Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Tested Product
2.2. Determination of MIC
2.3. Time–Kill Curve Assay
2.4. PAE and PA-SME Assays
3. Results
3.1. MIC Assay Results
3.2. Time–Kill Curve Results
3.3. PAE and PA-SME Assays Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- EFSA; European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Solarte, A.L.; Astorga, R.J.; De Aguiar, F.C.; De Frutos, C.; Barrero-Domínguez, B.; Huerta, B. Susceptibility Distribution to Essential Oils of Salmonella enterica Strains Involved in Animal and Public Health and Comparison of the Typhimurium and Enteritidis Serotypes. J. Med. Food 2018, 21, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Motta Felício, I.; Limongi de Souza, R.; de Oliveira Melo, C.; Gervázio Lima, K.Y.; Vasconcelos, U.; Olímpio de Moura, R.; Eleamen Oliveira, E. Development and characterization of a carvacrol nanoemulsion and evaluation of its antimicrobial activity against selected food-related pathogens. Lett. Appl. Microbiol. 2021, 72, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Campaniello, D.; Altieri, C.; Corbo, M.R.; Sinigaglia, M. Minimal Inhibitory Concentrations of Thymol and Carvacrol: Toward a Unified Statistical Approach to Find Common Trends. Microorganisms 2023, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Odenholt-Tornqvist, I.; Lowdin, E.; Cars, O. Postantibiotic effects and postantibiotic sub-MIC effects of roxithromycin, clarithromycin, and azithromycin on respiratory tract pathogens. Antimicrob. Agents Chemother. 1995, 39, 221–226. [Google Scholar] [CrossRef]
- Majtán, V.; Majtánová, L. Postantibiotic Effects and Postantibiotic Sub-MIC Effects of Ciprofloxacin, Pefloxacin and Amikacin on the Biological Properties of Salmonella Strains. Folia Microbiol. 1997, 42, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.C.; Alfaro, L.A.; Reinoso, J.C.; Mestre, M.J.G.; Rodríguez-Gascón, A. Análisis farmacocinético-farmacodinámico en microbiología: Herramienta para evaluar el tratamiento antimicrobiano. Enferm. Infecc. Microbiol. Clin. 2015, 33, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Nedbalcova, K.; Zouharova, M.; Sperling, D. Post-antibiotic effect of marbofloxacin, enrofloxacin and amoxicillin against selected respiratory pathogens of pigs. Vet. Med. 2019, 64, 67–77. [Google Scholar] [CrossRef]
- Peñalver, P.; Huerta, B.N.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS 2005, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zorn, B.; Goñi, J.G.; Rodríguez, L.J.D. El destino de Salmonella en el interior de un ser vivo. Porci 2004, 81, 23–28. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; VET01S; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Baroni, E.; Russi, N.; Rubio, M.; Picco, E.; Formentini, E. Actividad antibacteriana in vitro de ciprofloxacina sobre Escherichia coli: Efecto del suero bovino y tamaño del inóculo. InVet 2014, 16, 7–14. [Google Scholar]
- Sidhu, P.K.; Landoni, M.F.; Aliabadi, F.S.; Lees, P. PK-PD integration and modeling of marbofloxacin in sheep. Res. Vet. Sci. 2010, 88, 134–141. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Belato, K.K.; de Oliveira, J.R.; de Oliveira, F.S.; de Oliveira, L.D.; Camargo, S.E.A. Cytotoxicity and genotoxicity of thymol verified in murine macrophages (RAW 264.7) after antimicrobial analysis in Candida albicans, Staphylococcus aureus, and Streptococcus mutans. J. Funct. Foods 2018, 40, 455–460. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Harada, K.; Shimizu, T.; Kataoka, Y.; Takahashi, T. Post-antibiotic effect of orbifloxacin against Escherichia coli and Pseudomonas aeruginosa isolates from dogs. Acta Vet. Scand. 2012, 54, 16. [Google Scholar] [CrossRef]
- Labarca, J.L. New concepts in pharmacokinetics. Must we thinj again how to use the antibiotic? Rev. Chil. Infectol. 2002, 19, S33–S37. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Luque, I.; Maldonado, A.; Galán-Relaño, Á.; Huerta, B. Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiologyopen 2018, 7, e00613. [Google Scholar] [CrossRef] [PubMed]
- Wain, J.; Simpson, J.A.; Thi Diem Nga, L.; Song Diep, T.; Thanh Duy, P.; Baker, S.; Day, N.P.J.; White, N.J.; Parry, C.M. Bactericidal activities and post-antibiotic effects of ofloxacin and ceftriaxone against drug-resistant Salmonella enterica serovar Typhi. J. Antimicrob. Chemother. 2021, 76, 2606–2609. [Google Scholar] [CrossRef]
- Soren, O.; Brinch, K.S.; Patel, D.; Liu, Y.; Liu, A.; Coates, A.; Hu, Y. Antimicrobial peptide novicidin synergizes with rifampin, ceftriaxone, and ceftazidime against antibiotic-resistant Enterobacteriaceae in vitro. Antimicrob. Agents Chemother. 2015, 59, 6233–6240. [Google Scholar] [CrossRef]
- Akermi, S.; Smaoui, S.; Chaari, M.; Elhadef, K.; Gentile, R.; Hait, M.; Roymahapatra, G.; Mellouli, L. Combined in vitro/in silico approaches, molecular dynamics simulations and safety assessment of the multifunctional properties of thymol and carvacrol: A comparative insight. Chem. Biodivers. 2024, 21, e202301575. [Google Scholar] [CrossRef]
- Yan, S.; Bohach, G.A.; Stevens, D.L. Persistent acylation of high-molecular-weight penicillin-binding proteins by penicillin induces the postantibiotic effect in Streptococcus pyogenes. J. Infect. Dis. 1994, 170, 609–614. [Google Scholar] [CrossRef]
- Horváthová, E.; Sramková, M.; Lábaj, J.; Slamenová, D. Study of cytotoxic, genotoxic and DNA-protective effects of selected plant essential oils on human cells cultured in vitro. Neuro. Endocrinol. Lett. 2006, 27, 44–47. [Google Scholar] [CrossRef]
- Radha, R.; Latha, R.; Swaminathan, M.S. Chemical composition and bioactivity of essential oilfrom Syzygium travancoricum Gamble. Flavour Fragr. J. 2002, 17, 478. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of an essential oil from Origanum vulgare subsp. hirtum letsw. var. Vulkan when used as a sensory additive in feed for all animal species. EFSA J. 2017, 15, e05095. [Google Scholar] [CrossRef]
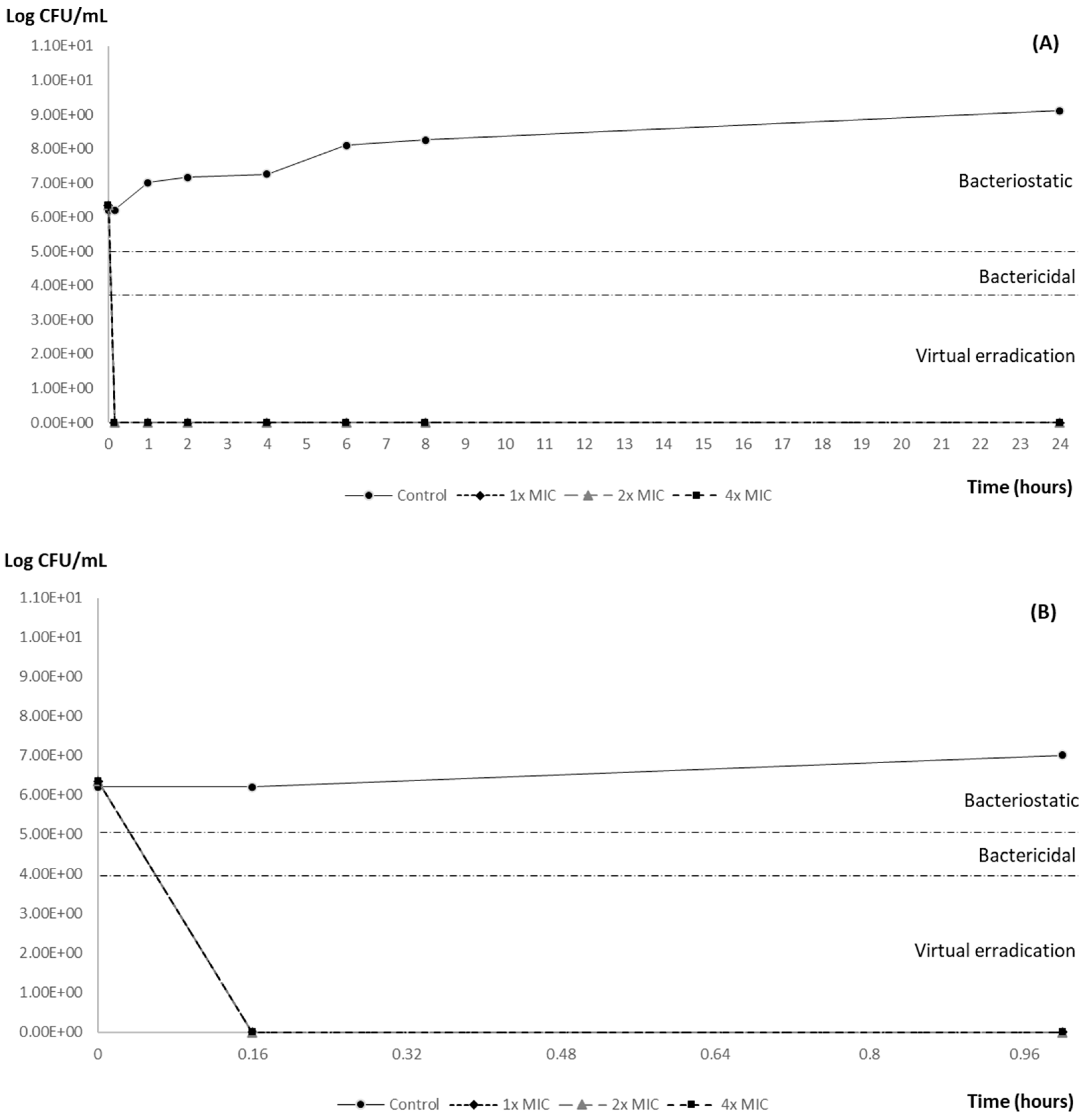
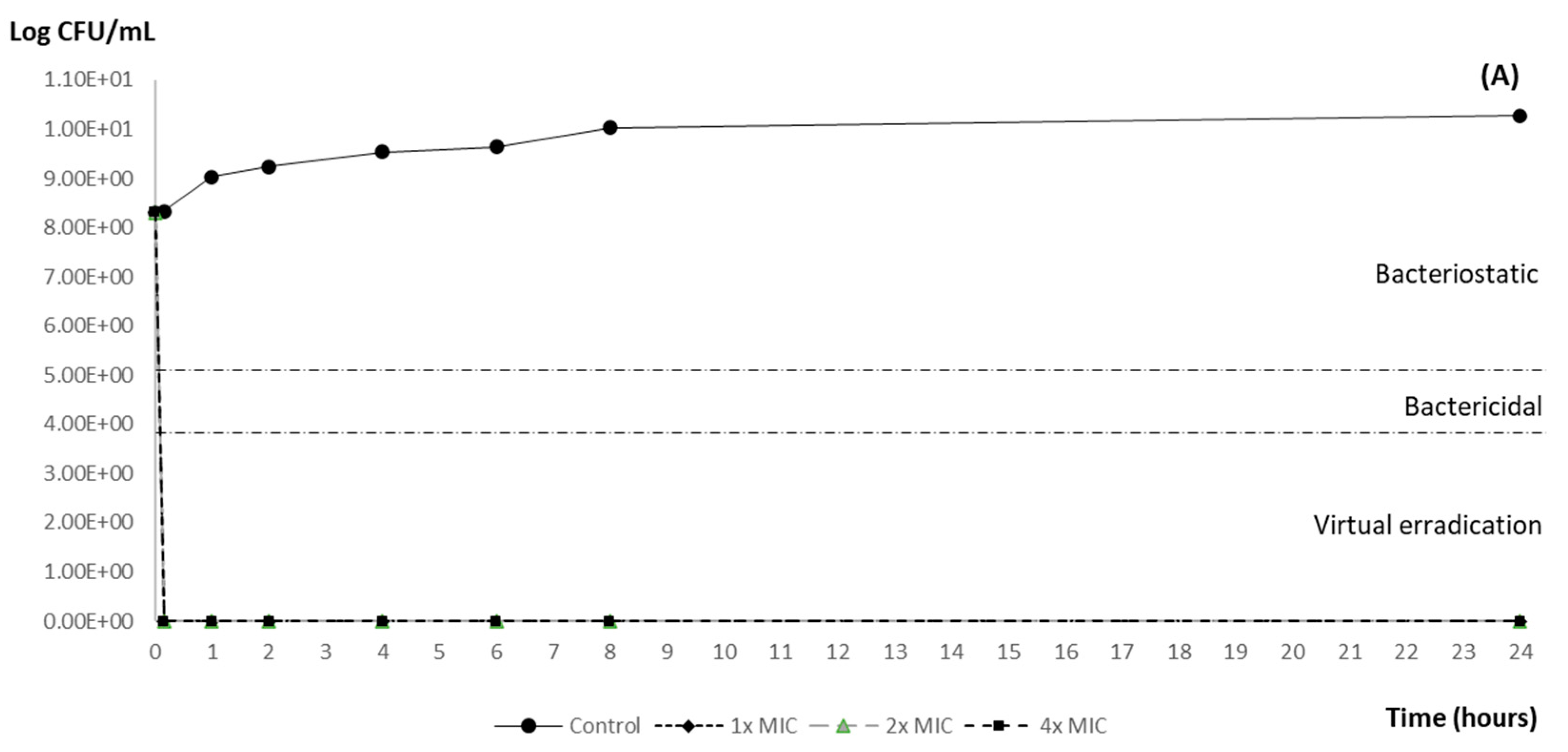

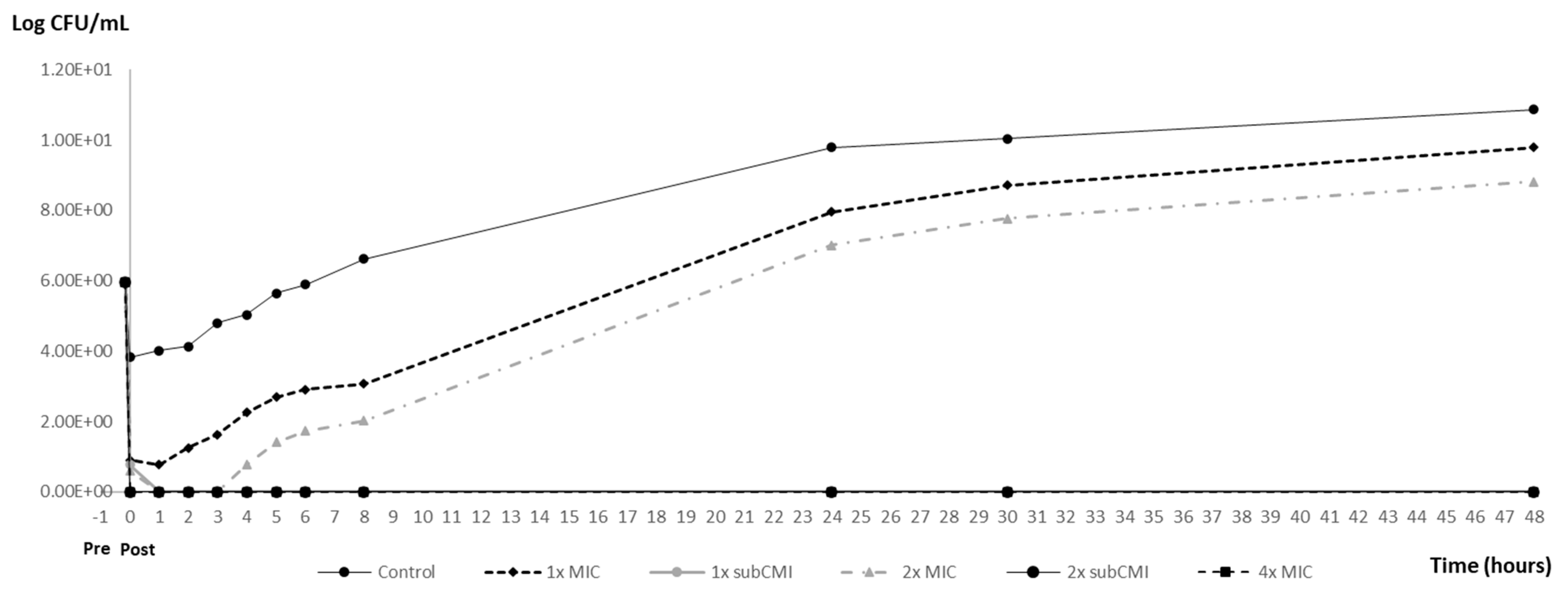
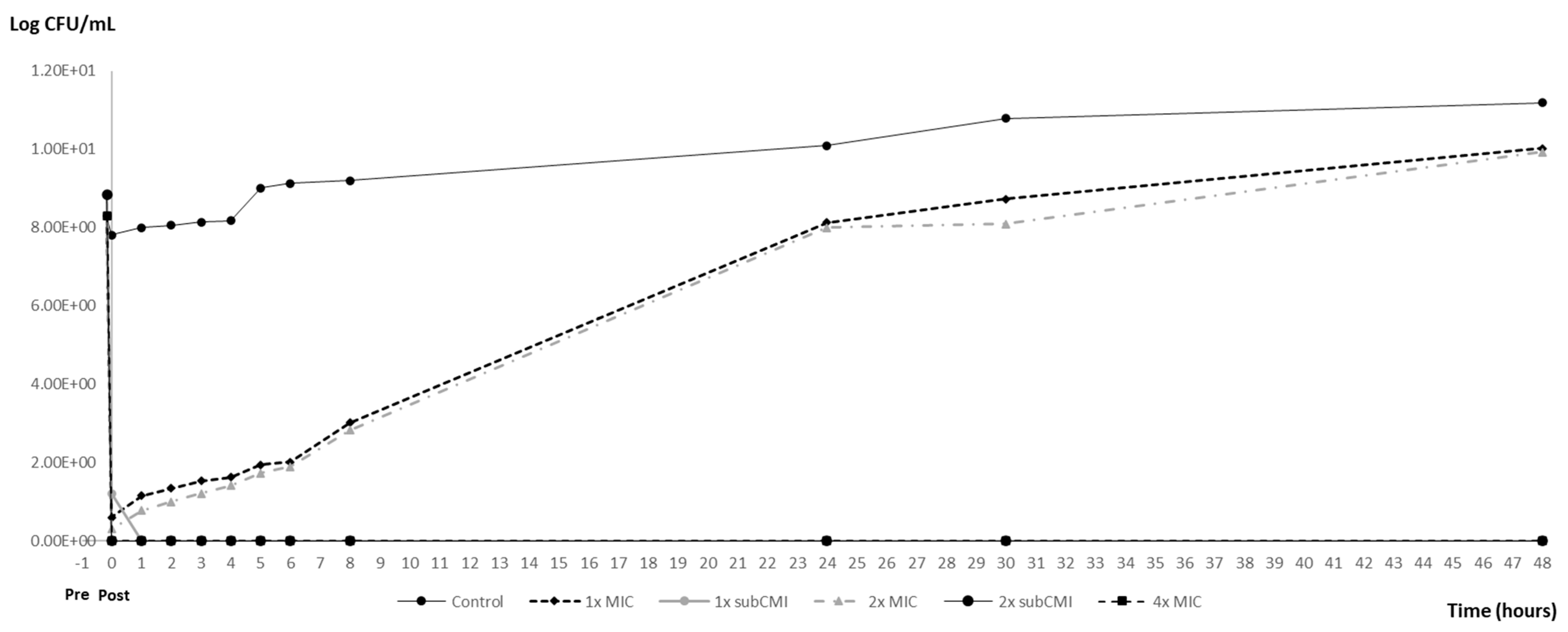
| Post-Exposition Time | Carvacrol Efficacy with Inoculum 106 | ||
| 1× CMI (0.6 mg/mL) | 2× CMI (1.2 mg/mL) | 4× CMI (2.4 mg/mL) | |
| 10 min | 100% | 100% | 100% |
| 1 h | 100% | 100% | 100% |
| 2 h | 100% | 100% | 100% |
| 4 h | 100% | 100% | 100% |
| 8 h | 100% | 100% | 100% |
| 24 h | 100% | 100% | 100% |
| Post-Exposition Time | Carvacrol Efficacy with Inoculum 108 | ||
| 1× CMI (0.6 mg/mL) | 2× CMI (1.2 mg/mL) | 4× CMI (2.4 mg/mL) | |
| 10 min | 100% | 100% | 100% |
| 1 h | 100% | 100% | 100% |
| 2 h | 100% | 100% | 100% |
| 4 h | 100% | 100% | 100% |
| 8 h | 100% | 100% | 100% |
| 24 h | 100% | 100% | 100% |
| Inoculum CFU/mL | PAEs (h) | PA-SMEs (h) | |||
|---|---|---|---|---|---|
| 1× MIC (0.6 mg/mL) | 2× MIC (1.2 mg/mL) | 4× MIC (2.4 mg/mL) | 1× + 0.25 MIC (0.6 + 0.15 mg/mL) | 2× + 0.25 MIC (1.2 + 0.15 mg/mL) | |
| 106 | 0 ± 0 | 2 ± 0 | >44.5 ± 0 | >44.5 ± 0 | >44.5 ± 0 |
| 108 | 0 ± 0 | 1 ± 0 | >43.5 ± 0 | >43.5 ± 0 | >43.5 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyer, E.; Galán-Relaño, Á.; Romero-Salmoral, A.; Barraza, P.; Gómez-Gascón, L.; Tarradas, C.; Luque, I.; de Aguiar, F.C.; Huerta Lorenzo, B. Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium. Animals 2024, 14, 2631. https://doi.org/10.3390/ani14182631
Boyer E, Galán-Relaño Á, Romero-Salmoral A, Barraza P, Gómez-Gascón L, Tarradas C, Luque I, de Aguiar FC, Huerta Lorenzo B. Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium. Animals. 2024; 14(18):2631. https://doi.org/10.3390/ani14182631
Chicago/Turabian StyleBoyer, Eva, Ángela Galán-Relaño, Antonio Romero-Salmoral, Paula Barraza, Lidia Gómez-Gascón, Carmen Tarradas, Inmaculada Luque, Fabiana Carolina de Aguiar, and Belén Huerta Lorenzo. 2024. "Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium" Animals 14, no. 18: 2631. https://doi.org/10.3390/ani14182631








