Microbial–Metabolomic Exploration of Tea Polyphenols in the Regulation of Serum Indicators, Liver Metabolism, Rumen Microorganisms, and Metabolism in Hu Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diet, and Experiment Design
2.3. Sample Collection
2.4. DNA Extraction and Analysis of Bacterial Community in Rumen
2.5. Metabolome Sequencing and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Dietary Supplementation with TPs on Serum and Rumen Fermentation in Hu Sheep
3.1.1. Effect of Dietary Supplementation with TPs on Serum Biochemistry, Immunity, and Antioxidants in Hu Sheep
3.1.2. Effect of Dietary Supplementation with TPs on Rumen Fermentation in Hu Sheep
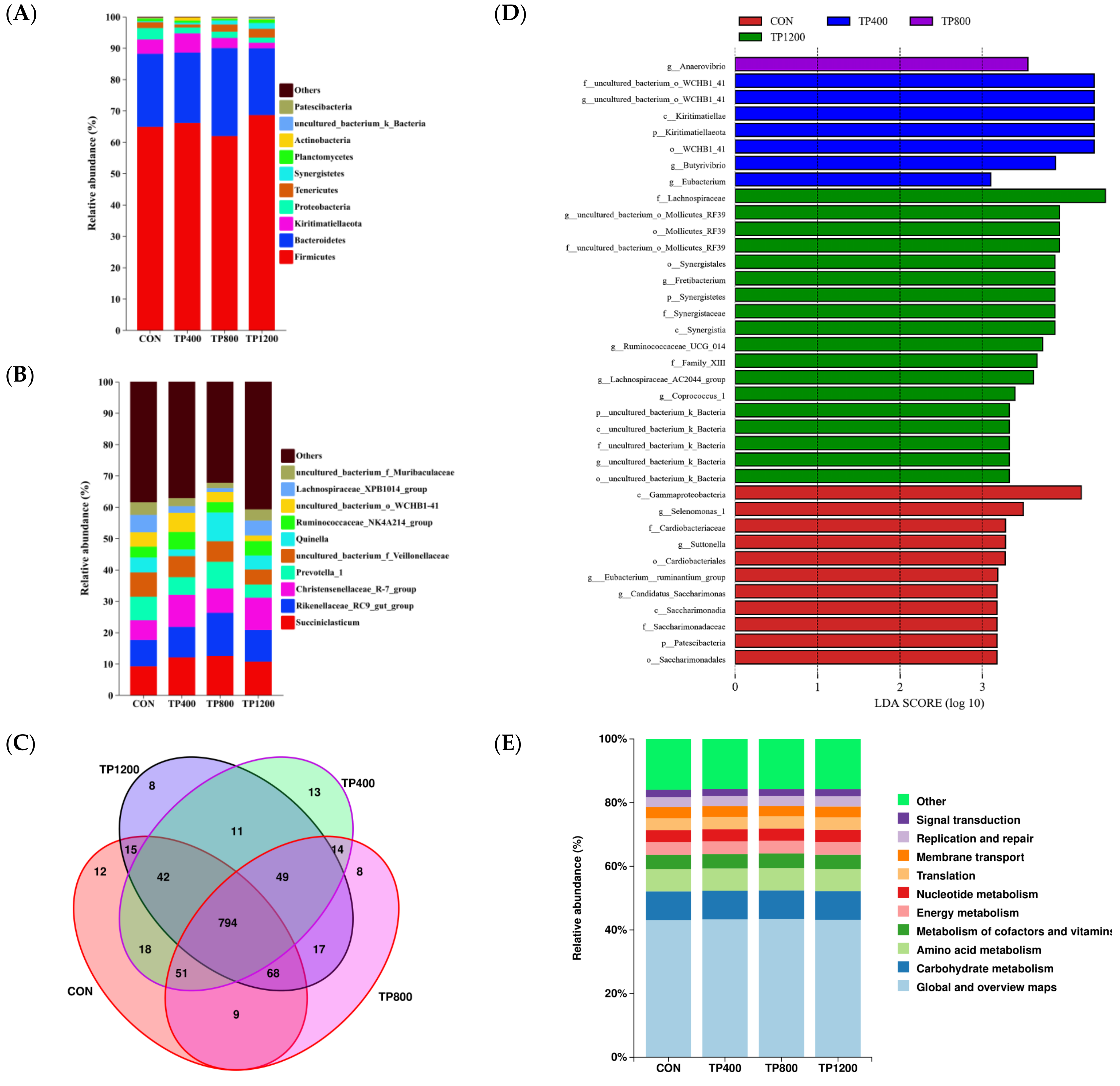
3.2. Effect of Feed Supplementation with Tea Polyphenols on Rumen Bacteria in Hu Sheep
3.2.1. Analysis of Rumen Microbial Diversity
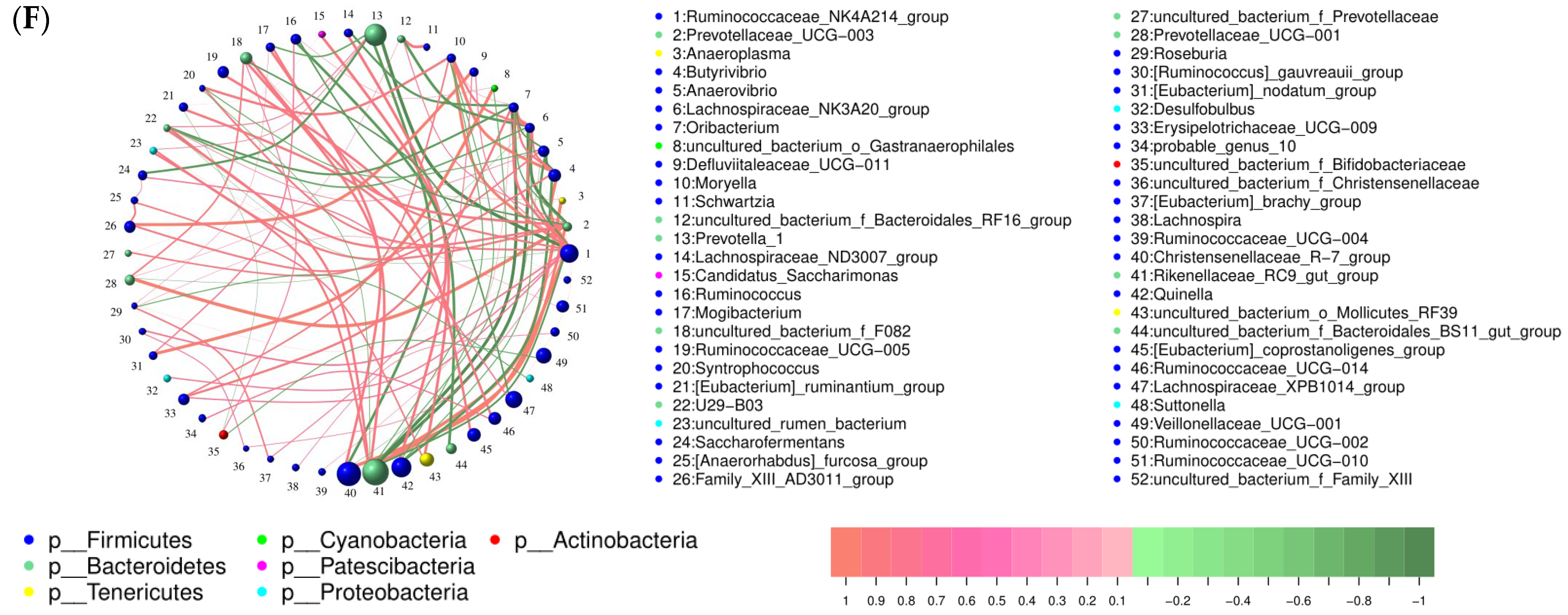
3.2.2. Effect of Feed Supplementation with TPs on the Composition and Function of Rumen Microorganisms
3.3. Effect of Feed Supplementation with TPs on Rumen Metabolism in Hu Sheep
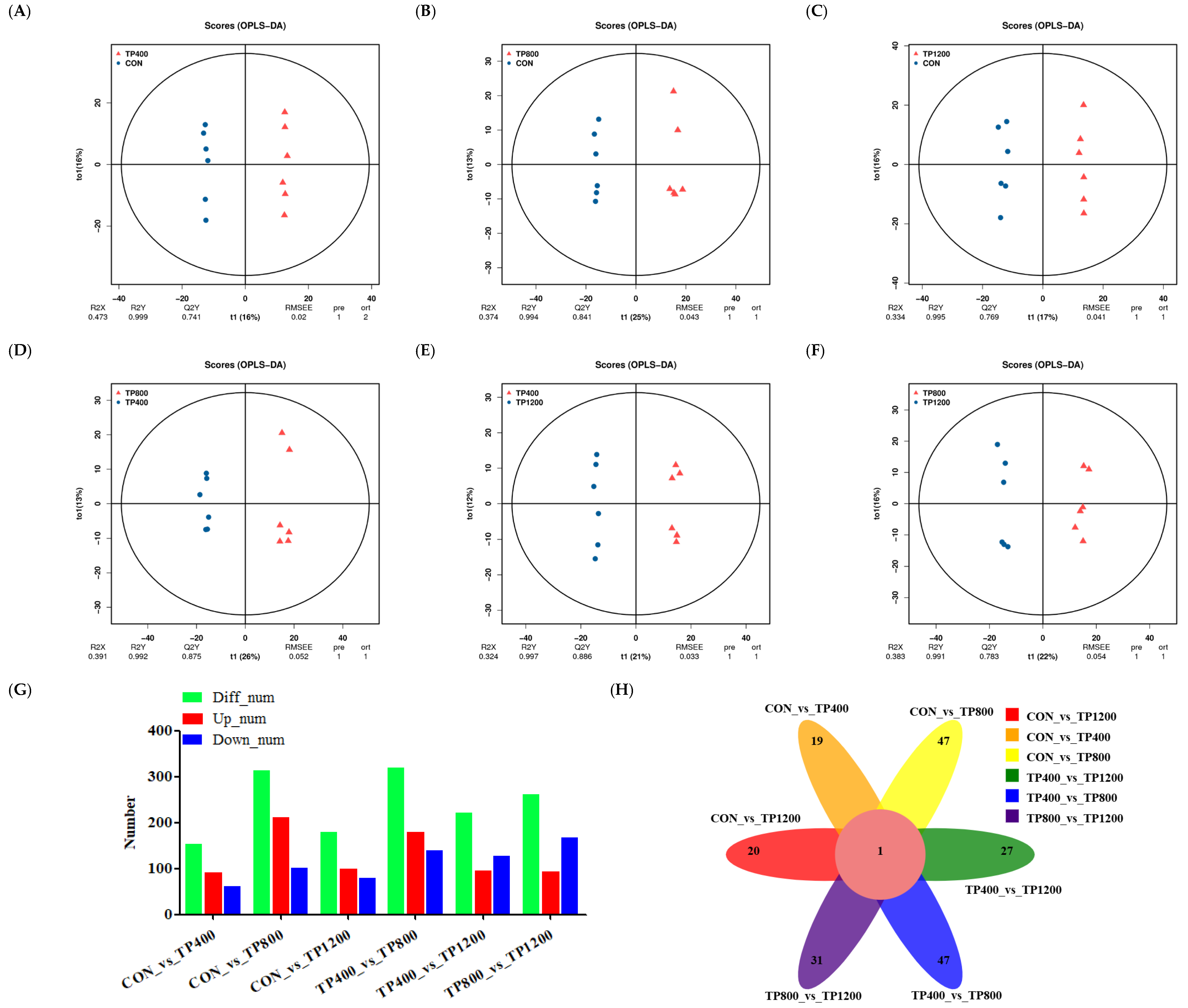
3.3.1. Analysis of Rumen Differential Metabolites across the Four Groups
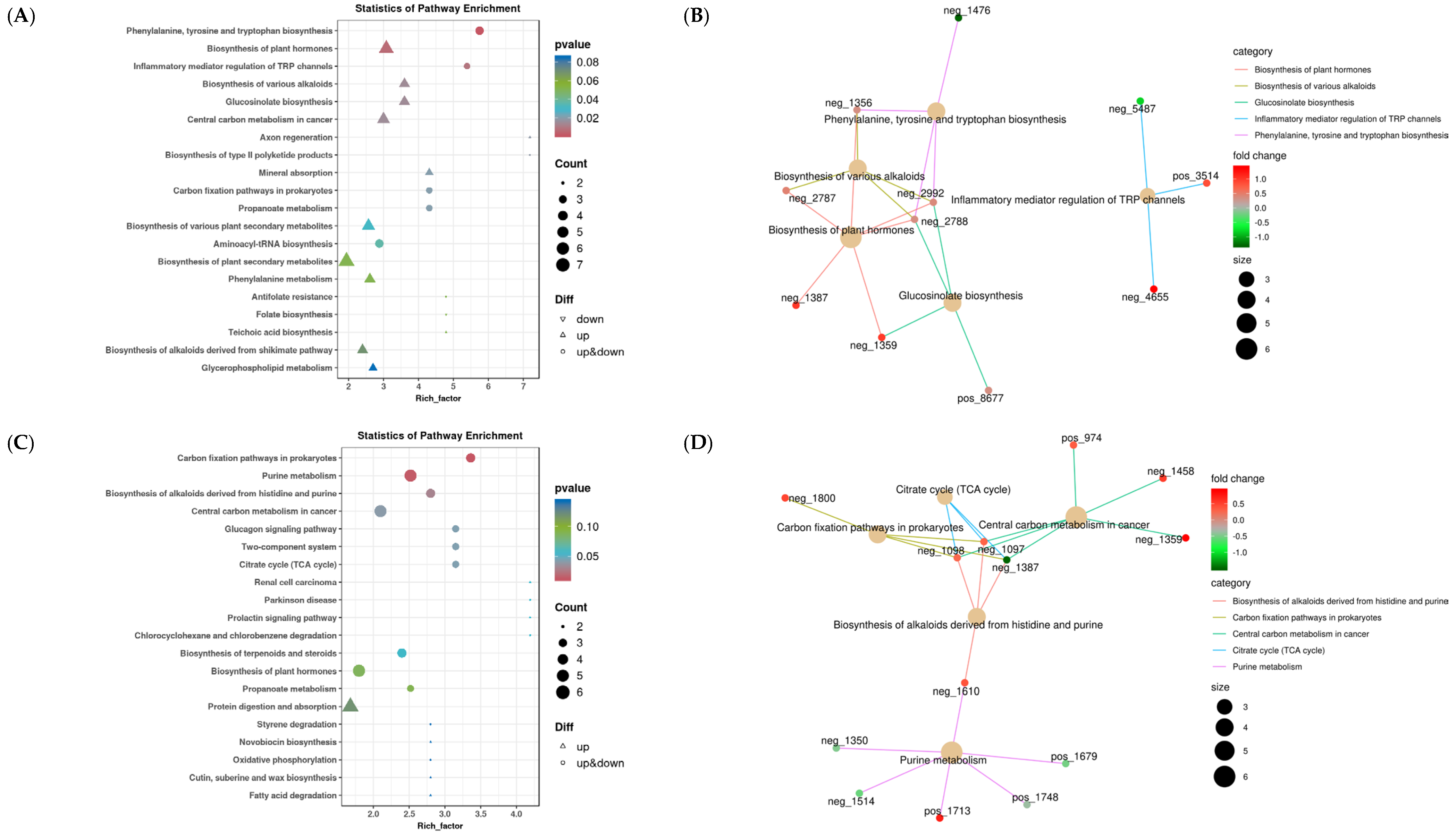
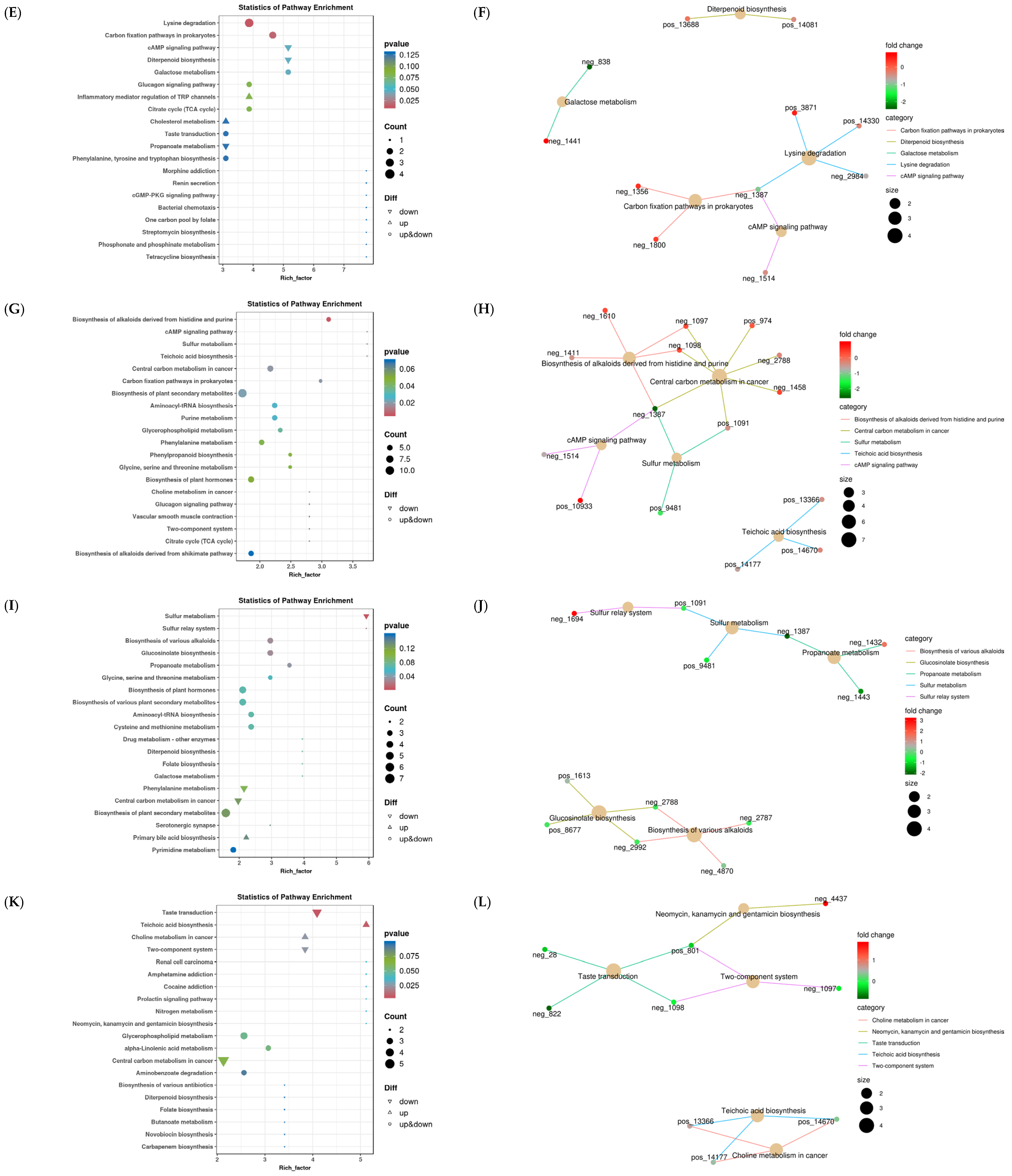
3.3.2. Rumen Functional Enrichment Analysis of Differential Metabolites
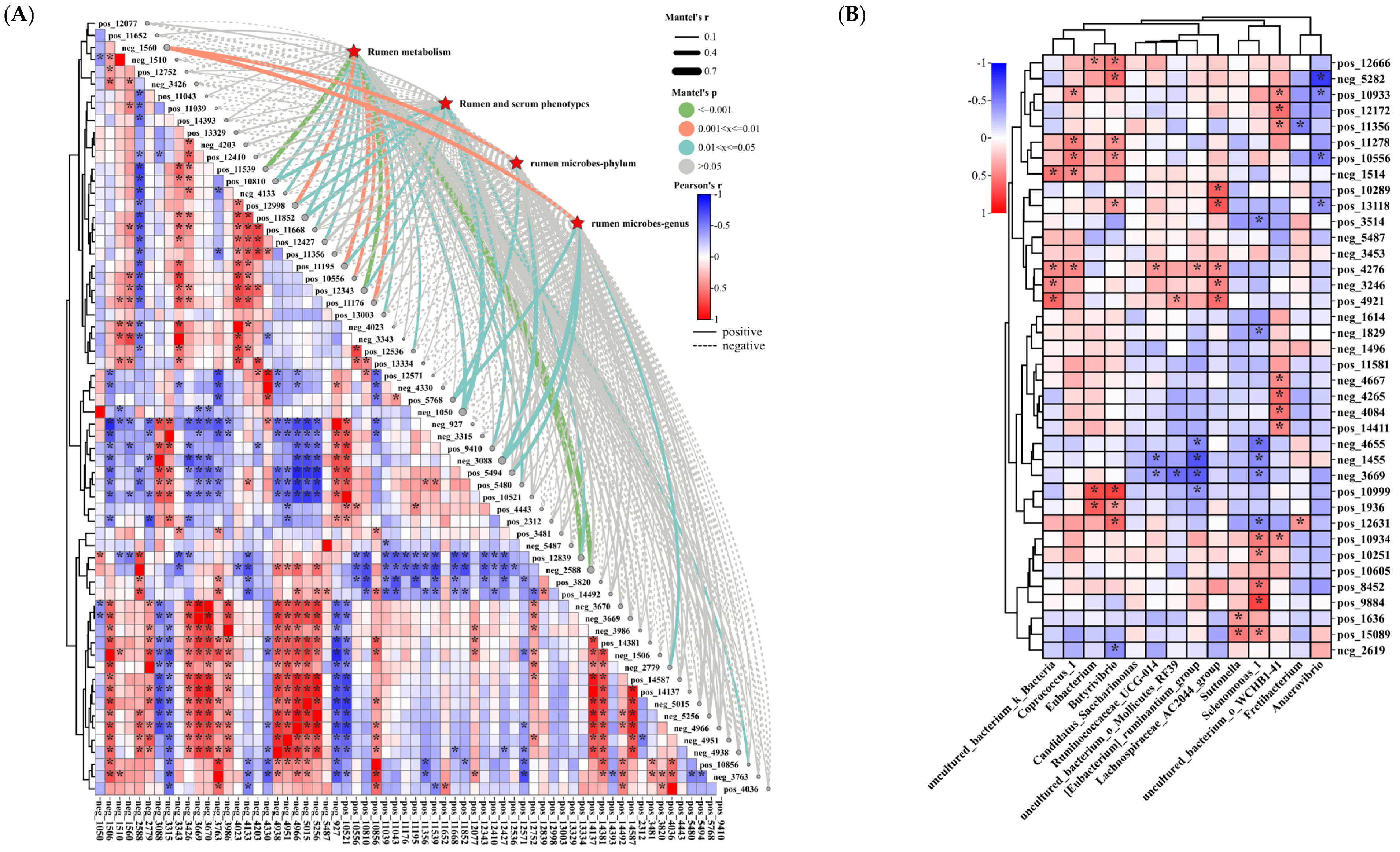
3.3.3. Analysis of the Correlation between Rumen Differential Metabolites and Liver Metabolism, Rumen Microbiology, and Phenotype
3.4. Effect of Feed Supplementation with TPs on Liver Metabolism in Hu Sheep
3.4.1. Analysis of Liver Differential Metabolites across the Four Groups
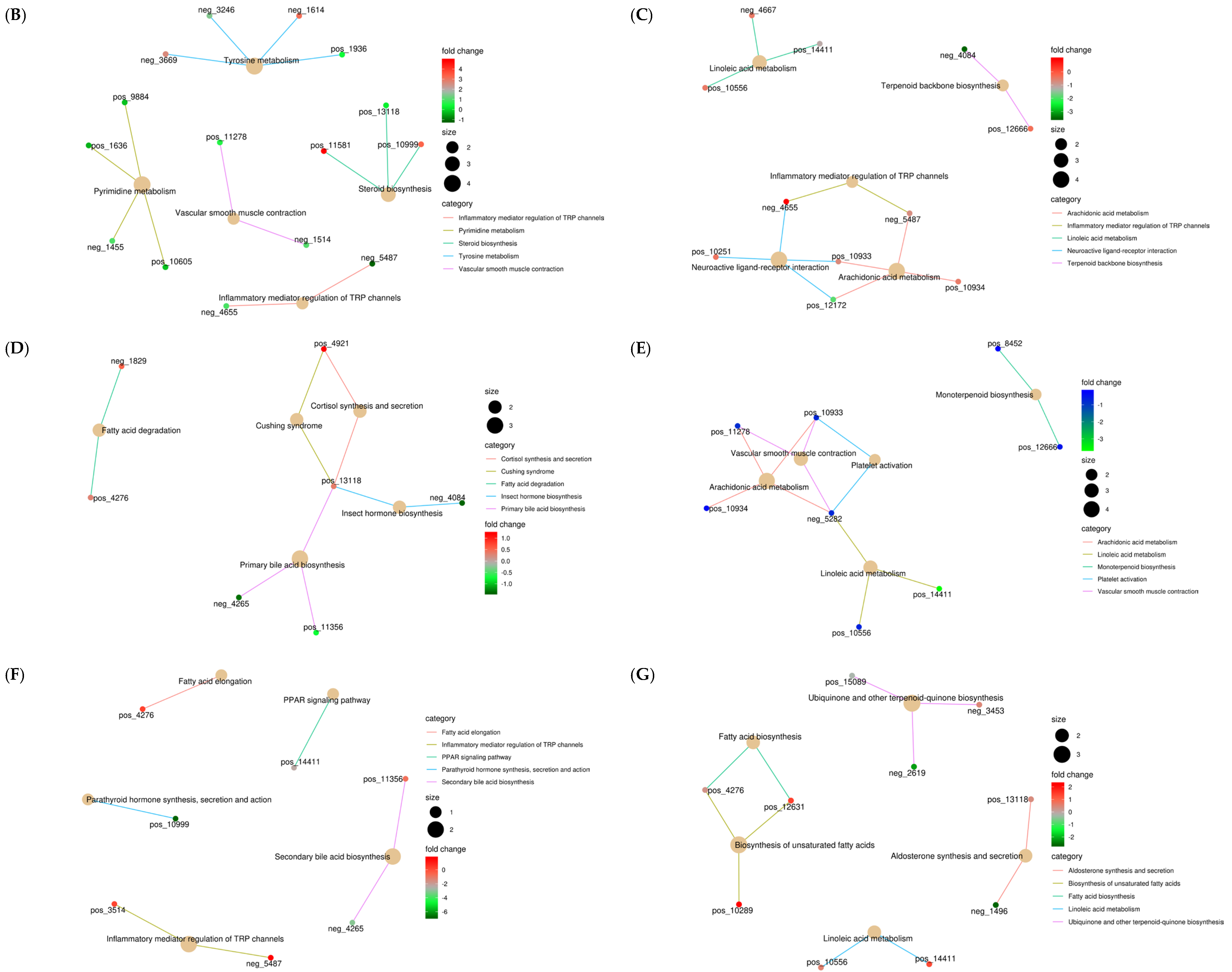
3.4.2. Functional Enrichment Analysis of Differential Metabolites in Liver
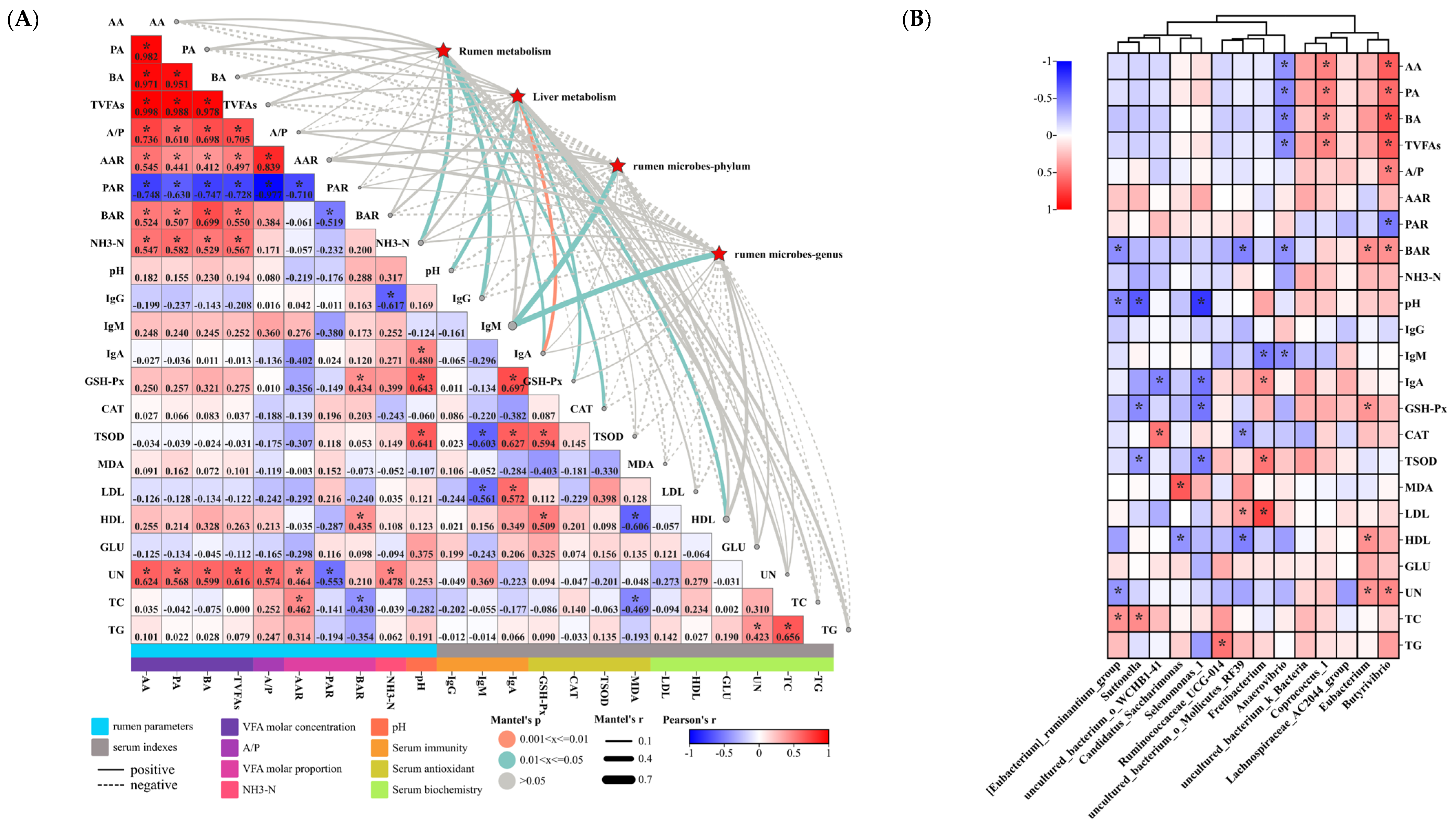
3.4.3. Analysis of the Correlation between Liver Differential Metabolites and Rumen Metabolism, Rumen Microbiology, and Phenotype
3.5. Correlation Analysis of Rumen and Liver Differential Metabolites
3.6. Microbiome and Metabolome in Relation to Serum Indices and Rumen Parameters in Hu Sheep
4. Discussion
4.1. Effect of TPs on Serum Biochemistry, Immunity, and Antioxidant Fermentation in Hu Sheep
4.2. Effect of TPs on Rumen Microorganisms in Hu Sheep
4.3. Effect of TPs on Rumen and Liver Metabolism in Hu Sheep
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Righi, F.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Quarantelli, A.; De Marchi, M.; Tsiplakou, E. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on poultry performances, health, and oxidative status: A review of the literature in the last 20 years. Antioxidants 2021, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wang, Z.; Liang, M.; Sha, Y.; Liu, M.; Liu, J.; Wang, T.; Zhao, C.; Wang, Z.; Guo, D.; et al. Effects of dietary supplementation with tea polyphenols and probiotics on laying performance, biochemical parameters intestinal morphology and microflora of laying hens. Int. J. Biol. Macromol. 2024, 256 Pt 1, 128368. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Oanh, N.C.; Thu, C.T.T.; Hong, N.T.; Giang, N.T.P.; Hornick, J.L.; Dang, P.K. Growth performance, meat quality, and blood characteristics of finisher crossbred pigs fed diets supplemented with different levels of green tea (Camellia sinensis) by-products. Vet. World 2023, 16, 27–34. [Google Scholar] [CrossRef]
- Miao, S.; Wei, Y.; Chen, J.; Wei, X. Extraction methods, physiological activities and high value applications of tea residue and its active components: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 12150–12168. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Sharangi, A.B.; Singh, J.P.; Thakur, P.K.; Ayala-Zavala, J.F.; Singh, A.; Dhua, R.S. Antimicrobial properties of teas and their extracts in vitro. Crit. Rev. Food Sci. Nutr. 2016, 56, 1428–1439. [Google Scholar] [CrossRef]
- Wang, N.; Lan, C.; Mehmood, M.A.; He, M.; Xiao, X.; Li, L.; Liao, D.; Xu, K.; Mo, S.; Zhang, P.; et al. Effects of Pu-erh and Dian Hong tea polyphenols on the gut-liver axis in mice. AMB Express 2023, 3, 53. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Li, Z.; Guo, Y.; Granato, D. Green tea polyphenols upregulate the Nrf2 signaling pathway and suppress oxidative stress and inflammation markers in D-Galactose-Induced liver aging in mice. Front. Nutr. 2022, 9, 836112. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, J.; Fu, C.; Zhang, X. The neuroprotective effect of tea polyphenols on the regulation of intestinal flora. Molecules 2021, 26, 3692. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, B.; Hu, Y.; Wang, J.; Liu, J.; Qin, R.; Lv, S.; Wang, S. Correlation analysis of intestinal redox state with the gut microbiota reveals the positive intervention of tea polyphenols on hyperlipidemia in high fat diet fed mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Yang, H.; Yang, X. Tea polyphenols regulate gut microbiota dysbiosis induced by antibiotic in mice. Food Res. Int. 2021, 141, 110153. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Wang, J.; Chen, Q.; Zeng, J.; Liu, A.; Chen, Z.; Lu, X. Oral administration of green tea polyphenols (TP) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J. Funct. Foods 2020, 64, 103654. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Jiang, K.; Shi, B.; Liu, L.; Hou, R.; Chen, G.; Farag, M.A.; Yan, N.; Liu, L. Dietary polyphenols regulate appetite mechanism via gut-brain axis and gut homeostasis. Food Chem. 2024, 446, 138739. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Shen, C.L.; Wang, J.S. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague-Dawley rats. J. Nutr. Biochem. 2020, 81, 108395. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Ding, L.; Xu, P.; Zhou, J. Matcha green tea targets the gut-liver axis to alleviate obesity and metabolic disorders induced by a high-fat diet. Front. Nutr. 2022, 9, 931060. [Google Scholar] [CrossRef]
- Wen, J.J.; Li, M.Z.; Chen, C.H.; Hong, T.; Yang, J.R.; Huang, X.J.; Geng, F.; Hu, J.L.; Nie, S.P. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota. Food Chem. 2023, 404 Pt A, 134591. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Jelveh, K.; Rasouli, B.; Kadim, I.T.; Slozhenkina, M.I.; Gorlov, I.F.; Seidavi, A.; Phillips, C.J.C. The effects of green tea in the diet of broilers challenged with coccidiosis on their performance, carcass characteristics, intestinal mucosal morphology, blood constituents and ceca microflora. Vet. Med. Sci. 2022, 8, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ding, X.; Wang, J.; Bai, S.; Zeng, Q.; Su, Z.; Xuan, Y.; Zhang, K. Tea polyphenols increase the antioxidant status of laying hens fed diets with different levels of ageing corn. Anim. Nutr. 2021, 7, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.Z.; Li, H.Y.; Fang, Y.; Sun, H.X.; Zhou, D.W. Effects of dietary supplementation with green tea polyphenols on digestion and meat quality in lambs infected with Haemonchus contortus. Meat Sci. 2015, 105, 1–7. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Y.; Song, L.; Li, M.; Dai, H.; Bao, H.; Zhang, G.; Zhao, L.; Zhang, C.; Yi, J.; et al. Green tea polyphenols supplementation alters immunometabolism and oxidative stress in dairy cows with hyperketonemia. Anim. Nutr. 2021, 7, 206–215. [Google Scholar] [CrossRef]
- Chao, Y.M.; Chen, D.W.; Yu, B.; He, J.; Yu, J.; Mao, X.B.; Luo, Y.H.; Huang, Z.Q.; Luo, J.Q.; Wang, S.H.; et al. Effects of tea polyphenol on growth performance, antioxidant capacity, carcass performance and meat quality of finishing pigs. Chin. J. Anim. Nutr. 2016, 28, 3996–4005. [Google Scholar]
- Wang, H.; Zhan, J.; Jia, H.; Jiang, H.; Pan, Y.; Zhong, X.; Zhao, S.; Huo, J.H. Relationship between rumen microbial differences and phenotype traits among Hu sheep and crossbred offspring sheep. Animals 2024, 14, 1509. [Google Scholar] [CrossRef]
- Li, X.X.; Wang, Z.W.; Qin, W.N.; Gao, X.M.; Wu, J.P.; Zhao, S.G.; Jiao, T. Effects of oregano essential oil, cobalt and synergistic of both of them on rumen degradation rate and fermentation characteristics for corn silage. Ital. J. Anim. Sci. 2022, 21, 1476–1488. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, J.; Jiang, H.; Jia, H.; Pan, Y.; Zhong, X.; Huo, J.; Zhao, S. Metagenomics-Metabolomics exploration of three-way-crossbreeding effects on rumen to provide basis for crossbreeding improvement of sheep microbiome and metabolome of sheep. Animals 2024, 14, 2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhang, T.; Shen, X.T.; Liu, J.; Zhao, D.L.; Sun, Y.W.; Wang, L.; Liu, Y.J.; Gong, X.Y.; Liu, Y.X. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Reznik, E.; Lee, C.H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Langille, M.G.I. Picrust2: An improved and extensible approach for metagenome inference. bioRxiv 2019, 672295. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Zhang, K.Y.; Ding, X.M.; Luo, Y.H.; Bai, S.P.; Zeng, Q.F.; Wang, J.P. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult. Sci. 2016, 95, 1709–1717. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Zhou, Y.; Zhang, Z.; Xie, Z.; Zhang, J.; Wan, X. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013, 61, 8565–8572. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Qu, J.T.; Lin, H.; Zhang, Z.Y.; He, Y.K.; Liu, Y.H.; Zhang, P.H. Effects of tea polyphenols on performance, blood indexes and antioxidant function of dairy goats. Chin. J. Anim. Nutr. 2021, 33, 4533–4540. [Google Scholar]
- Shah, A.M.; Cai, Y.; Zou, H.; Zhang, X.; Wang, L.; Xue, B.; Yu, P.; Wang, Z.; Peng, Q. Effects of supplementation of branches and leaves trimmed from tea plant on growth performance, rumen fermentation and meat composition of Nanjiang Yellow Goats. Animals 2019, 9, 590. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, F.; Wang, J.; Wu, P.; Qiu, X.; He, X.; Xiao, Y.; Gan, S. Effect of tea polyphenols on intestinal barrier and immune function in weaned lambs. Front. Vet. Sci. 2024, 11, 1361507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhang, X.; Zhao, S.; Zou, K.; Xie, J.; Wang, X.; Liu, C.; Wang, J.; Wang, Y. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine 2018, 38, 90–97. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Gessner, D.K.; Brock, C.; Hof, L.M.; Most, E.; Koch, C.; Eder, K. Effects of supplementation of green tea extract on the milk performance of peripartal dairy cows and the expression of stress response genes in the liver. J. Anim. Sci. Biotechnol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X. Interactions of tea polyphenols with intestinal microbiota and their implication for anti-obesity. J. Sci. Food Agric. 2020, 100, 897–903. [Google Scholar] [CrossRef]
- Yan, R.; Ho, C.T.; Zhang, X. Interaction between tea polyphenols and intestinal microbiota in host metabolic diseases from the perspective of the gut-brain axis. Mol. Nutr. Food Res. 2020, 64, e2000187. [Google Scholar] [CrossRef]
- Shtossel, O.; Koren, O.; Shai, I.; Rinott, E.; Louzoun, Y. Gut microbiome-metabolome interactions predict host condition. Microbiome 2024, 12, 24. [Google Scholar] [CrossRef]
- Jansma, J.; El Aidy, S. Understanding the host-microbe interactions using metabolic modeling. Microbiome 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Zhang, X.; Li, C.; Yuan, L.; Zhang, D.; Zhao, Y.; Li, X.; Cheng, J.; Lin, C.; et al. Heritability and recursive influence of host genetics on the rumen microbiota drive body weight variance in male Hu sheep lambs. Microbiome 2023, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Furman, O.; Shenhav, L.; Sasson, G.; Kokou, F.; Honig, H.; Jacoby, S.; Hertz, T.; Cordero, O.X.; Halperin, E.; Mizrahi, I. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat. Commun. 2020, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota-host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Szeligowska, N.; Cholewińska, P.; Czyż, K.; Wojnarowski, K.; Janczak, M. Inter and intraspecies comparison of the level of selected bacterial phyla in in cattle and sheep based on feces. BMC Vet. Res. 2021, 17, 224. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Xu, D.; Zhang, D.; Zhang, Y.; Song, Q.; Li, X.; Zhao, Y.; Zhao, L.; Li, W.; et al. Relationship between rumen microbial differences and traits among Hu sheep, Tan sheep, and Dorper sheep. J. Anim. Sci. 2022, 100, skac261. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Jewell, K.A.; McCormick, C.A.; Odt, C.L.; Weimer, P.J.; Suen, G. Ruminal bacterial community composition in dairy cows is dynamic over the course of two lactations and correlates with feed efficiency. Appl. Environ. Microbiol. 2015, 81, 4697–4710. [Google Scholar] [CrossRef]
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked seasonal variation in structure and function of gut microbiota in forest and alpine musk deer. Front. Microbiol. 2021, 12, 699797. [Google Scholar] [CrossRef]
- Chen, A.; Ma, T.; Zhong, Y.; Deng, S.; Zhu, S.; Fu, Z.; Huang, Y.; Fu, J. Effect of tea polyphenols supplement on growth performance, antioxidation, and gut microbiota in squabs. Front. Microbiol. 2024, 14, 1329036. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Celi, P.; Yan, L.; Ding, X.; Bai, S.; Zeng, Q.; Mao, X.; Feng, B.; Xu, S.; et al. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants 2019, 8, 503. [Google Scholar] [CrossRef]
- Lv, C.; Cheng, L.; Feng, W.; Xie, H.; Kou, J.; Wang, L.; Shi, M.; Song, X.; Wang, X.; Chen, S.; et al. Targeting microbiota-immune-synaptic plasticity to explore the effect of tea polyphenols on improving memory in the aged type 2 diabetic rat model. Nutr. Neurosci. 2024, 1–17. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Z.; Cui, Y.; Peng, C.; Fan, Y.; Tan, C.; Wang, Q.; Liu, Z.; Gong, J. Pu-erh tea and theabrownin ameliorate metabolic syndrome in mice via potential microbiota-gut-liver-brain interactions. Food Res. Int. 2022, 162 Pt B, 112176. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Wei, Y.H.; Ma, X.; Zhao, J.C.; Wang, X.Q.; Gao, C.Q. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes 2023, 15, 2190300. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, Y.; Xu, J.; Liang, B.; Cai, X.; Zhu, C.; Wang, L.; Wang, S.; Zhu, X.; Gao, P.; et al. Succinate promotes skeletal muscle protein synthesis via Erk1/2 signaling pathway. Mol. Med. Rep. 2017, 16, 7361–7366. [Google Scholar] [CrossRef]
- Kong, L.; Yue, Y.; Li, J.; Yang, B.; Chen, B.; Liu, J.; Lu, Z. Transcriptomics and metabolomics reveal improved performance of Hu sheep on hybridization with Southdown sheep. Food Res. Int. 2023, 173 Pt 1, 113240. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Ravnskjaer, K.; Madiraju, A.; Montminy, M. Role of the cAMP Pathway in glucose and lipid metabolism. Handb. Exp. Pharmacol. 2016, 233, 29–49. [Google Scholar] [CrossRef]
- Yan, K.; Gao, L.N.; Cui, Y.L.; Zhang, Y.; Zhou, X. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (Review). Mol. Med. Rep. 2016, 13, 3715–3723. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green tea polyphenols modulate colonic microbiota diversity and lipid metabolism in high-fat diet treated HFA mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef]
- Suo, X.; Yan, X.; Tan, B.; Pan, S.; Li, T.; Liu, H.; Huang, W.; Zhang, S.; Yang, Y.; Dong, X. Effect of tea polyphenols, α-lipoic acid and their joint use on the antioxidant and lipid metabolism performance of hybrid grouper (♀Epinephelus fuscoguttatus × ♂E. lanceolatu) fed with high-lipid diets. Aquac. Nutr. 2023, 2023, 1393994. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, Y.; Wan, B.; Wang, Q.; Wan, X. Green tea polyphenols alter lipid metabolism in the livers of broiler chickens through increased phosphorylation of AMP-activated protein kinase. PLoS ONE 2017, 12, e0187061. [Google Scholar] [CrossRef]
- Nie, Q.; Luo, X.; Wang, K.; Ding, Y.; Jia, S.; Zhao, Q.; Li, M.; Zhang, J.; Zhuo, Y.; Lin, J.; et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell 2024, 187, 2717–2734. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Hornef, M.W.; Schaap, F.G.; Cerovic, V.; Clavel, T.; Bruns, T. Gut-liver axis: Barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 447–461. [Google Scholar] [CrossRef]
- Beldowska, A.; Barszcz, M.; Dunislawska, A. State of the art in research on the gut-liver and gut-brain axis in poultry. J. Anim. Sci. Biotechnol. 2023, 14, 37. [Google Scholar] [CrossRef]





| Ingredients | Content (%) | Nutrient Levels (2) | Content (%) |
|---|---|---|---|
| Corn | 19.50 | DE (MJ/Kg) | 11.15 |
| Wheat bran | 16.00 | CP | 16.57 |
| Soybean meal | 18.60 | NDF | 33.14 |
| NaCl | 0.40 | ADF | 24.09 |
| CaCO3 | 0.50 | Ca | 1.24 |
| Premix (1) | 5.00 | P | 0.44 |
| Peanut vine | 40.00 | ||
| Total | 100.00 |
| Items | CON | TP400 | TP800 | TP1200 | SEM | p Value | ||
|---|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | ||||||
| LDL, μmol/L | 242.05 | 241.88 | 267.06 | 271.96 | 7.50 | 0.384 | 0.099 | 0.866 |
| HDL, μmol/L | 49.13 | 55.04 | 50.16 | 53.35 | 0.89 | 0.056 | 0.287 | 0.403 |
| GLU, mmol/L | 2.83 | 3.48 | 3.30 | 3.18 | 0.13 | 0.377 | 0.473 | 0.159 |
| UN, mmol/L | 0.12 | 0.13 | 0.12 | 0.11 | 0.01 | 0.665 | 0.538 | 0.492 |
| TC, mmol/L | 0.20 | 0.17 | 0.15 | 0.17 | 0.01 | 0.382 | 0.251 | 0.226 |
| TG, mmol/L | 2.70 | 2.62 | 2.32 | 3.06 | 0.22 | 0.726 | 0.707 | 0.384 |
| Items | CON | TP400 | TP800 | TP1200 | SEM | p Value | ||
|---|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | ||||||
| IgG, ug/mL | 2903.28 | 3056.56 | 3253.38 | 2903.28 | 105.75 | 0.758 | 0.842 | 0.261 |
| IgM, μg/mL | 39.71 | 36.41 | 36.41 | 37.11 | 1.71 | 0.457 | 0.646 | 0.598 |
| IgA, μg/mL | 23.65 c | 31.46 b | 34.97 ab | 41.61 a | 1.56 | <0.001 | <0.001 | 0.732 |
| GSH−Px, pmol/mL | 88.70 c | 102.79 ab | 95.64 bc | 105.74 a | 1.48 | <0.001 | <0.001 | 0.102 |
| CAT, ng/L | 474.52 ab | 549.76 a | 378.98 c | 432.48 bc | 15.68 | 0.002 | 0.002 | 0.569 |
| TSOD, pg/mL | 204.12 b | 240.05 a | 233.97 a | 254.02 a | 5.12 | 0.003 | <0.001 | 0.294 |
| MDA, nmol/mL | 18.60 | 15.72 | 17.94 | 16.00 | 0.58 | 0.053 | 0.271 | 0.678 |
| Items | CON | TP400 | TP800 | TP1200 | SEM | p Value | ||
|---|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | ||||||
| pH | 7.38 b | 7.57 a | 7.56 a | 7.55 a | 0.02 | <0.001 | <0.001 | <0.001 |
| NH3−N | 23.22 | 25.65 | 23.89 | 26.55 | 0.76 | 0.398 | 0.238 | 0.940 |
| VFA molar concentration (mmol/L) | ||||||||
| AA | 14.90 | 17.37 | 11.96 | 17.26 | 1.19 | 0.348 | 0.875 | 0.555 |
| PA | 4.01 | 4.49 | 3.11 | 4.54 | 0.50 | 0.216 | 0.926 | 0.377 |
| BA | 2.75 | 3.74 | 2.26 | 3.40 | 0.26 | 0.195 | 0.841 | 0.883 |
| TVFAs | 23.59 | 27.94 | 19.05 | 27.66 | 3.38 | 0.284 | 0.838 | 0.559 |
| AA/PA | 3.72 | 3.71 | 3.72 | 3.73 | 0.12 | 1.000 | 0.936 | 0.935 |
| VFA molar proportion | ||||||||
| AAR | 63.23 | 61.41 | 62.09 | 61.73 | 0.85 | 0.320 | 0.242 | 0.318 |
| PAR | 17.03 | 16.74 | 16.82 | 16.63 | 0.34 | 0.924 | 0.561 | 0.921 |
| BAR | 11.34 b | 13.04 a | 11.65 b | 12.22 ab | 0.23 | 0.047 | 0.513 | 0.192 |
| Items | CON | TP400 | TP800 | TP1200 | SEM | p Value |
|---|---|---|---|---|---|---|
| ACE | 817.14 | 789.96 | 824.73 | 821.02 | 16.28 | 0.884 |
| Chao1 | 813.52 | 790.63 | 824.13 | 813.80 | 15.88 | 0.906 |
| Simpson | 0.98 | 0.98 | 0.98 | 0.98 | 0.00 | 0.411 |
| Shannon | 7.39 | 7.53 | 7.35 | 7.63 | 0.08 | 0.644 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhan, J.; Zhao, S.; Jiang, H.; Jia, H.; Pan, Y.; Zhong, X.; Huo, J. Microbial–Metabolomic Exploration of Tea Polyphenols in the Regulation of Serum Indicators, Liver Metabolism, Rumen Microorganisms, and Metabolism in Hu Sheep. Animals 2024, 14, 2661. https://doi.org/10.3390/ani14182661
Wang H, Zhan J, Zhao S, Jiang H, Jia H, Pan Y, Zhong X, Huo J. Microbial–Metabolomic Exploration of Tea Polyphenols in the Regulation of Serum Indicators, Liver Metabolism, Rumen Microorganisms, and Metabolism in Hu Sheep. Animals. 2024; 14(18):2661. https://doi.org/10.3390/ani14182661
Chicago/Turabian StyleWang, Haibo, Jinshun Zhan, Shengguo Zhao, Haoyun Jiang, Haobin Jia, Yue Pan, Xiaojun Zhong, and Junhong Huo. 2024. "Microbial–Metabolomic Exploration of Tea Polyphenols in the Regulation of Serum Indicators, Liver Metabolism, Rumen Microorganisms, and Metabolism in Hu Sheep" Animals 14, no. 18: 2661. https://doi.org/10.3390/ani14182661





