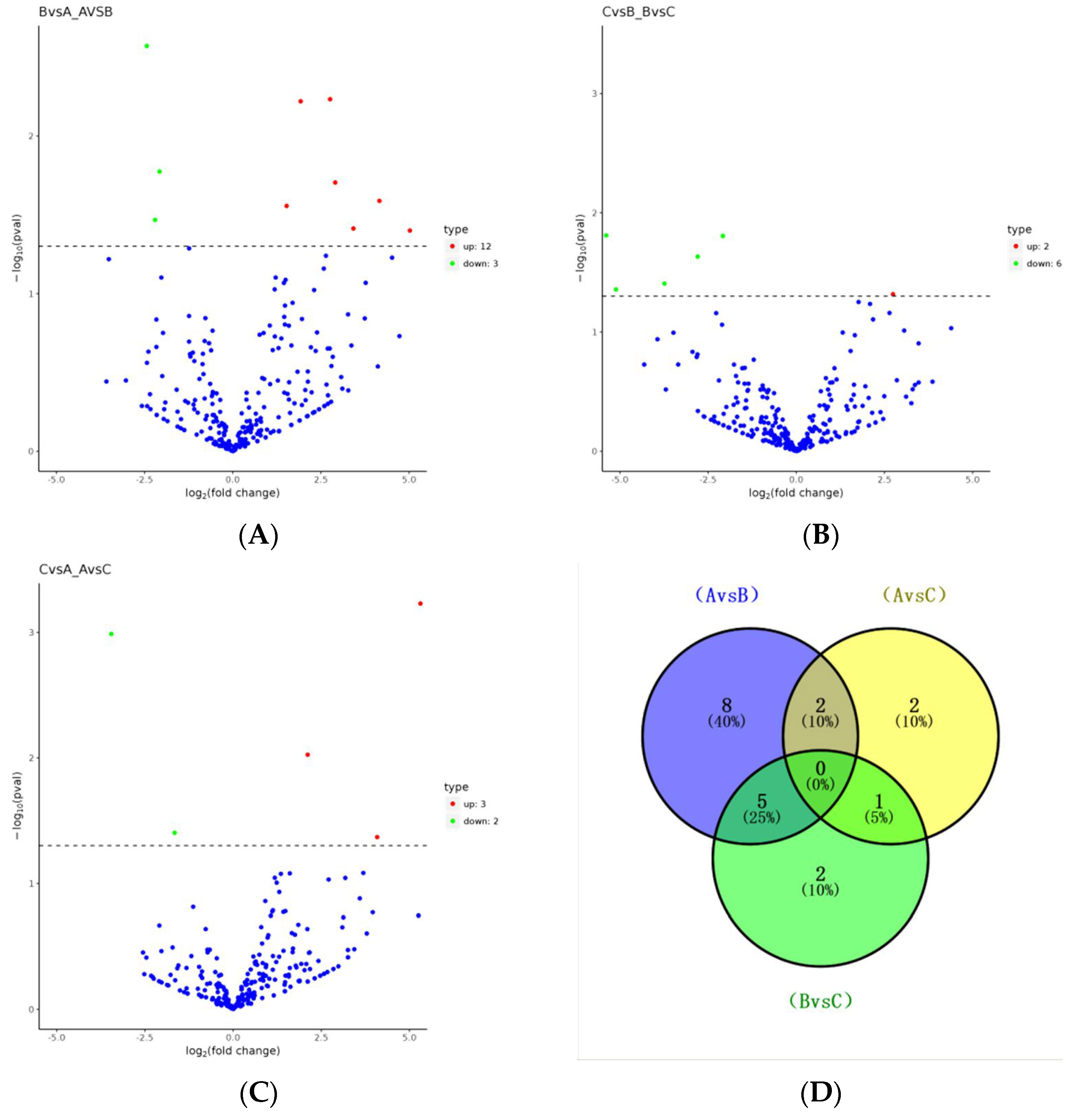
3.3. Selection of DEmiRNAs
3.4. qPCR Validation of the RNA-Seq Data
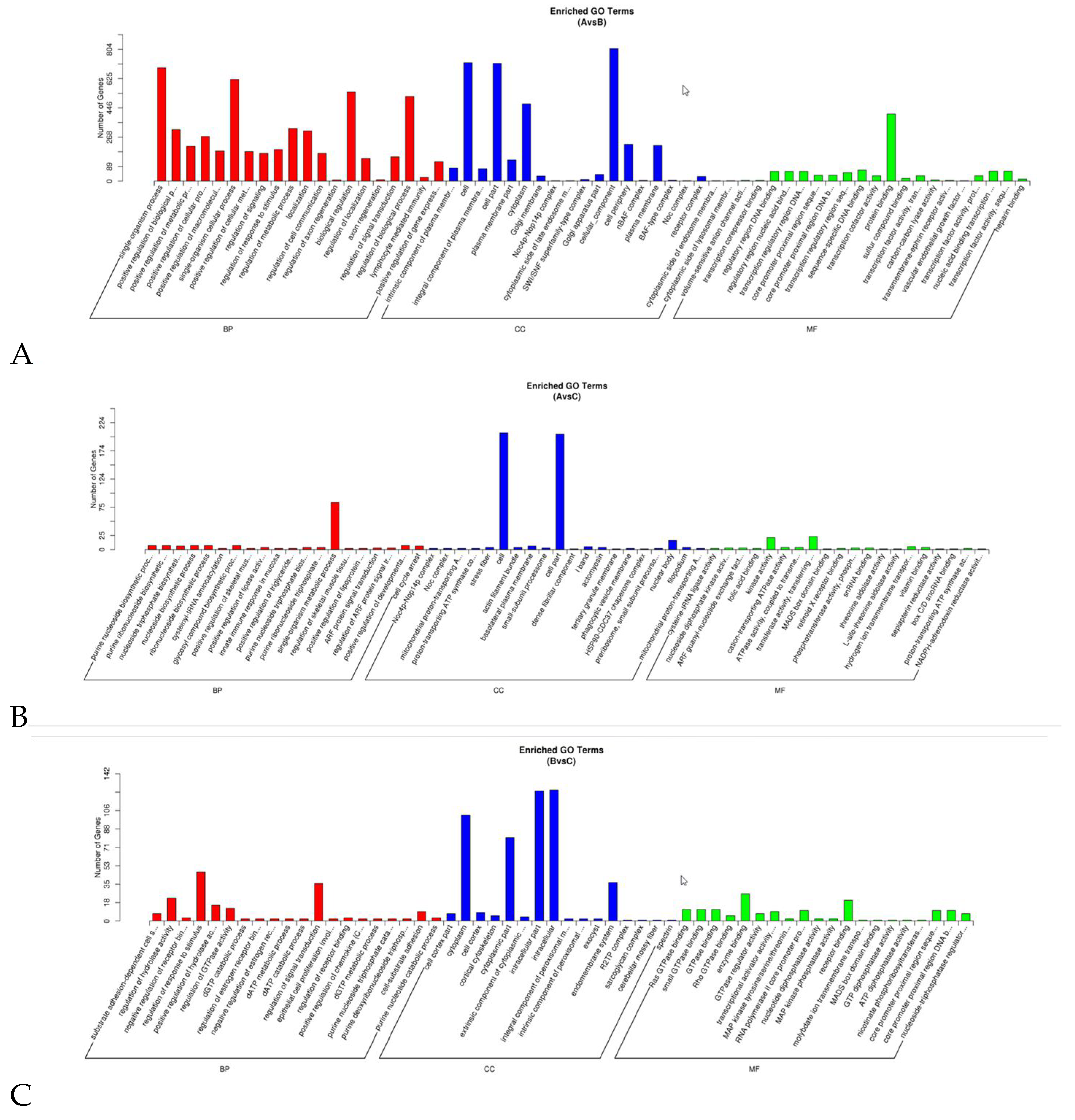
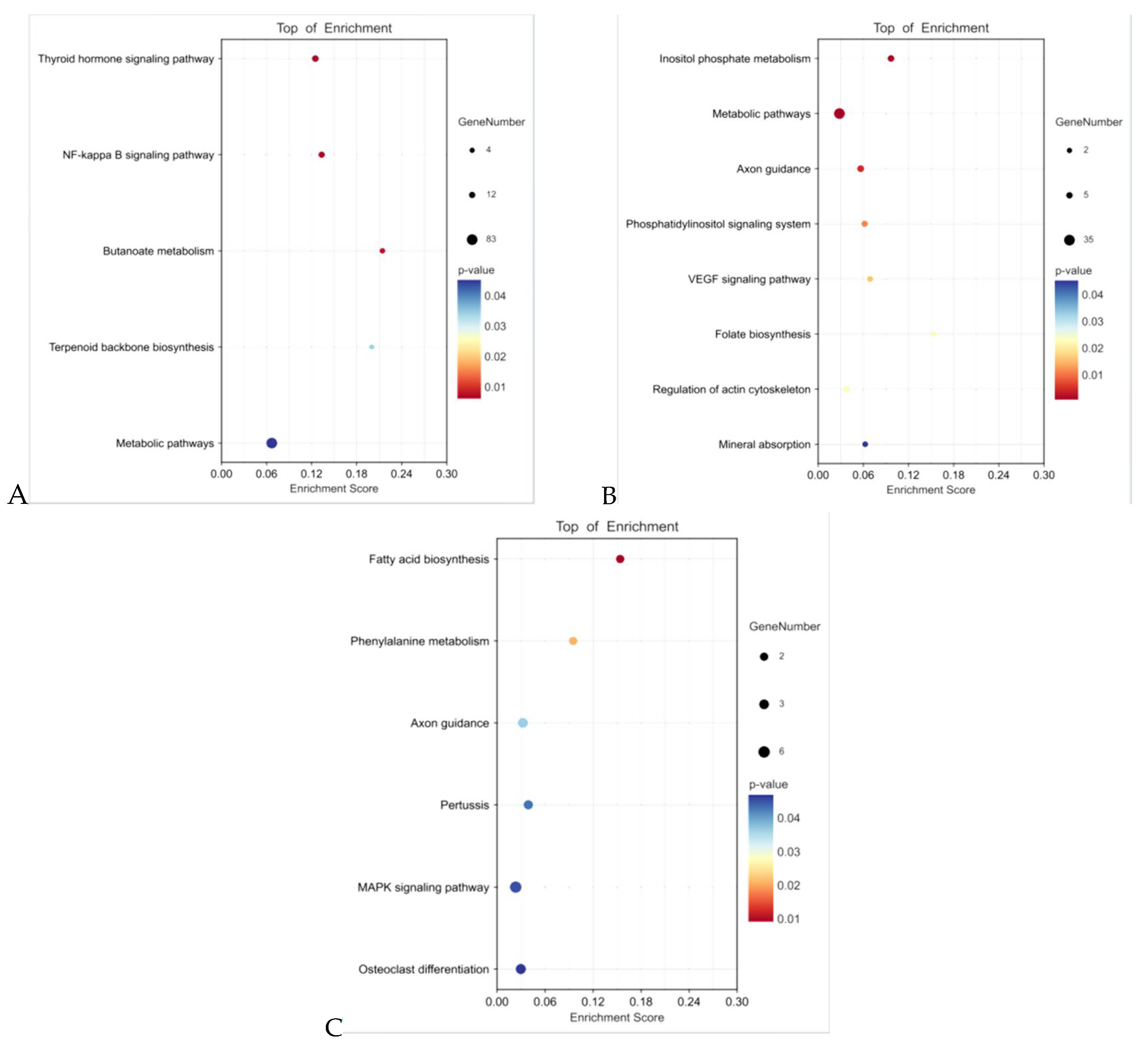
3.5. Functional Enrichment Analysis of Target Genes of DEmiRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Board Invited Review: Post-transfer consequences of invitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Kou, H.; Chen, X.; Lu, Y.; Li, L.; Wang, D. Early pregnancy diagnoses based on physiological indexes of dairy cattle: A review. Trop. Anim. Health Prod. 2020, 52, 2205–2212. [Google Scholar] [CrossRef]
- Liao, X.G.; Li, Y.L.; Gao, R.F.; Geng, Y.Q.; Chen, X.M.; Liu, X.Q.; Ding, Y.B.; Mu, X.Y.; Wang, Y.X.; He, J.L. Folate deficiency decreases apoptosis of endometrium decidual cells in pregnant mice via the mitochondrial pathway. Nutrients 2015, 7, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Wilczynska, A.; Bushell, M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Kolluru, G.K.; Ahmed, A. Small molecule, big prospects: microRNA in pregnancy and its complications. J. Pregnancy 2017, 2017, 6972732. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Giacomini, E.; Scotti, G.M.; Vanni, V.S.; Lazarevic, D.; Makieva, S.; Privitera, L.; Signorelli, S.; Cantone, L.; Bollati, V.; Murdica, V.; et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum. Reprod. 2021, 36, 2249–2274. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. The role of placental exosomes in reproduction. Am. J. Reprod. Immunol. 2010, 63, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Arosh, J.; Chapdelaine, P.; Fortier, M. Expression of prostaglandin transporter in the bovine uterus and fetal membranes during pregnancy. Biol. Reprod. 2005, 73, 230–236. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Nagaeva, O.; Chen, T.; Stendahl, U.; Antsiferova, J.; Mogren, I.; Hernestal, J.; Baranov, V. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: A possible novel immune escape mechanism for fetal survival. J. Immunol. 2006, 176, 3585–3592. [Google Scholar] [CrossRef]
- Markkandan, K.; Ahn, K.; Lee, D.J.; Kim, T.I.; Dang, C.; Hong, S.-E.; Yoon, H.-B.; Lim, H.-J.; Hong, C.P. Profiling and identification of pregnancy-associated circulating microRNAs in dairy cattle. Genes Genom. 2018, 40, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liang, G.; Sun, X. Comparative miRNAome analysis revealed different miRNA expression profiles in bovine sera and exosomes. BMC Genom. 2016, 17, 630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yang, C.; Yang, J.; Liu, P.; Jiang, K.; Shaukat, A.; Wu, H.; Deng, G. Placental exosome-mediated Bta-miR-499-Lin28B/let-7 axis regulates inflammatory bias during early pregnancy. Cell Death Dis. 2018, 9, 704. [Google Scholar] [CrossRef]
- Mamo, S.; Mehta, J.P.; McGettigan, P.; Fair, T.; Spencer, T.E.; Bazer, F.W.; Lonergan, P. RNA sequencing reveals novel gene clusters in bovine conceptuses associated with maternal recognition of pregnancy and implantation. Biol. Reprod. 2011, 85, 1143–1151. [Google Scholar] [CrossRef]
- Martins, J.P.; Policelli, R.K.; Pursley, J.R. Luteolytic effects of cloprostenol sodium in lactating dairy cows treated with G6G/Ovsynch. J. Dairy Sci. 2011, 94, 2806–2814. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, H.M.; Chen, L.H.; Hsu, L.T.; Lai, C.H. Immune Tolerance of Embryo Implantation and Pregnancy: The role of human decidual stromal cell- and embryonic-derived extracellular vesicles. Int. J. Mol. Sci. 2022, 23, 13382. [Google Scholar] [CrossRef]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental exosomes as early biomarker of preeclampsia: Potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef]
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol. Reprod. 2016, 94, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Wang, H.; Duan, E. Uterine fluid in pregnancy:a biological and clinical outlook. Trends Mol. Med. 2017, 23, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, M.A.; Gijbels, K.; Visser, A.; Craeye, D.; Walbers, S.; Pinxteren, J.; Deans, R.J.; Annaert, W.; Vaes, B.L. Using miRNA-mRNA interaction analysis to link biologically relevant miRNAs to stem cell identity testing for next-generation culturing development. Stem Cells Transl. Med. 2016, 5, 709–722. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Zhai, Y.; Shi, Q.; Chu, Q.; Chen, F.; Feng, Y.; Zhang, Z.; Qi, X.; Arends, D.; Brockmann, G.A.; Wang, E.; et al. miRNA profiling in intrauterine exosomes of pregnant cattle on day 7. Front. Vet. Sci. 2022, 9, 1078394. [Google Scholar] [CrossRef]
- Klohonatz, K.M.; Cameron, A.D.; Hergenreder, J.R.; da Silveira, J.C.; Belk, A.D.; Veeramachaneni, D.N.R.; Bouma, G.J.; Bruemmer, J.E. Circulating miRNAs as potential alternative cell signaling associated with maternal recognition of pregnancy in the mare. Biol. Reprod. 2016, 95, 124. [Google Scholar] [CrossRef]
- Ioannidis, J.; Donadeu, F.X. Circulating miRNA signatures of early pregnancy in cattle. BMC Genom. 2016, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Shekibi, M.; Heng, S.; Nie, G. MicroRNAs in the regulation of endometrial receptivity for embryo implantation. Int. J. Mol. Sci. 2022, 23, 6210. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Zou, X.; Lin, H.J.; Zhao, B.C.; Zhang, M.L.; Luo, C.H.; Fu, S.X. miRNA-185 regulates the VEGFA signaling pathway in dairy cows with retained fetal membranes. Theriogenology 2018, 110, 116–121. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Zou, X.; Zhao, B.C.; Zhang, M.L.; Lin, H.J.; Luo, C.H.; Xu, Z.M.; Shao, L.Y.; Fu, S.X. miRNA-185 regulates retained fetal membranes of cattle by targeting STIM1. Theriogenology 2019, 126, 166–171. [Google Scholar] [CrossRef]
- Fan, X.; Muruganandan, S.; Shallie, P.D.; Dhal, S.; Petitt, M.; Nayak, N.R. VEGF maintains maternal vascular space homeostasis in the mouse placenta through modulation of trophoblast giant cell functions. Biomolecules 2021, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sirohi, V.K.; Kumari, S.; Shukla, V.; Manohar, M.; Popli, P.; Dwivedi, A. Sorcin is involved during embryo implantation via activating VEGF/PI3K/Akt pathway in mice. J. Mol. Endocrinol. 2018, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Zhu, C.; Jin, Y.; Li, H. Discovering the role of VEGF signaling pathway in mesendodermal induction of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2021, 553, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Kimura, K.; Aoki, M.; Hirako, M. Vascular endothelial growth factor (VEGF) promotes the early development of bovine embryo in the presence of cumulus cells. J. Vet. Med. Sci. 2002, 64, 967–971. [Google Scholar] [CrossRef]
- Hamada, K.; Oike, Y.; Takakura, N.; Ito, Y.; Jussila, L.; Dumont, D.J.; Alitalo, K.; Suda, T. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood 2000, 96, 3793–3800. [Google Scholar] [CrossRef] [PubMed]
- Beijar, E.C.; Mallard, C.; Powell, T.L. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta 2006, 27, 322–326. [Google Scholar] [CrossRef]
- Feng, R.; Qin, X.; Li, Q.; Adeniran, S.O.; Huang, F.; Li, Y.; Zhao, Q.; Zheng, P. Progesterone regulates inflammation and receptivity of cells via the NF-kappaB and LIF/STAT3 pathways. Theriogenology 2022, 186, 50–59. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Zhang, J.; Zhang, K.; Hu, X.; Wang, L.; Wang, X.; Li, J. High mobility group box-1 regulates expression of EGFR, VEGF, StAR and TIMP1/2 in bovine granulosa cells through a mechanism involving TLR2/NF-kappaB. Anim. Reprod. Sci. 2022, 247, 107152. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Guo, S.; Zhang, T.; Ma, X.; Jiang, K.; Guo, X.; Deng, G. IFN-tau Attenuates LPS-Induced endometritis by restraining HMGB1/NF-kappaB activation in bEECs. Inflammation 2021, 44, 1478–1489. [Google Scholar] [CrossRef]
- Sigdel, A.; Bisinotto, R.S.; Penagaricano, F. Genes and pathways associated with pregnancy loss in dairy cattle. Sci. Rep. 2021, 11, 13329. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, J.; Bazer, F.W.; Song, G. Stimulatory Effect of vascular endothelial growth factor on proliferation and migration of porcine trophectoderm cells and their regulation by the phosphatidylinositol-3-Kinase-AKT and mitogen-activated protein kinase cell signaling pathways. Biol. Reprod. 2014, 90, 50. [Google Scholar] [CrossRef] [PubMed]
- Olabarrieta, E.; Totorikaguena, L.; Agirregoitia, N.; Agirregoitia, E. Implication of mu opioid receptor in the in vitro maturation of oocytes and its effects on subsequent fertilization and embryo development in mice. Mol. Reprod. Dev. 2019, 86, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, A.Q.; Ozawa, M.; Hansen, P.J. Timing and dependence upon mitogen-activated protein kinase signaling for pro-developmental actions of insulin-like growth factor 1 on the preimplantation bovine embryo. Growth Horm. IGF Res. 2011, 21, 107–111. [Google Scholar] [CrossRef]
- Hambruch, N.; Haeger, J.-D.; Dilly, M.; Pfarrer, C. EGF stimulates proliferation in the bovine placental trophoblast cell line F3 via Ras and MAPK. Placenta 2010, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]

| miRNA Name | Reverse Transcription Primer Sequence (5′→3′) | qPCR Primer Sequence (5′→3′) | Product Length (bp) |
|---|---|---|---|
| bta-miR-185 | RT:GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCAGGAAC | F:GAATACTGGAGAGAAAGGCA R:GCAATTGCACTGGATACG | 48 |
| bta-miR-199a-3p | RT:GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAACAGGTA | F:GCTCGCCCCAGTGTTCAGAC R:CGTGTCGTGGAGTCGGCA | 62 |
| bta-miR-29a | RT:GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTAACCGAT | F:GCCTCACTAGCACCATCTGAA R: TATCCAGTGCGTGTCGTG | 73 |
| bta-miR-128 | RT:GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAGAGAC | F:TGCTCATCACAGTGAACCG R:AGTGCAGGGTCCGAGGTATT | 62 |
| bta-miR-3604 | RT:GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAGTAGT | R:GCCTCATAACCAATGTGCAG F:AGTGCAGGGTCCGAGGTATT | 63 |
| bta-miR-218 | RT:GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACATGGT | F:GCAGCATTGTGCTTGATCTA R: GAGGTATTCGCACTGGATA | 51 |
| bta-miR-122 | RT:GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCAAACACC | F:GCAGCATGGAGTGTGACAAT R:TATCCAGTGCGTGTCGTG | 72 |
| Group | Sample | Reads | Bases | Error Rate | Q20 | Q30 | GC Content |
|---|---|---|---|---|---|---|---|
| 0 d | A1 | 10,824,395 | 0.541 G | 0.01% | 99.46% | 97.45% | 52.56% |
| A2 | 11,291,469 | 0.565 G | 0.01% | 99.52% | 98.04% | 51.60% | |
| A3 | 10,828,966 | 0.541 G | 0.01% | 99.57% | 98.20% | 52.43% | |
| 14 d | B1 | 11,827,970 | 0.591 G | 0.01% | 99.48% | 97.72% | 52.63% |
| B2 | 11,464,028 | 0.573 G | 0.01% | 99.54% | 97.94% | 51.85% | |
| B3 | 15,418,305 | 0.771 G | 0.01% | 99.50% | 97.80% | 52.57% | |
| 21 d | C1 | 10,487,519 | 0.524 G | 0.01% | 99.42% | 96.99% | 52.73% |
| C2 | 10,822,832 | 0.541 G | 0.01% | 99.58% | 98.58% | 52.35% | |
| C3 | 12,057,138 | 0.603 G | 0.01% | 99.53% | 97.99% | 52.62% |
| Group | Sample | Total sRNA | Mapped sRNA | “+”Mapped sRNA | “-”Mapped sRNA |
|---|---|---|---|---|---|
| A1 | 10,211,425 (100%) | 8,058,781 (78.92%) | 5,475,561 (53.62%) | 2,583,220 (25.30%) | |
| 0 d | A2 | 10,898,020 (100%) | 9,768,913 (89.64%) | 6,700,448 (61.48%) | 3,068,465 (28.16%) |
| A3 | 9,859,695 (100%) | 7,924,069 (80.37%) | 5,629,459 (57.10%) | 2,294,610 (23.27%) | |
| B1 | 11,478,340 (100%) | 8,827,964 (76.91%) | 5,880,868 (51.23%) | 2,947,096 (25.68%) | |
| 14 d | B2 | 11,151,053 (100%) | 8,976,220 (80.50%) | 5,902,948 (52.94%) | 3,073,272 (27.56%) |
| B3 | 15,246,813 (100%) | 1,300,366 (85.29%) | 9,797,584 (64.26%) | 3,206,078 (21.03%) | |
| C1 | 10,174,282 (100%) | 7,719,617 (75.87%) | 5,055,172 (49.69%) | 2,664,445 (26.19%) | |
| 21 d | C2 | 10,430,486 (100%) | 8,148,185 (78.12%) | 5,504,272 (52.77%) | 2,643,913 (25.35%) |
| C3 | 10,137,855 (100%) | 7,906,125 (77.99%) | 5,336,579 (52.64%) | 2,569,546 (25.35%) |
| Group | Sample | Known miRNA Matrices | Known miRNA Precursors | Novel miRNA Matrices | Novel miRNA Precursors |
|---|---|---|---|---|---|
| 0 d | A1 | 210 | 277 | 5 | 5 |
| A2 | 207 | 255 | 3 | 3 | |
| A3 | 192 | 239 | 2 | 2 | |
| 14 d | B1 | 228 | 292 | 3 | 3 |
| B2 | 257 | 342 | 4 | 4 | |
| B3 | 143 | 190 | 2 | 2 | |
| 21 d | C1 | 195 | 251 | 5 | 5 |
| C2 | 224 | 289 | 3 | 3 | |
| C3 | 193 | 257 | 3 | 4 | |
| Total | 367 | 472 | 9 | 10 |
| Group | miRNA Name | log2 Fold Change | pval |
|---|---|---|---|
| 0 d vs. 14 d | |||
| bta-miR-1 | −3.419840451 | 0.0386019 | |
| bta-miR-107 | −4.157990081 | 0.025785178 | |
| bta-miR-122 | 2.435598687 | 0.002682208 | |
| bta-miR-1298 | −5.783528305 | 0.010463213 | |
| bta-miR-133a | −6.184832579 | 0.006337687 | |
| bta-miR-185 | −2.760173877 | 0.005839627 | |
| bta-miR-199a-3p | −1.924179333 | 0.006023499 | |
| bta-miR-199a-5p | 2.203766067 | 0.034036793 | |
| bta-miR-202 | −5.021631332 | 0.039784364 | |
| bta-miR-218 | −2.904624102 | 0.019721384 | |
| bta-miR-2889 | −6.002972001 | 0.011132547 | |
| bta-miR-29a | −1.52695064 | 0.02776566 | |
| bta-miR-3432a | 2.075160579 | 0.016811504 | |
| bta-miR-3604 | −1.924179333 | 0.006023499 | |
| bta-miR-493 | −6.028330221 | 0.006258652 | |
| 0 d vs. 14 d | |||
| bta-miR-107 | −4.08341007 | 0.042774401 | |
| bta-miR-128 | 1.658418829 | 0.039524034 | |
| bta-miR-199a-5p | 3.454122921 | 0.001027989 | |
| bta-miR-205 | −5.30962881 | 0.000588845 | |
| bta-miR-486 | −2.112590613 | 0.009433274 | |
| 14 d vs. 21 d | |||
| bta-miR-1 | 3.738895476 | 0.039250249 | |
| bta-miR-1298 | 5.390119257 | 0.015470609 | |
| bta-miR-145 | 5.113806788 | 0.043988941 | |
| bta-miR-199a-3p | 2.08290067 | 0.015652709 | |
| bta-miR-205 | −6.652315352 | 0.000397973 | |
| bta-miR-218 | 2.796429934 | 0.023303866 | |
| bta-miR-3604 | 2.08290067 | 0.015652709 | |
| bta-miR-409b | −2.740989268 | 0.048214807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Bao, B.; Wang, Y.; Wang, Z.; Yang, Y.; Xu, J.; Wang, X.; Luoreng, Z. RNA Sequencing Reveals the Involvement of Serum Exosomal miRNAs in Early Pregnancy in Cattle. Animals 2024, 14, 2600. https://doi.org/10.3390/ani14172600
Ji Z, Bao B, Wang Y, Wang Z, Yang Y, Xu J, Wang X, Luoreng Z. RNA Sequencing Reveals the Involvement of Serum Exosomal miRNAs in Early Pregnancy in Cattle. Animals. 2024; 14(17):2600. https://doi.org/10.3390/ani14172600
Chicago/Turabian StyleJi, Zhongxiang, Binwu Bao, Yumei Wang, Zhengxing Wang, Yi Yang, Jinrui Xu, Xingping Wang, and Zhuoma Luoreng. 2024. "RNA Sequencing Reveals the Involvement of Serum Exosomal miRNAs in Early Pregnancy in Cattle" Animals 14, no. 17: 2600. https://doi.org/10.3390/ani14172600





