The Ruminal Microbiome Alterations Associated with Diet-Induced Milk Fat Depression and Milk Fat Globule Size Reduction in Dairy Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Animals, Diets, and Sampling
2.3. MFG Size Analysis
2.4. Ruminal Microbial DNA Extraction and PCR Amplification
2.5. Illumina MiSeq Sequencing
2.6. Processing of Sequencing Data
2.7. Statistical Analysis
3. Results
3.1. Effect of CLA on the Production Performance of Dairy Goats
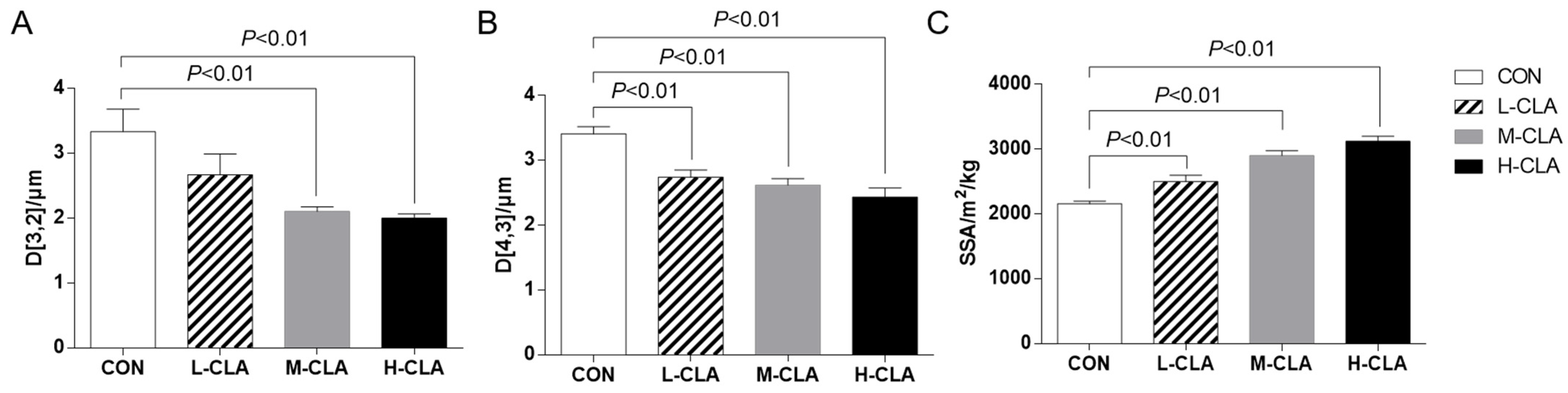
3.2. The Effect of CLA on MFG Size Parameters
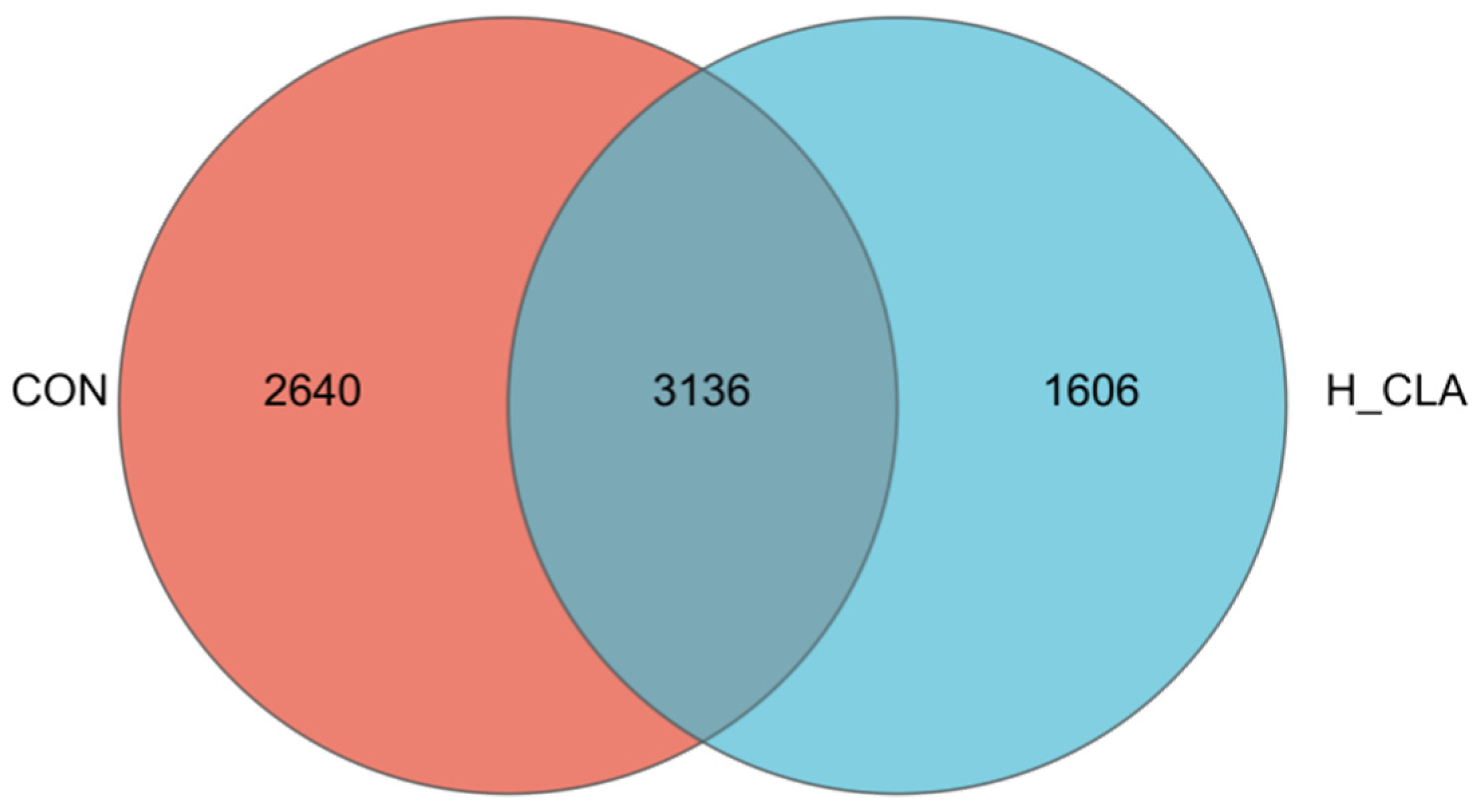
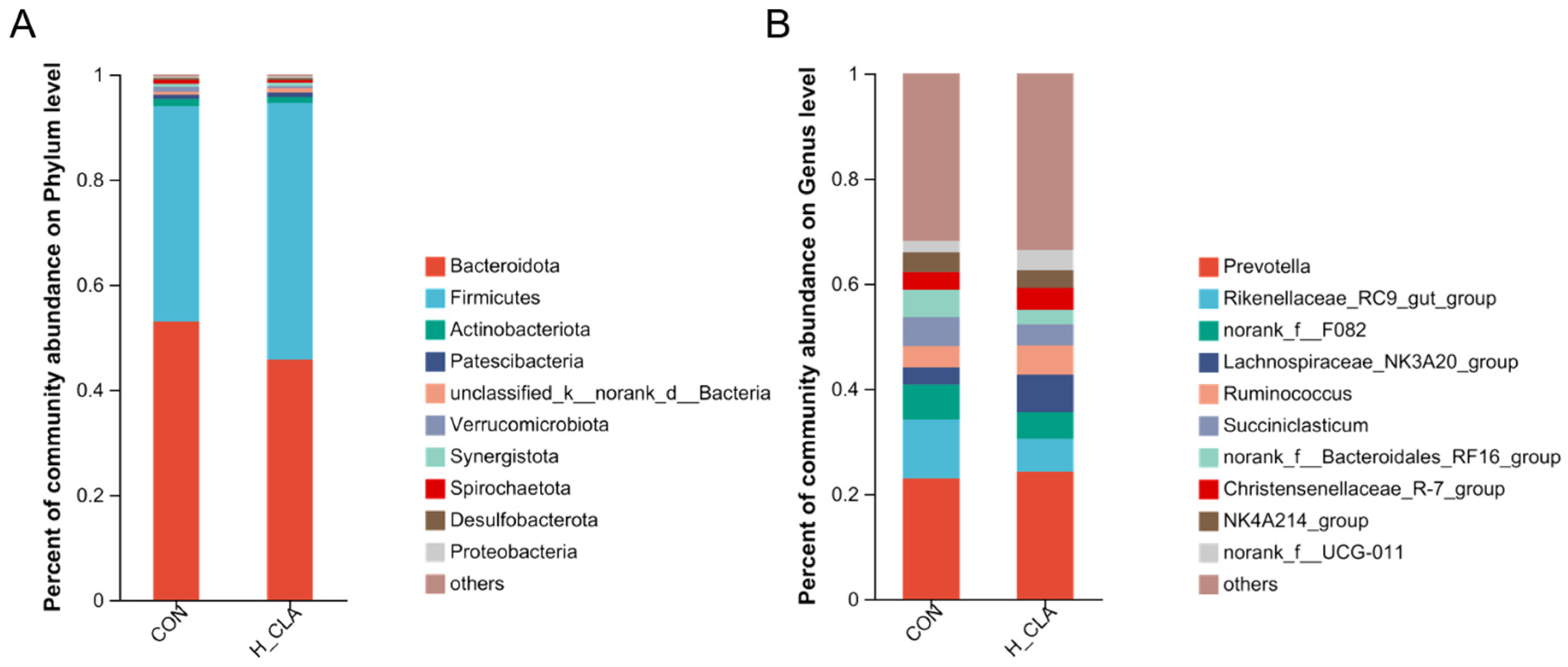
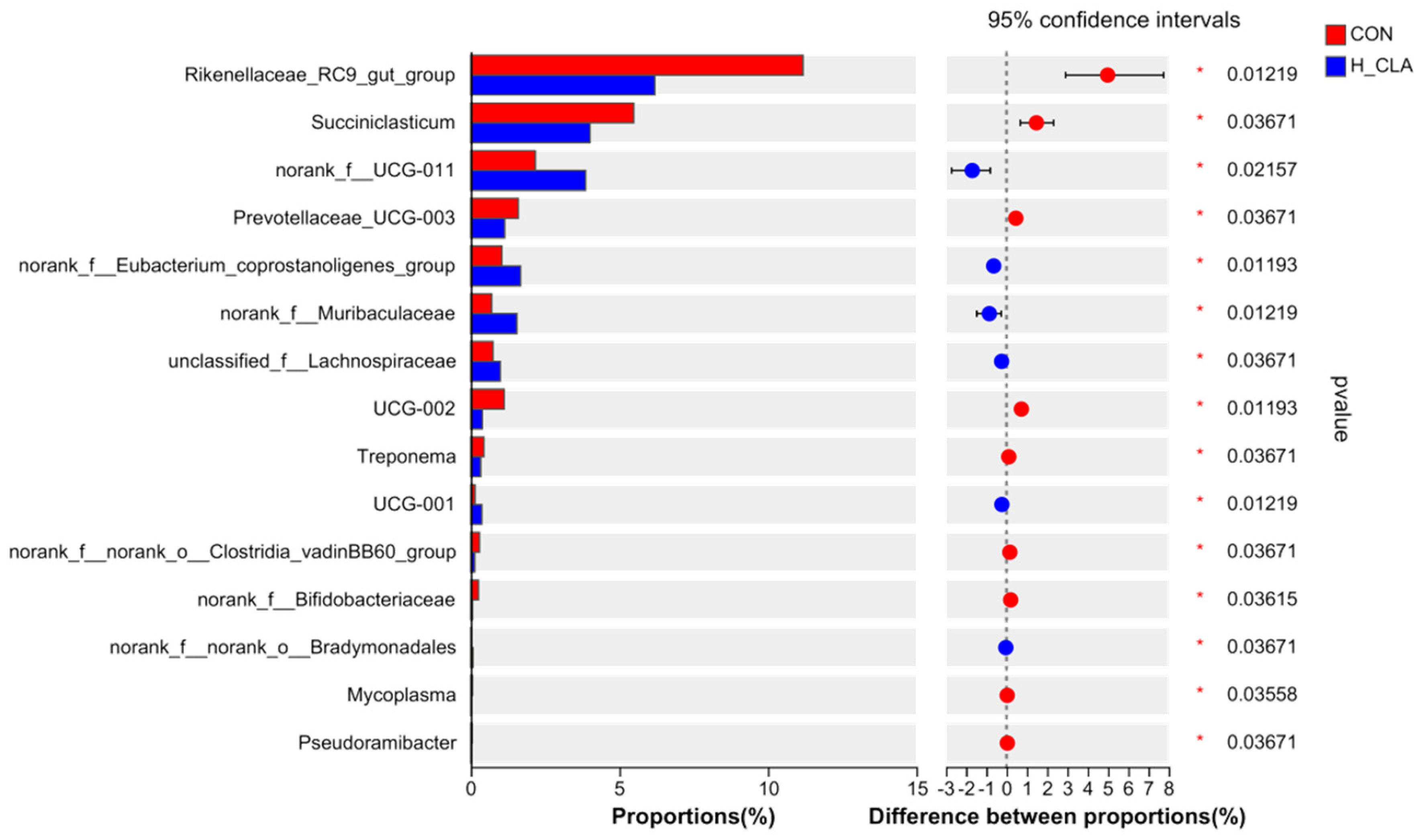
3.3. The Effect of CLA on the Composition of Ruminal Bacteria
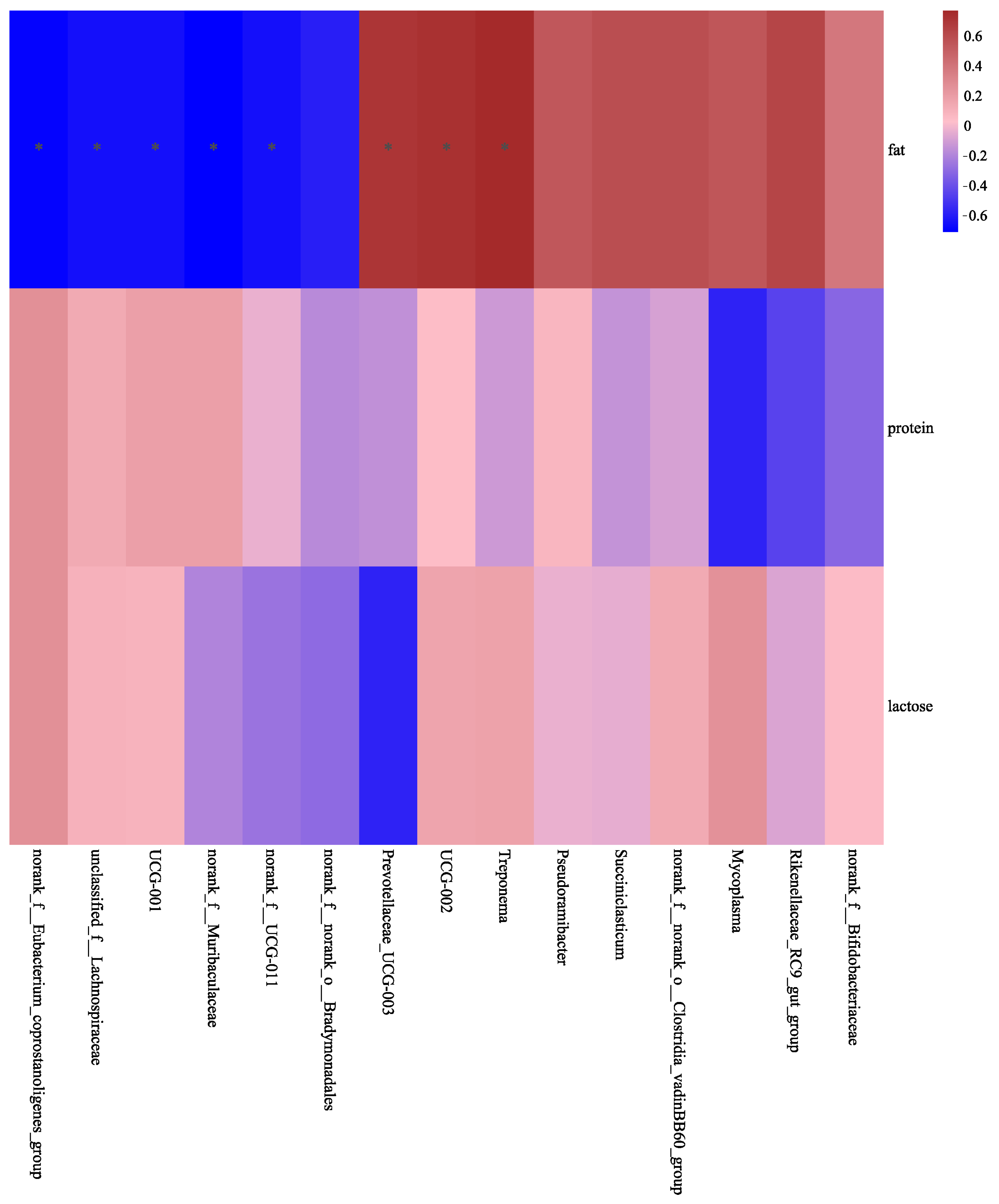
3.4. Correlation of Differential Ruminal Bacteria with Goat Milk Composition and MFG Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashes, J.R.; Gulati, S.K.; Scott, T.W. Potential to alter the content and composition of milk fat through nutrition. J. Dairy Sci. 1997, 80, 2204–2212. [Google Scholar] [CrossRef]
- Bauman, D.E.; Harvatine, K.J.; Lock, A.L. Nutrigenomics, Rumen-Derived Bioactive Fatty Acids, and the Regulation of Milk Fat Synthesis. Annu. Rev. Nutr. 2011, 31, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, J.; Yang, D.; Wang, X.Y.; Yang, Z.Y.; Jin, Q.Z.; Wang, M.Y.; Lai, J.Q.; Wang, X.G. Phospholipid Composition and Fat Globule Structure I: Comparison of Human Milk Fat from Different Gestational Ages, Lactation Stages, and Infant Formulas. J. Agric. Food Chem. 2019, 67, 13922–13928. [Google Scholar] [CrossRef]
- Michalski, M.C.; Gassi, J.Y.; Famelart, M.H.; Leconte, N.; Camier, B.; Michel, F.; Briard, V. The size of native milk fat globules affects physico-chemical and sensory properties of Camembert cheese. Lait 2003, 83, 131–143. [Google Scholar] [CrossRef]
- Michalski, M.C.; Ollivon, M.; Briard, V.; Leconte, N.; Lopez, C. Native fat globules of different sizes selected from raw milk: Thermal and structural behavior. Chem. Phys. Lipids 2004, 132, 247–261. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, D.L. Changes in milk fat phospholipids during lactation. J. Dairy Sci. 1990, 73, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, S.; Hurtaud, C.; Marnet, P.G.; Faverdin, P.; Peyraud, J.L. Composition of milk fat from cows selected for milk fat globule size and offered either fresh pasture or a corn silage-based diet. J. Dairy Sci. 2007, 90, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.; Auldist, M.; Greenwood, J.; Day, L. Natural variation of bovine milk fat globule size within a herd. J. Dairy Sci. 2014, 97, 4072–4082. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Corl, B.A.; Dwyer, D.A.; Saebo, A.; Bauman, D.E. Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R179–R184. [Google Scholar] [CrossRef]
- Lock, A.L.; Rovai, M.; Gipson, T.A.; de Veth, M.J.; Bauman, D.E. A conjugated linoleic acid supplement containing trans-10, cis-12 conjugated linoleic acid reduces milk fat synthesis in lactating goats. J. Dairy Sci. 2008, 91, 3291–3299. [Google Scholar] [CrossRef]
- Shi, H.P.; Zhang, T.Y.; Li, C.; Wang, J.J.; Huang, J.T.; Li, Z.Y. trans-10,cis-12-Conjugated Linoleic Acid Affects Expression of Lipogenic Genes in Mammary Glands of Lactating Dairy Goats. J. Agric. Food Chem. 2017, 65, 9460–9467. [Google Scholar] [CrossRef]
- Xing, Z.Y.; Zhang, M.L.; Wang, Y.Y.; Yang, G.Y.; Han, L.Q.; Loor, J.J. Short communication: A decrease in diameter of milk fat globules accompanies milk fat depression induced by conjugated linoleic acid supplementation in lactating dairy cows. J. Dairy Sci. 2020, 103, 5143–5147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Xing, Z.Y.; Huang, Q.X.; Han, L.Q. Effect of conjugated linoleic acid supplementation on fat globule size in raw milk. Int. Dairy J. 2021, 115, 104919. [Google Scholar] [CrossRef]
- Rico, D.E.; Preston, S.H.; Risser, J.M.; Harvatine, K.J. Rapid changes in key ruminal microbial populations during the induction of and recovery from diet-induced milk fat depression in dairy cows. Br. J. Nutr. 2015, 114, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Su, X.D.; Bai, H.X.; Yang, Y.T.; Wang, H.R.; Dan, Z.; Lu, J.B.; Wu, S.R.; Cai, C.J.; Cao, Y.C.; et al. Specific enrichment of microbes and increased ruminal propionate production: The potential mechanism underlying the high energy efficiency of Holstein heifers fed steam-flaked corn. Amb Express 2019, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.B.; Guo, C.Z.; Sun, D.M.; Seddik, H.E.; Mao, S.Y. The Ruminal Microbiome and Metabolome Alterations Associated with Diet-Induced Milk Fat Depression in Dairy Cows. Metabolites 2019, 9, 154. [Google Scholar] [CrossRef]
- Zou, C.X.; Gu, Q.C.; Zhou, X.K.; Xia, Z.S.; Muhammad, W.I.; Tang, Q.F.; Liang, M.Z.; Lin, B.; Qin, G.S. Ruminal microbiota composition associated with ruminal fermentation parameters and milk yield in lactating buffalo in Guangxi, China-A preliminary study. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1374–1379. [Google Scholar] [CrossRef]
- Pitta, D.W.; Indugu, N.; Vecchiarelli, B.; Rico, D.E.; Harvatine, K.J. Alterations in ruminal bacterial populations at induction and recovery from diet-induced milk fat depression in dairy cow. J. Dairy Sci. 2018, 101, 295–309. [Google Scholar] [CrossRef]
- Lu, J.; Argov-Argaman, N.; Anggrek, J.; Boeren, S.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K.A. The protein and lipid composition of the membrane of milk fat globules depends on their size. J Dairy Sci 2016, 99, 4726–4738. [Google Scholar] [CrossRef]
- Harvatine, K.J.; Boisclair, Y.R.; Bauman, D.E. Time-dependent effect of trans-10, cis-12 conjugated linoleic acid on gene expression of lipogenic enzymes and regulators in mammary tissue of dairy cows. J. Dairy Sci. 2018, 101, 7585–7592. [Google Scholar] [CrossRef]
- Perfield, J.W.; Lock, A.L.; Griinari, J.M.; Sæbo, A.; Delmonte, P.; Dwyer, D.A.; Bauman, D.E. trans-9, cis-11 conjugated linoleic acid reduces milk fat synthesis in lactating dairy cows. J. Dairy Sci. 2007, 90, 2211–2218. [Google Scholar] [CrossRef]
- Peterson, D.G.; Matitashvili, E.A.; Bauman, D.E. Diet-induced milk fat depression is characterized by increased milk fat content of t10, c12 CLA and corresponding reductions in mammary tissue expression of lipogenic genes. Faseb J. 2002, 16, A232. [Google Scholar]
- Baumgard, L.H.; Sangster, J.K.; Bauman, D.E. Milk fat synthesis in dairy cows is progressively reduced by increasing supplemental amounts of trans-10, cis-12 conjugated linoleic acid (CLA). J. Nutr. 2001, 131, 1764–1769. [Google Scholar] [CrossRef]
- Viswanadha, S.; Giesy, J.G.; Hanson, T.W.; McGuire, M.A. Dose response of milk fat to intravenous administration of the trans-10, cis-12 isomer of conjugated linoleic acid. J. Dairy Sci. 2003, 86, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Liu, Z.T.; Kang, F.Y.; Wu, K.X.; Ni, H.; Han, Y.Q.; Yang, Y.B.; Fu, T.; Yang, G.Y.; Gao, T.Y.; et al. Is milk fat globule size correlated with milk fat content in Ruminants? Food Chem. 2024, 439, 138101. [Google Scholar] [CrossRef] [PubMed]
- Tzirkel-Hancock, N.; Sharabi, L.; Argov-Argaman, N. Milk fat globule size: Unraveling the intricate relationship between metabolism, homeostasis, and stress signaling. Biochimie 2023, 215, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Suwal, S.; Andersen, U.; Otte, J.; Ahrn, L. Effects of pulsed electric field on fat globule structure, lipase activity, and fatty acid composition in raw milk and milk with different fat globule sizes. Innov. Food Sci. Emerg. Technol. 2021, 67, 102548. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Riedl, K.; García-Cano, I.; Chaves, F.; Walsh, K.R.; Jimenez-Flores, R.; Failla, M.L. Efficient in vitro digestion of lipids and proteins in bovine milk fat globule membrane ingredient (MFGMi) and whey-casein infant formula with added MFGMi. J. Dairy Sci. 2023, 106, 3086–3097. [Google Scholar] [CrossRef]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Mida, K.; Cohen, B.C.; Visker, M.; Hettinga, K. Milk fat content and DGAT1 genotype determine lipid composition of the milk fat globule membrane. PLoS ONE 2013, 8, e68707. [Google Scholar] [CrossRef]
- Mesilati-Stahy, R.; Moallem, U.; Magen, Y.; Argov-Argaman, N. Altered concentrate to forage ratio in cows ration enhanced bioproduction of specific size subpopulation of milk fat globules. Food Chem. 2015, 179, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, T.; Huang, Q.; Xing, Z.; Yang, J.; Lu, W.; Hu, M.; Han, L.Q.; Loor, J.J.; Gao, T.Y. Size, number and phospholipid composition of milk fat globules are affected by dietary conjugated linoleic acid. J. Anim. Physiol. Anim. Nutr. 2022, 107, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.E.; Steele, M.A.; Northwood, K.S.; Dijkstra, J.; France, J.; Wright, A.D.G.; McBride, B.W. Impact of subacute ruminal acidosis (SARA) adaptation and recovery on the density and diversity of bacteria in the rumen of dairy cows. Fems Microbiol. Ecol. 2011, 78, 275–284. [Google Scholar] [CrossRef]
- Huo, W.J.; Zhu, W.Y.; Mao, S.Y. Impact of subacute ruminal acidosis on the diversity of liquid and solid-associated bacteria in the rumen of goats. World J. Microbiol. Biotechnol. 2014, 30, 669–680. [Google Scholar] [CrossRef]
- Sadet, S.; Martin, C.; Meunier, B.; Morgavi, D.P. PCR-DGGE analysis reveals a distinct diversity in the bacterial population attached to the rumen epithelium. Animal 2007, 1, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Griinari, J.M.; Dwyer, D.A.; McGuire, M.A.; Bauman, D.E.; Palmquist, D.L.; Nurmela, K.V. Trans-octadecenoic acids and milk fat depression in lactating dairy cows. J. Dairy Sci. 1998, 81, 1251–1261. [Google Scholar] [CrossRef]
- Piperova, L.S.; Teter, B.B.; Bruckental, I.; Sampugna, J.; Mills, S.E.; Yurawecz, M.P.; Fritsche, J.; Ku, K.; Erdman, R.A. Mammary lipogenic enzyme activity, trans fatty acids and conjugated linoleic acids are altered in lactating dairy cows fed a milk fat-depressing diet. J. Nutr. 2000, 130, 2568–2574. [Google Scholar] [CrossRef]
- Rico, D.E.; Holloway, A.W.; Harvatine, K.J. Effect of diet fermentability and unsaturated fatty acid concentration on recovery from diet-induced milk fat depression. J. Dairy Sci. 2015, 98, 7930–7943. [Google Scholar] [CrossRef]
- Huws, S.A.; Kim, E.J.; Cameron, S.J.S.; Girdwood, S.E.; Davies, L.; Tweed, J.; Vallin, H.; Scollan, N.D. Characterization of the rumen lipidome and microbiome of steers fed a diet supplemented with flax and echium oil. Microb. Biotechnol. 2015, 8, 331–341. [Google Scholar] [CrossRef]
- Lourenço, M.; Ramos-Morales, E.; Wallace, R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef]
- Liu, K.Z.; Zhang, Y.D.; Huang, G.X.; Zheng, N.; Zhao, S.G.; Wang, J.Q. Ruminal bacterial community is associated with the variations of total milk solid content in Holstein lactating cows. Anim. Nutr. 2022, 9, 175–183. [Google Scholar] [CrossRef]
- Naas, A.E.; Mackenzie, A.K.; Mravec, J.; Schückel, J.; Willats, W.G.T.; Eijsink, V.G.H.; Pope, P.B. Do Rumen Bacteroidetes Utilize an Alternative Mechanism for Cellulose Degradation? Mbio 2014, 5, e01401-14. [Google Scholar] [CrossRef]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H.; Global Rumen Census, C. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567, Erratum in Sci. Rep. 2016, 6, 19175. [Google Scholar] [CrossRef] [PubMed]
- Kopecny, J.; Zorec, M.; Mrázek, J.; Kobayashi, Y.; Marinsek-Logar, R. Butyrivibrio hungatei sp nov and Pseudobutyrivibrio xylanivorans sp nov., butyrate-producing bacteria from the rumen. Int. J. Syst. Evol. Microbiol. 2003, 53, 201–209. [Google Scholar] [CrossRef]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E.; Bacteria, N.A.C.R. Comparative Genome Analysis of Prevotella ruminicola and Prevotella bryantii: Insights into Their Environmental Niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef]
- Seshadri, R.; Leahy, S.C.; Attwood, G.T.; Teh, K.H.; Lambie, S.C.; Cookson, A.L.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Hadjithomas, M.; Varghese, N.J.; et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 2018, 36, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.Y.; Huang, S.C.; Liu, T.T.; Geng, A.L. Bacterial xylose isomerases from the mammal gut Bacteroidetes cluster function in Saccharomyces cerevisiae for effective xylose fermentation. Microb. Cell Factories 2015, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Guan, L.L.; Liu, J.X. Assessment of Rumen Microbiota from a Large Dairy Cattle Cohort Reveals the Pan and Core Bacteriomes Contributing to Varied Phenotypes. Appl. Environ. Microbiol. 2018, 84, e00970-18. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Guan, L.L.; Liu, J.X. Assessment of rumen bacteria in dairy cows with varied milk protein yield. J. Dairy Sci. 2019, 102, 5031–5041. [Google Scholar] [CrossRef]
- Pitta, D.W.; Kumar, S.; Vecchiarelli, B.; Shirley, D.J.; Bittinger, K.; Baker, L.D.; Ferguson, J.D.; Thomsen, N. Temporal dynamics in the ruminal microbiome of dairy cows during the transition period. J. Anim. Sci. 2014, 92, 4014–4022. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar]



| Fatty Acid | Total Fatty Acids (%) |
|---|---|
| Palmitic acid | 3.0 |
| Stearic acid | 2.7 |
| Oleic acid | 11.3 |
| Linoleic acid | 1.3 |
| Conjugated linoleic acid | 80.5 |
| C18:2 cis-9, trans-11 | 38.5 |
| C18:2 trans-10, cis-12 | 38.1 |
| Other CLA isomers | 3.8 |
| Others | <0.1 |
| Item | CON | L-CLA | M-CLA | H-CLA | SEM | p-Value |
|---|---|---|---|---|---|---|
| Milk yield (kg/day) | 1.21 | 1.13 | 1.23 | 1.22 | 0.097 | 0.501 |
| Milk fat (g/100 mL) | 3.63 a | 2.69 b | 1.85 c | 1.74 c | 0.252 | <0.01 |
| Milk protein (g/100 mL) | 3.52 | 3.54 | 3.48 | 3.79 | 0.193 | 0.676 |
| Milk lactose (g/100 mL) | 4.21 | 4.23 | 4.29 | 4.25 | 0.090 | 0.920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, Z.; Wu, K.; Zhang, C.; Fu, T.; Sun, Y.; Gao, T.; Han, L. The Ruminal Microbiome Alterations Associated with Diet-Induced Milk Fat Depression and Milk Fat Globule Size Reduction in Dairy Goats. Animals 2024, 14, 2614. https://doi.org/10.3390/ani14172614
Zhang M, Liu Z, Wu K, Zhang C, Fu T, Sun Y, Gao T, Han L. The Ruminal Microbiome Alterations Associated with Diet-Induced Milk Fat Depression and Milk Fat Globule Size Reduction in Dairy Goats. Animals. 2024; 14(17):2614. https://doi.org/10.3390/ani14172614
Chicago/Turabian StyleZhang, Menglu, Zhentao Liu, Kuixian Wu, Chuankai Zhang, Tong Fu, Yu Sun, Tengyun Gao, and Liqiang Han. 2024. "The Ruminal Microbiome Alterations Associated with Diet-Induced Milk Fat Depression and Milk Fat Globule Size Reduction in Dairy Goats" Animals 14, no. 17: 2614. https://doi.org/10.3390/ani14172614






