Endoplasmic Reticulum Stress Contributes to Intestinal Injury in Intrauterine Growth Restriction Newborn Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Serum Glucose Analysis
2.3. Morphological Analysis
2.4. Electron Microscope Analysis
2.5. Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick End Labeling (TUNEL) Assay
2.6. Jejunal Digestive Enzyme Measurement
2.7. RNA Extraction and Real-Time PCR Assay
2.8. Protein Expression Measurement with Western Blot
2.9. Statistical Analysis
3. Results
3.1. Jejunal Morphology
3.2. Serum Glucose Level and Jejunal Digestive Enzyme Activities
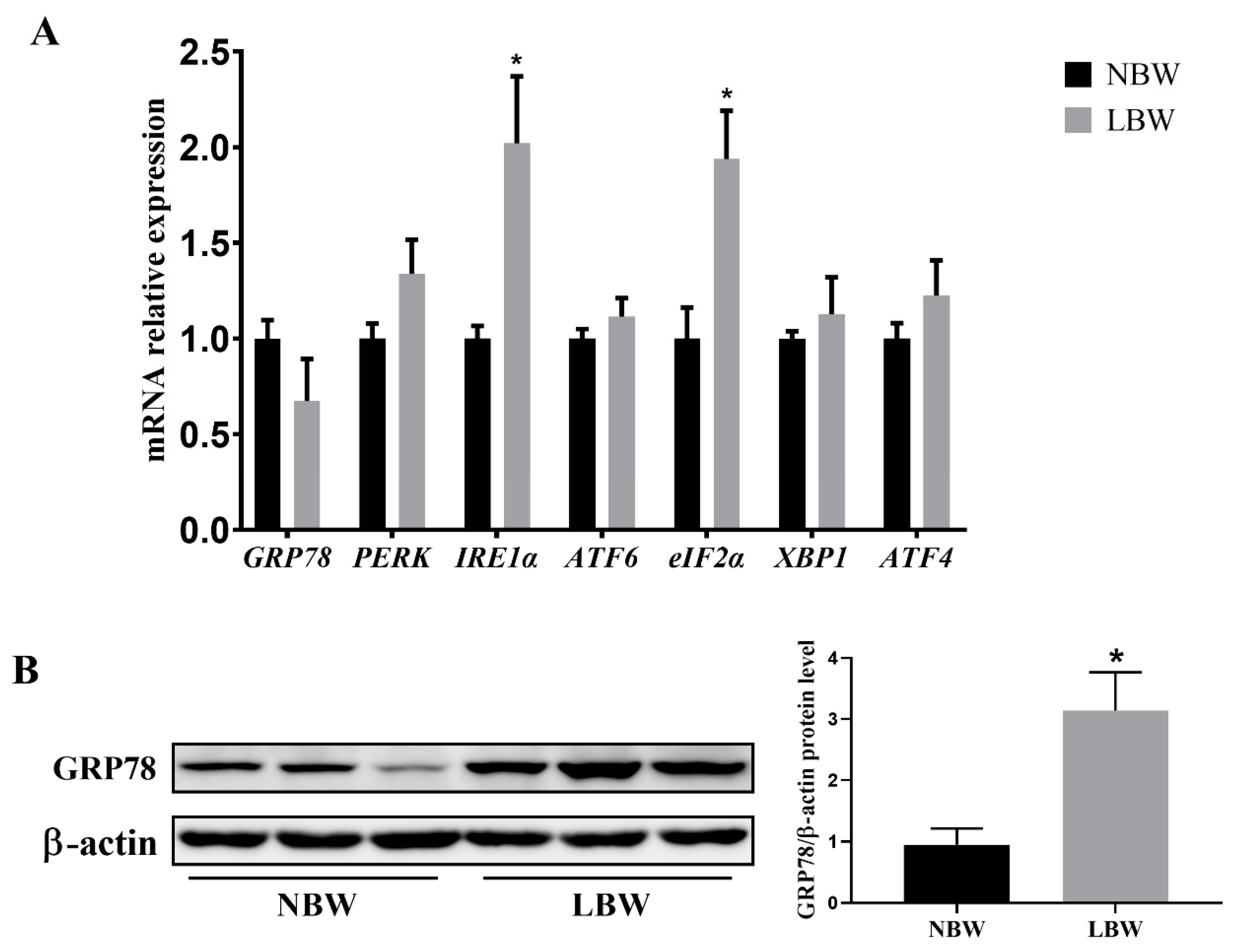
3.3. Jejunal ERS-Related Gene Expression and GRP78 Protein Level
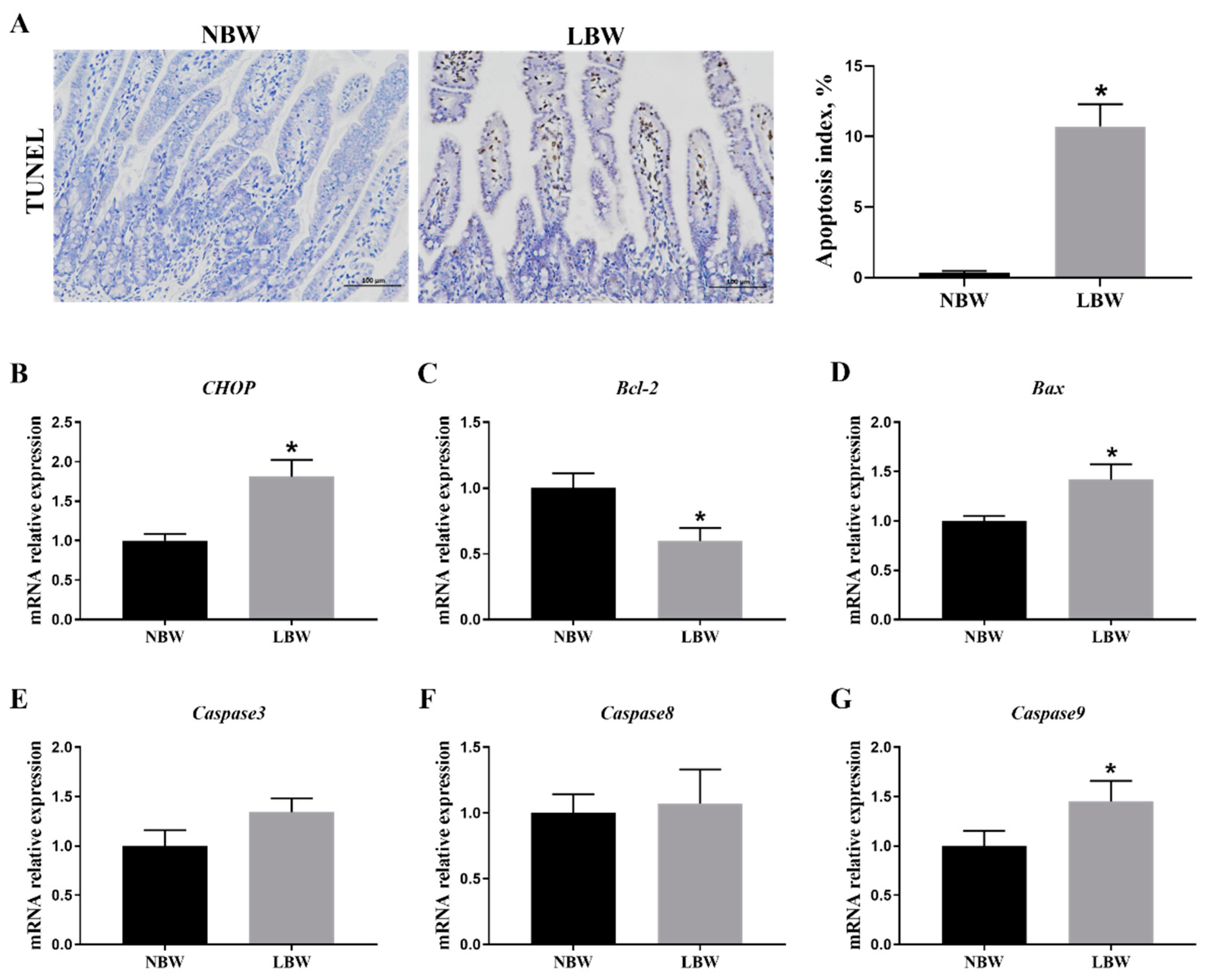
3.4. Jejunal Apoptosis
3.5. Intestinal Barrier Gene Expression in the Jejunum
3.6. Expression Levels of Cytokine Genes in the Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Huygelen, V.; De Vos, M.; Willemen, S.; Fransen, E.; Casteleyn, C.; Van Cruchten, S.; Van Ginneken, C. Age-related differences in mucosal barrier function and morphology of the small intestine in low and normal birth weight piglets. J. Anim. Sci. 2014, 92, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Xuan, Y.; Han, F.; Tian, G.; Fang, Z.; Lin, Y.; et al. Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Brit. J. Nutr. 2015, 114, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Peng, X.; Chen, H.; Yan, C.; Liu, Y.; Xu, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur. J. Nutr. 2017, 56, 1753–1765. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Su, W.; Ying, Z.; Chen, Y.; Zhang, L.; Lu, Z.; Wang, T. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 22. [Google Scholar] [CrossRef]
- D’Inca, R.; Gras-Le Guen, C.; Che, L.; Sangild, P.T.; Le Huërou-Luron, I. Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 2011, 99, 208–216. [Google Scholar] [CrossRef]
- Thongsong, B.; Wiyaporn, M.; Kalandakanond-Thongsong, S. Blood glucose, amino acid profiles and nutrient transporter gene expressions in the small intestine of low and normal birthweight piglets during the early suckling period. Vet. J. 2019, 247, 1–7. [Google Scholar] [CrossRef]
- Olszewski, J.; Zabielski, R.; Skrzypek, T.; Matyba, P.; Wierzbicka, M.; Adamski, A.; Grzesiuk, E.; Sady, M.; Gajewski, Z.; Ferenc, K. Differences in intestinal barrier development between intrauterine growth restricted and normal birth weight piglets. Animals 2021, 11, 990. [Google Scholar] [CrossRef]
- Dong, L.; Zhong, X.; Ahmad, H.; Li, W.; Wang, Y.; Zhang, L.; Wang, T. Intrauterine growth restriction impairs small intestinal mucosal immunity in neonatal piglets. J. Histochem. Cytochem. 2014, 62, 510–518. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Li, Y.; Zhang, T.; Ying, Z.; Su, W.; Zhang, L.; Wang, T. l-Threonine improves intestinal mucin synthesis and immune function of intrauterine growth–retarded weanling piglets. Nutrition 2019, 59, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Wu, Z.H.; Li, T.T.; Liu, C.; Han, D.D.; Tao, S.Y.; Pi, Y.; Li, N.; Wang, J.J. Perturbation of the lipid metabolism and intestinal inflammation in growing pigs with low birth weight is associated with the alterations of gut microbiota. Sci. Total Environ. 2020, 719, 137382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Lin, G.; Li, D.; Wu, G.; Wang, J. Temporal proteomic analysis reveals continuous impairment of intestinal development in neonatal piglets with intrauterine growth restriction. J. Proteome Res. 2010, 9, 924–935. [Google Scholar] [CrossRef]
- Coleman, O.I.; Haller, D. ER stress and the UPR in shaping intestinal tissue homeostasis and immunity. Front. Immunol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Feng, C.; Lin, G.; Wu, G.; Li, D.; Wang, J. Innate differences and colostrum-induced alterations of jejunal mucosal proteins in piglets with intra-uterine growth restriction. Brit. J. Nutr. 2018, 119, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Wang, J.; Li, P.; Chen, L.; Wu, G.; Yin, Y.; Hu, W.; Dangott, L.J. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, S.; Wang, F.; Wang, H.; Xu, D.; Li, G. Microcystin-LR exposure decreased the fetal weight of mice by disturbance of placental development and ROS-mediated endoplasmic reticulum stress in the placenta. Environ. Pollut. 2020, 256, 113362. [Google Scholar] [CrossRef]
- Fang, T.; Yao, Y.; Tian, G.; Chen, D.; Wu, A.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Luo, Y.; et al. Chitosan oligosaccharide attenuates endoplasmic reticulum stress-associated intestinal apoptosis via the Akt/mTOR pathway. Food Funct. 2021, 12, 8647–8658. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Morise, A.; Louveau, I.; Le Huërou-Luron, I. Growth and development of adipose tissue and gut and related endocrine status during early growth in the pig: Impact of low birth weight. Animal 2008, 2, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Gene. Dev. 2007, 21, 2861. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Degroote, J.; Van Ginneken, C.; Van Poucke, M.; Vergauwen, H.; Dam, T.M.T.; Vanrompay, D.; Peelman, L.J.; De Smet, S.; Michiels, J. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2015, 30, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Eugene, S.P.; Reddy, V.S.; Trinath, J. Endoplasmic reticulum stress and intestinal inflammation: A perilous union. Front. Immunol. 2020, 11, 543022. [Google Scholar] [CrossRef] [PubMed]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M.U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Yang, N.; Huang, Y.; Hu, T.; Rao, C. Endoplasmic reticulum stress-mediated cell death in liver injury. Cell. Death. Dis. 2022, 13, 1051. [Google Scholar] [CrossRef]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: Personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef]
- Ferenc, K.; Pilżys, T.; Skrzypek, T.; Garbicz, D.; Marcinkowski, M.; Dylewska, M.; Gładysz, P.; Skorobogatov, O.; Gajewski, Z.; Grzesiuk, E. Structure and function of enterocyte in intrauterine growth retarded pig neonates. Dis. Markers 2017, 2017. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Van Meerveld, B.G.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroent. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, D.; Begun, J. The role of barrier function, autophagy, and cytokines in maintaining intestinal homeostasis. Semin. Cell Dev. Biol. 2017, 61, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, Y.; Zha, X.; Ma, Y.; Liu, X.; Elsabagh, M.; Wang, H.; Wang, M. Dietary L-Arginine or N-Carbamylglutamate alleviates colonic barrier injury, oxidative stress, and inflammation by modulation of intestinal microbiota in intrauterine growth-retarded suckling lambs. Antioxidants. 2022, 11, 2251. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, N.; Liu, C.; Li, T.; Wang, W.; Jiang, L.; Li, Z.; Han, D.; Tao, S.; Wang, J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Ren, S.; Elsabagh, M.; Wang, M.; Wang, H. Dietary N-carbamylglutamate or l-arginine supplementation improves hepatic energy status and mitochondrial function and inhibits the AMP-activated protein kinase-peroxisome proliferator-activated receptor γ coactivator-1α-transcription factor A pathway in intrauterine-growth-retarded suckling lambs. Anim. Nutr. 2021, 7, 859–867. [Google Scholar] [CrossRef]

| Gene | Primer Sequences (5′-3′) | Size (bp) | Accession No. |
|---|---|---|---|
| IL-1β | F: CGTGCAATGATGACTTTGTCTGT | 112 | NM_214055.1 |
| R: AGAGCCTTCAGCATGTGTGG | |||
| IL-6 | F: TTCACCTCTCCGGACAAAAC | 122 | NM_001252429.1 |
| R: TCTGCCAGTACCTCCTTGCT | |||
| IL-8 | F: AGTTTTCCTGCTTTCTGCAGCT | 144 | NM_213867.1 |
| R: TGGCATCGAAGTTCTGCACT | |||
| IL-10 | F: TAATGCCGAAGGCAGAGAGT | 134 | NM_214041.1 |
| R: GGCCTTGCTCTTGTTTTCAC | |||
| TNF-α | F: CGTGAAGCTGAAAGACAACCAG | 121 | NM_214022.1 |
| R: GATGGTGTGAGTGAGGAAAACG | |||
| IFN-λ | F: TGCATCACATCCACGTCGAA | 131 | NM_001142837.1 |
| R: GCAGCCTTGGGACTCTTTCT | |||
| GRP78 | F: ACCAAAATCGCCTGACACCT | 90 | XM_001927795.5 |
| R:TGCGCTCCTTGAGCTTTTTG | |||
| IRE1α | F:GAGCAGCCTTAACCCACACT | 80 | XM_005668695.1 |
| R:GTACCCGCCAGACACTCAAA | |||
| eIF2α | F:GCGAAAACTAAAGATGGCGAGA | 101 | XM_005656337.1 |
| R:AGACCCGGCATTCATAGAGT | |||
| XBP1 | F: GCTTGGGGATGGATGCCTTA | 116 | NM_001142836.1 |
| R: CTGCAGAGGTGCACGTAGTC | |||
| PERK | F:AGACTGTGACTTGGAGGACG | 151 | NM_001161638.1 |
| R:GGATGCGTTATCACAGCCAG | |||
| ATF4 | F:TGGCGTATTAGAGGCAGCAG | 146 | NM_001123078.1 |
| R:TTTGTCGGTTACAGCAACGC | |||
| ATF6 | F:CCGAAGAGAAGAGCCATCTG | 127 | XM_001924512.4 |
| R:TCCTTTGATTTGCAGGGTTC | |||
| Mucin2 | F: GGTCATGCTGGAGCTGGACAGT | 181 | XM_003122394.1 |
| R: TGCCTCCTCGGGGTCGTCAC | |||
| Claudin-1 | F: ATTTCAGGTCTGGCTATCTTAGTTGC | 214 | NM_001244539.1 |
| R: AGGGCCTTGGTGTTGGGTAA | |||
| Occludin | F: AACTTCCACTGATGTCCCCCGT | 138 | NM_001163647.2 |
| R: CCTAGACTTTCCTGCTCTGCCC | |||
| ZO-1 | F: CGTGTCAACGCCACTATCA | 90 | NM_001206404.1 |
| R: TTGTCTTCCAAAGCCCCT | |||
| CHOP | F: GTCATTGCCTTTCTCCTTCGG | 139 | NM_001144845.1 |
| R: GGTTTTTGACTCCTCCTCATTTCC | |||
| Bax | F:CTGACGGCAACTTCAACTGG | 200 | XM_003127290.5 |
| R:CGTCCCAAAGTAGGAGAGGA | |||
| Bcl-2 | F:AGCATGCGGCCTCTATTTGA | 120 | XM_021099593.1 |
| R:GGCCCGTGGACTTCACTTAT | |||
| Caspase3 | F: TGTGTGCTTCTAAGCCATGG | 158 | NM_214131.1 |
| R: AGTTCTGTGCCTCGGCAG | |||
| Caspase8 | F: AGACAAGGGCATCATCTACGG | 103 | NM_001031779.2 |
| R: GGGTTTACCAAGAAGGGAAGG | |||
| Caspase9 | F: AATGCCGATTTGGCTTACGT | 195 | XM_003127618.4 |
| R:CATTTGCTTGGCAGTCAGGTT | |||
| β-actin | F: GGATGACGATATTGCTGCGC | 190 | XM_003124280.5 |
| R: GATGCCTCTCTTGCTCTGGG |
| Item | NBW | LBW | p-Value |
|---|---|---|---|
| Glucose, mmol/L | 7.02 ± 0.27 | 3.99 ± 0.27 * | 0.00 |
| Lipase, U/mg protein | 2.40 ± 0.60 | 1.31 ± 0.14 | 0.11 |
| Lactase, U/mg protein | 63.22 ± 9.64 | 30.96 ± 5.12 * | 0.02 |
| Trypsin, U/mg protein | 6.54 ± 1.36 | 7.06 ± 1.49 | 0.80 |
| Item | NBW | LBW | p-Value |
|---|---|---|---|
| Mucin2 | 1.00 ± 0.13 | 0.58 ± 0.07 * | 0.02 |
| Claudin1 | 1.00 ± 0.14 | 0.40 ± 0.02 * | 0.00 |
| Occludin | 1.00 ± 0.05 | 0.31 ± 0.04 * | 0.00 |
| ZO-1 | 1.00 ± 0.09 | 0.25 ± 0.01 * | 0.00 |
| Item | NBW | LBW | p-Value |
|---|---|---|---|
| IL-1β | 1.00 ± 0.21 | 1.42 ± 0.14 | 0.13 |
| IL-6 | 1.00 ± 0.20 | 2.30 ± 0.33 * | 0.00 |
| IL-8 | 1.00 ± 0.20 | 1.14 ± 0.17 | 0.59 |
| IL-10 | 1.00 ± 0.19 | 0.65 ± 0.08 | 0.16 |
| TNF-α | 1.00 ± 0.08 | 1.55 ± 0.29 | 0.09 |
| IFN-γ | 1.00 ± 0.19 | 1.56 ± 0.12 * | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, T.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Mao, X.; Yan, H.; Yu, B. Endoplasmic Reticulum Stress Contributes to Intestinal Injury in Intrauterine Growth Restriction Newborn Piglets. Animals 2024, 14, 2677. https://doi.org/10.3390/ani14182677
Fang T, Tian G, Chen D, He J, Zheng P, Mao X, Yan H, Yu B. Endoplasmic Reticulum Stress Contributes to Intestinal Injury in Intrauterine Growth Restriction Newborn Piglets. Animals. 2024; 14(18):2677. https://doi.org/10.3390/ani14182677
Chicago/Turabian StyleFang, Tingting, Gang Tian, Daiwen Chen, Jun He, Ping Zheng, Xiangbing Mao, Hui Yan, and Bing Yu. 2024. "Endoplasmic Reticulum Stress Contributes to Intestinal Injury in Intrauterine Growth Restriction Newborn Piglets" Animals 14, no. 18: 2677. https://doi.org/10.3390/ani14182677






