Proteomics and Microbiota Conjoint Analysis in the Nasal Mucus: Revelation of Differences in Immunological Function in Manis javanica and Manis pentadactyla
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pangolin Sample Collection and Preparation
2.2. TMT Labeling and LC-MS/MS Analysis
2.3. Protein Database Search and Bioinformatics Analysis
2.4. DNA Extraction and Data Analysis of Microbiome
2.5. Integrated Analysis of Proteomics and Microbiotas
2.6. Bioinformatics Analysis of Complement C5 in Pangolins
2.7. RNA Extraction and cDNA Synthesis of Spleen from Pangolins
2.8. Recombinant Protein Vector Establishment and Expression
3. Results
3.1. Protein Identification and Relative Quantification
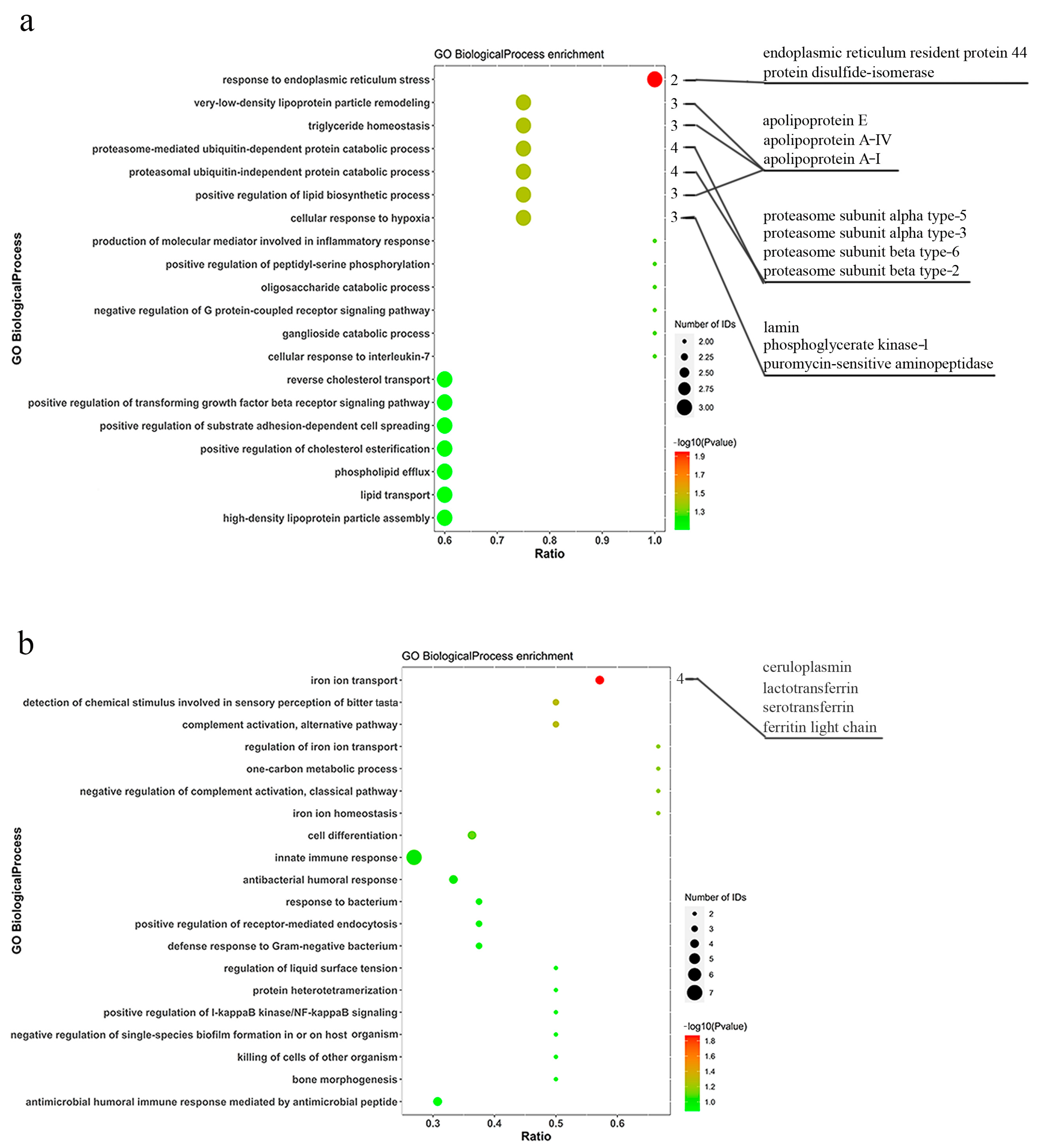
3.2. GO Functional Enrichment Analysis of Differentially Expressed Proteins
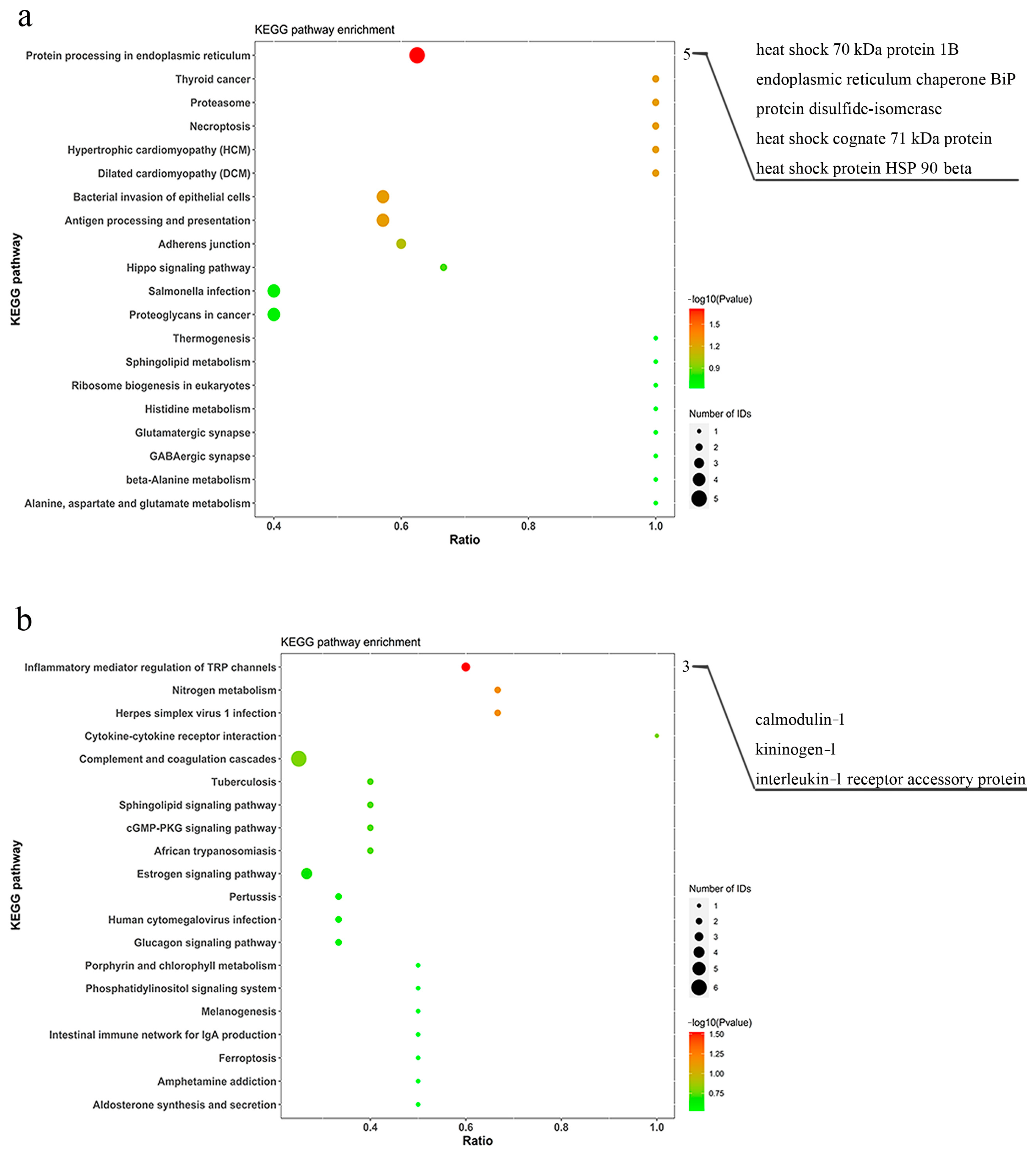
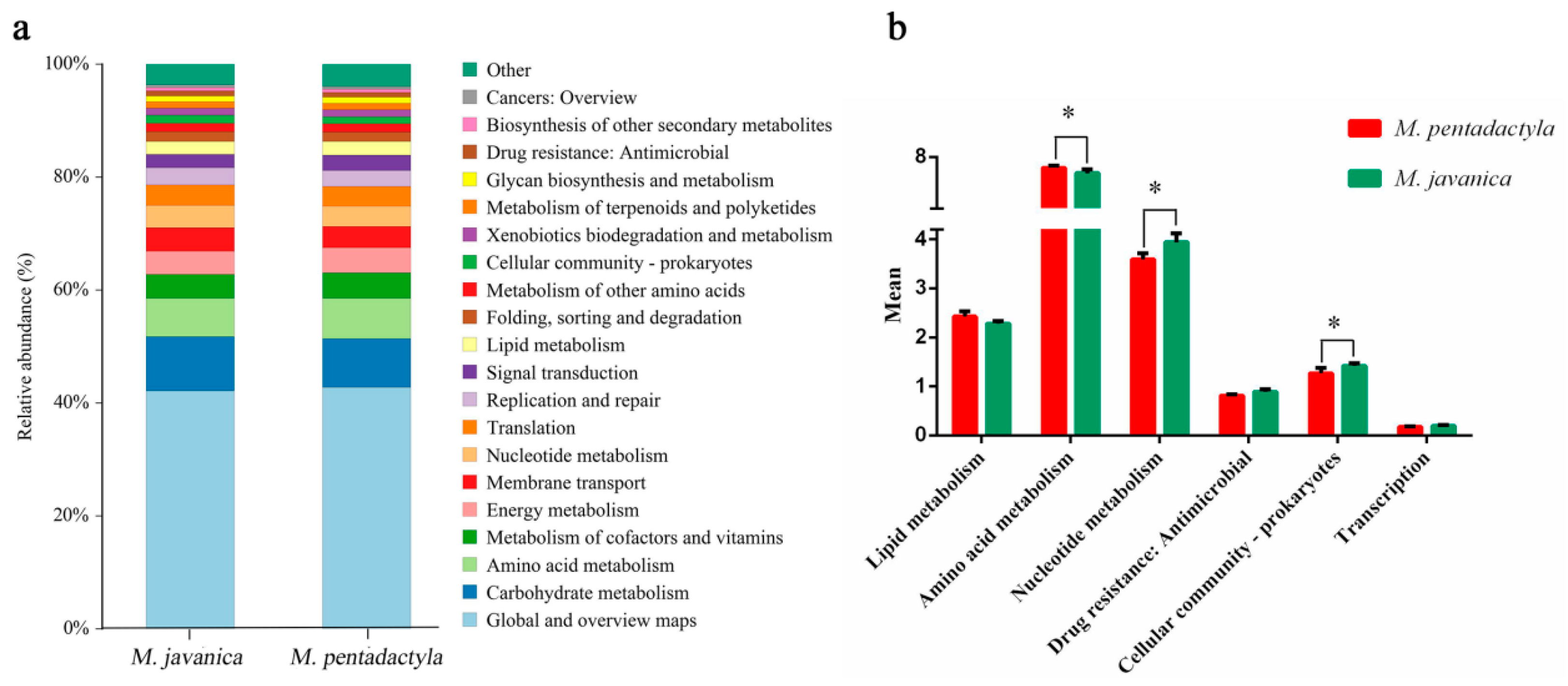
3.3. Pathway Enrichment Analysis of Differentially Expressed Proteins
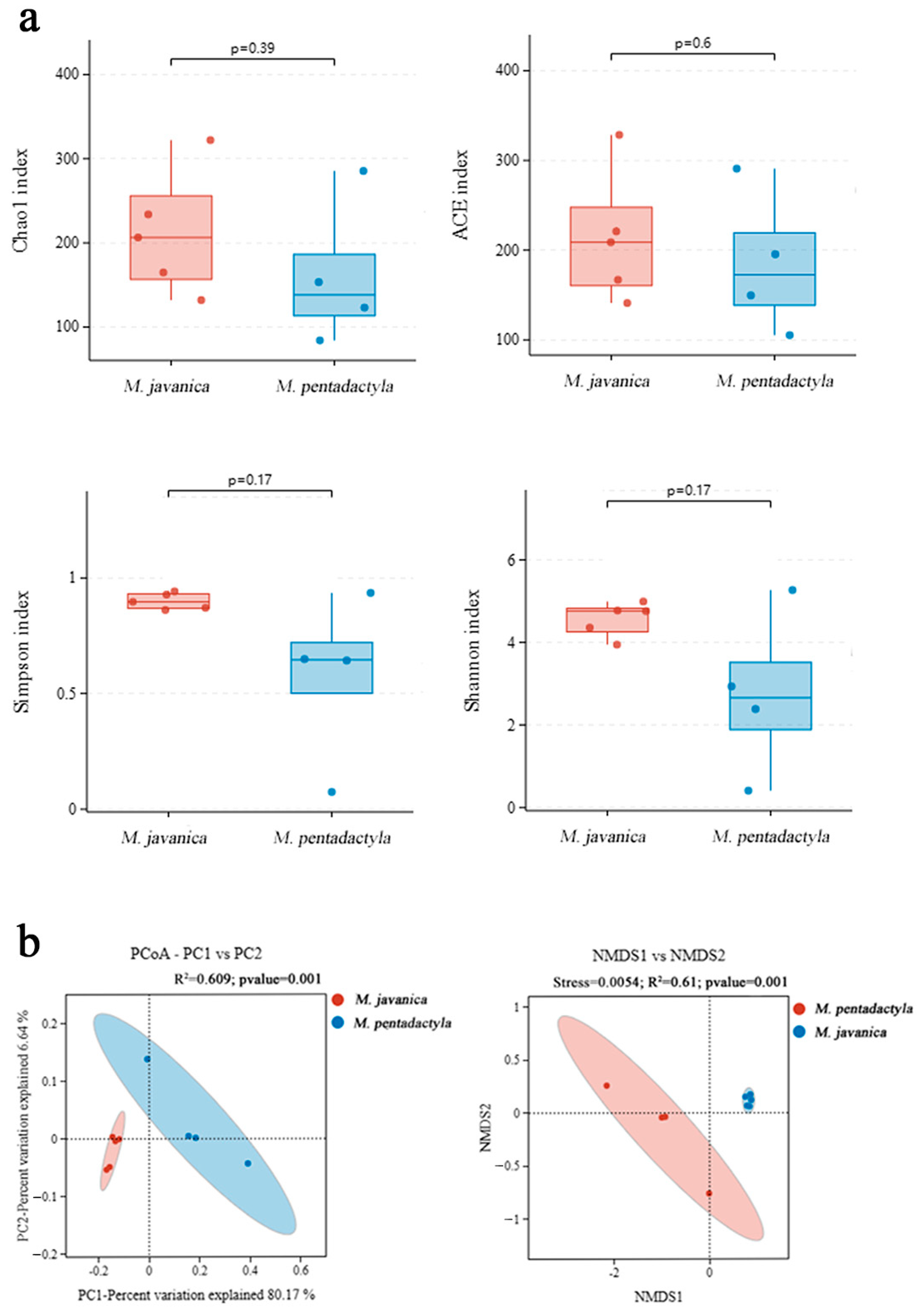
3.4. Nasal Microbiota Identification and Diversity Analysis
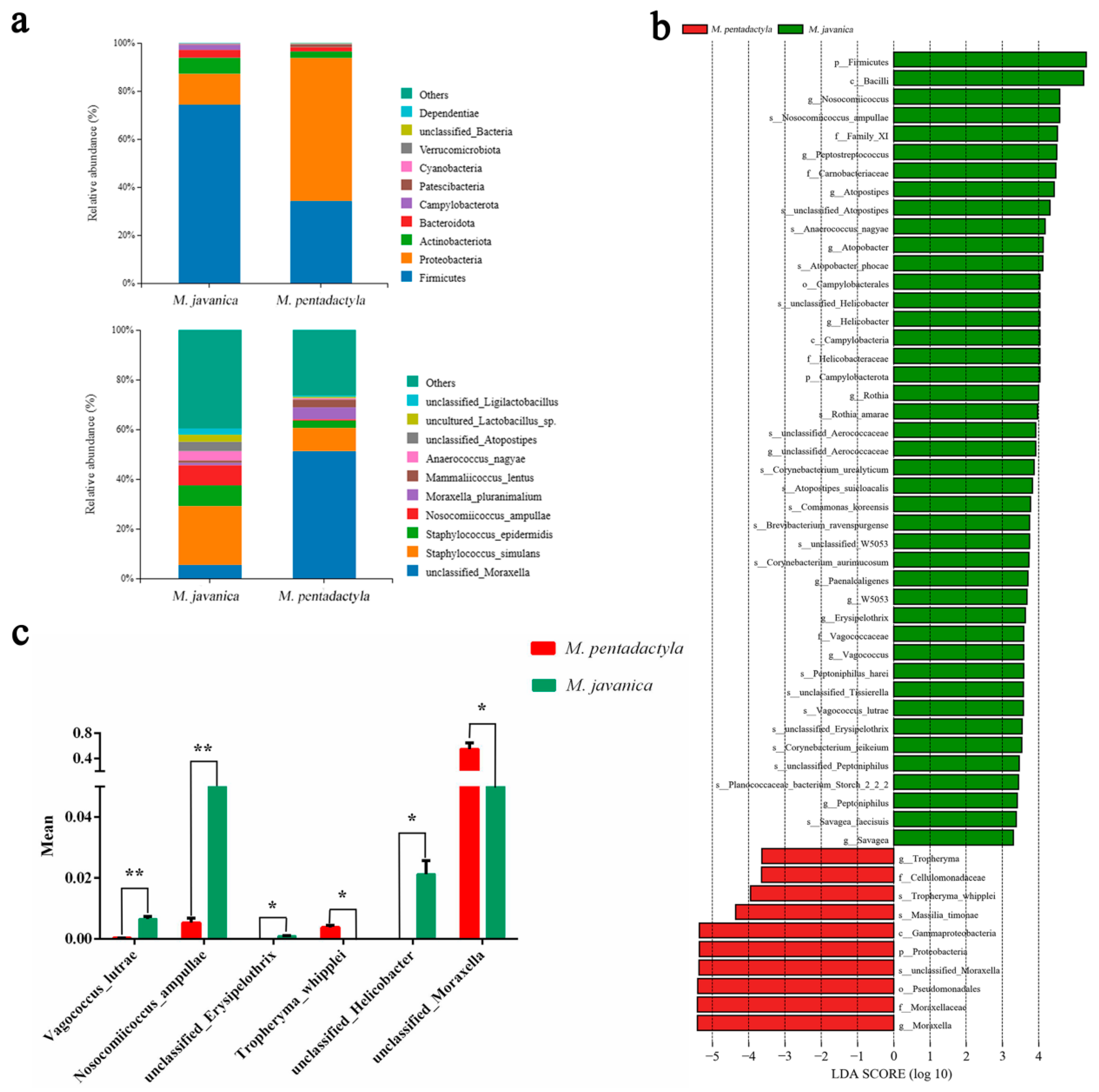
3.5. Differential Microbiota and Predicted Function between Pangolins
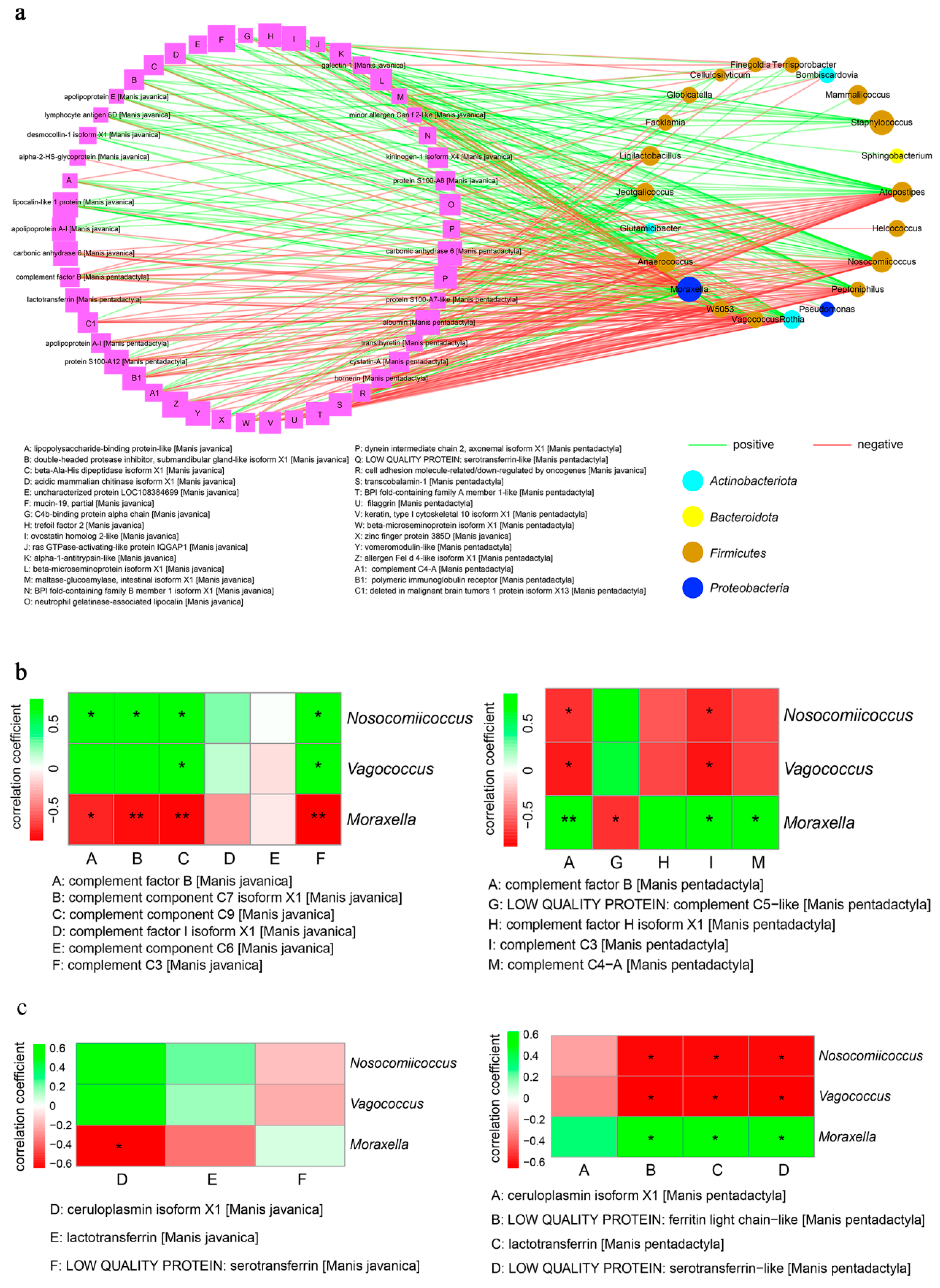
3.6. Integrated Analysis of Proteomics and Microbiotas between Pangolins
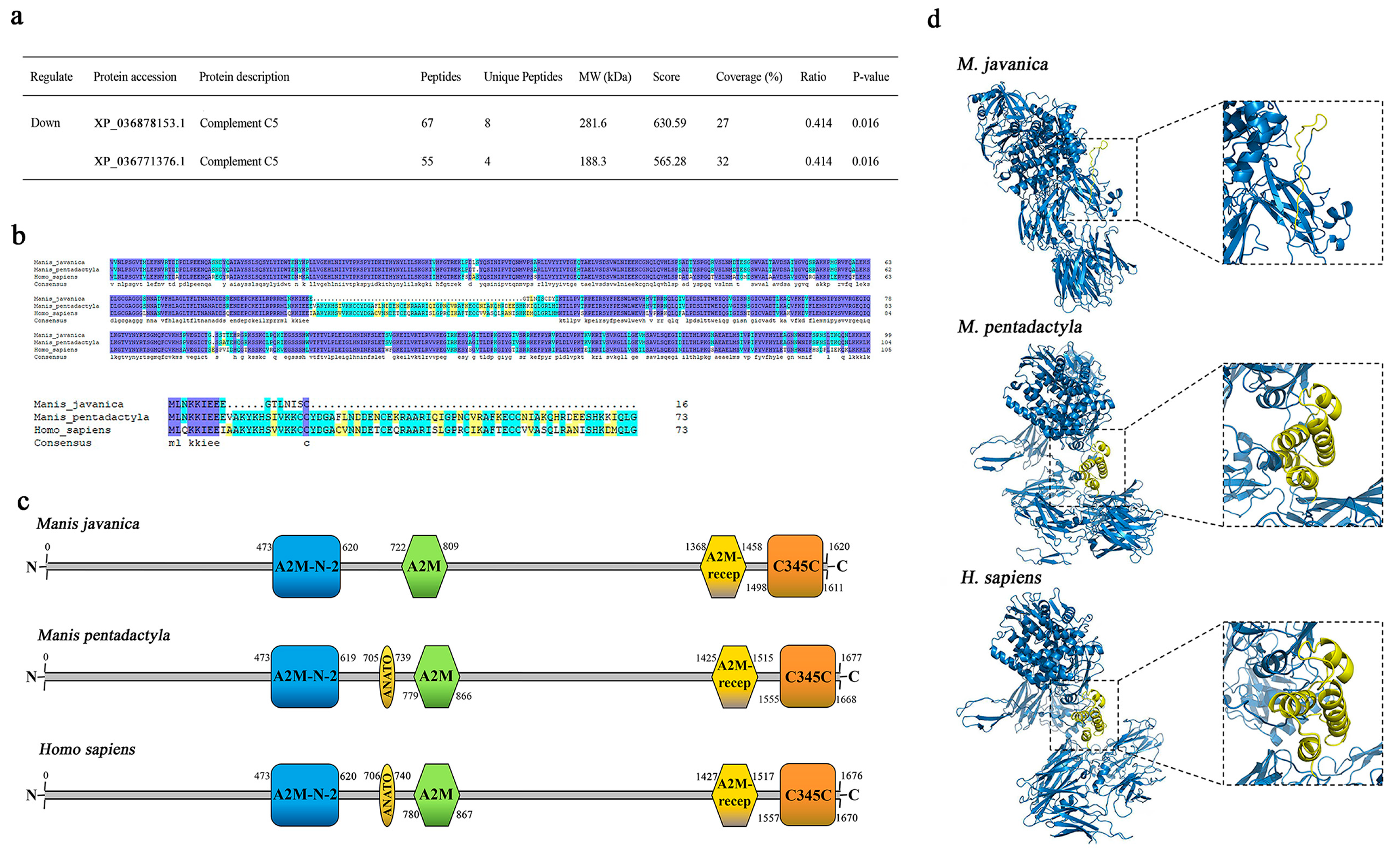
3.7. Multiple Sequence Alignment and 3D-Modeling of Complement C5 in Pangolins
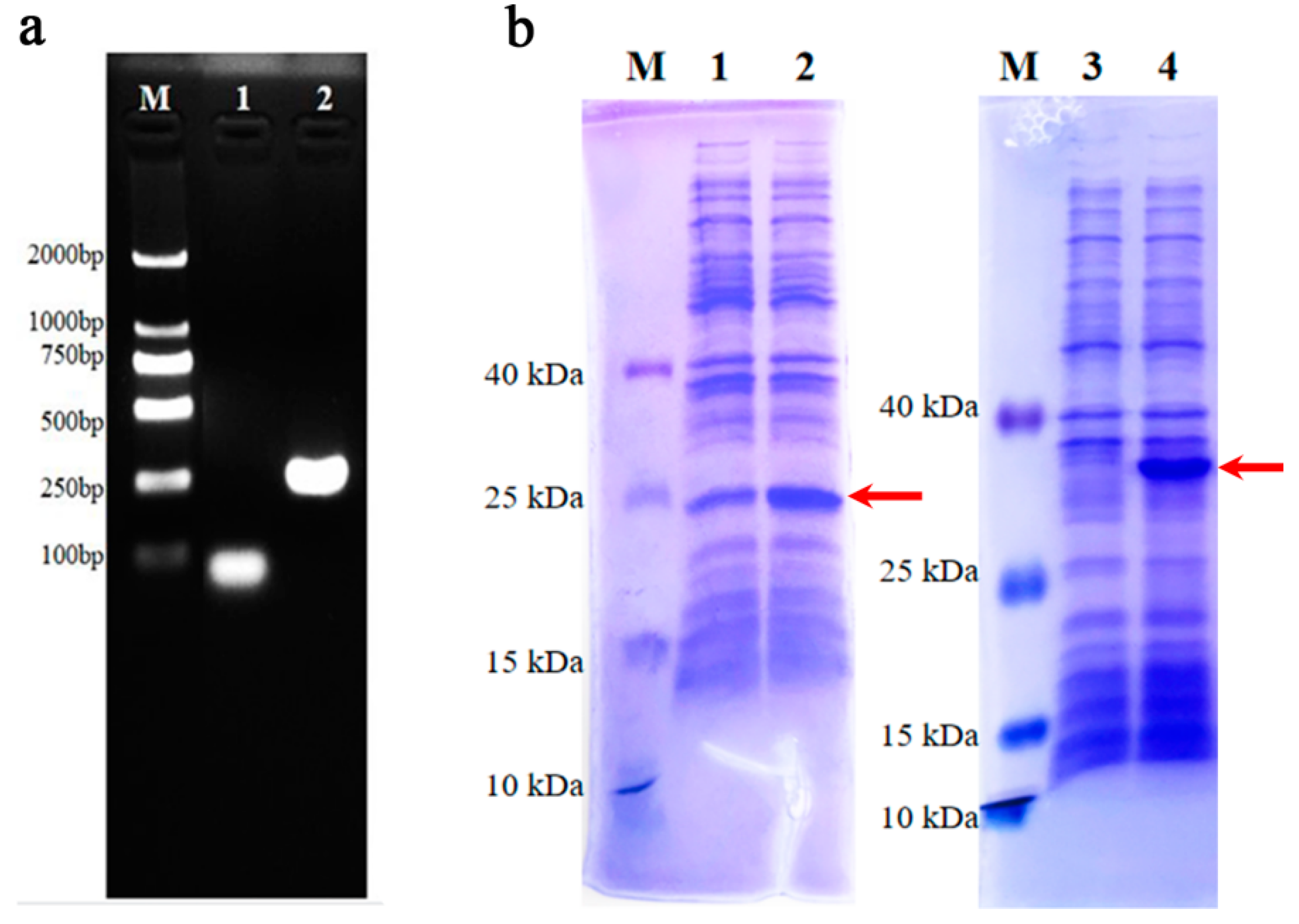
3.8. DNA Amplification and Recombinant Protein Verification of C5a in Pangolins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabana, F.; Tay, C. The addition of soil and chitin into Sunda pangolin (Manis javanica) diets affect digestibility, faecal scoring, mean retention time and body weight. Zoo. Biol. 2020, 39, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.E.; Jiang, H.Y.; Li, L.M.; Zhang, X.J.; Li, G.Y.; Li, H.M.; Jin, X.J.; Chen, J.P. The fecal metagenomics of Malayan pangolins identifies an extensive adaptation to myrmecophagy. Front. Microbiol. 2018, 9, 2793. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, W.; Chen, J.P. Viral metagenomics revealed Sendai virus and coronavirus infection of Malayan pangolins (Manis javanica). Viruses 2019, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Banik, A.; Sahu, S.K.; Panda, S.; Dangar, T.K. Parasites and bacteria associated with Indian pangolins Manis crassicaudata (Mammalia: Manidae). Glob. Ecol. Conserv. 2020, 23, e01042. [Google Scholar] [CrossRef]
- Chong, S.M.; Heng, Y.R.; Yeong, C.Y.F. Pyelonephritis and cystic endometrial hyperplasia in a captive Sunda pangolin (Mavis javanica). J. Comp. Pathol. 2021, 184, 101–105. [Google Scholar] [CrossRef]
- Sato, S.; Kiyono, H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2012, 2, 225–232. [Google Scholar] [CrossRef]
- Hasegawa, M.; Inohara, N. Regulation of the gut microbiota by the mucosal immune system in mice. Int. Immunol. 2014, 26, 481–487. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Uhr, T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann. N. Y. Acad. Sci. 2004, 1029, 36–43. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Casadei, E.; Shibasaki, Y.; Ding, Y.; Sauters, T.J.C.; Yu, Y.Y.; Salinas, I.; Sunyer, J.O. Specialization of mucosal immunoglibulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020, 5, eaay3254. [Google Scholar] [CrossRef]
- Meyer, W.; Liumsiricharoen, M.; Hornickel, I.; Suprasert, A.; Schnapper, A.; Fleischer, L.G. Demonstration of substances of innate immunity in the integument of the Malayan pangolin (Manis javanica). Eur. J. Wildl. Res. 2010, 56, 287–296. [Google Scholar] [CrossRef]
- Wang, Z.Y.; He, F.; Rao, W.L.; Ni, N.; Shen, Q.W.; Zhang, D.Q. Proteomic analysis of goat longissimus dorsi muscles with different drip loss values related to meat quality traits. Food Sci. Biotechnol. 2016, 25, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Park, D.; Reddy, A.; Wilmarth, P.A.; Jensen, J.T. Comparing endocervical mucus proteome of humans and rhesus macaques. Proteom. Clin. Appl. 2021, 15, e2100023. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.S.T.; Bassiouni, A.; Ramezanpour, M.; Chegeni, N.; Colella, A.; Chataway, T.; Wormald, P.; Vreugde, S.; Psaltis, A. Scoping review of chronic rhinosinusitis proteomics. Rhinology 2020, 58, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, N.S.; Salati, A.P.; Keyvanshokooh, S. The effects of bisphenol a on liver proteome and mucus vitellogenin in comparison to plasma as a non-invasive biomarker in immature siberian sturgeons (Acipenser baerii). Comp. Biochem. Phys. D 2021, 38, 100795. [Google Scholar] [CrossRef]
- Choo, S.W.; Rayko, M.; Tan, T.K.; Hari, R.; Komissarov, A.; Wee, W.Y.; Yurchenko, A.A.; Kliver, S.; Tamazian, G.; Antunes, A.; et al. Pangolin genomes and the evolution of mammalian scales and immunity. Genome Res. 2016, 26, 1312–1322. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez, A.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, J.N.; Wu, S.G.; Zhang, Z.; Li, Y.Z. Global geographic diversity and distribution of the myxobacteria. Microbiol. Spectr. 2021, 9, e00012-21. [Google Scholar] [CrossRef]
- Yang, Q.; Cahn, J.K.B.; Piel, J.; Song, Y.F.; Zhang, W.; Lin, H.W. Marine sponge endosymbionts: Structural and functional specificity of the microbiome within Euryspongia arenaria cells. Microbiol. Spectr. 2022, 10, e02296-21. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Bai, S.J.; Hou, G. Microbial communities on fish eggs from Acanthopagrus schlegelii and Halichoeres nigrescens at the Xuwen coral reef in the gulf of tonkin. PeerJ 2020, 8, e8517. [Google Scholar] [CrossRef]
- Gennaro, R.; Simonic, T.; Negri, A.; Mottola, C.; Secchi, C.; Ronchi, S.; Romeo, D. C5a fragment of bovine complement, purification, bioassays, amino-acid sequence and other structural studies. Eur. J. Biochem. 1986, 155, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.C.; Gahan, C.G.M.; Hill, C. Impact of selected Lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS Immunol. Med. Microbiol. 2007, 50, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Obrist, B.; Spoerk, S.; Lang-Loidolt, D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.E.; Wessling-Resnick, M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012, 14, 207–216. [Google Scholar] [CrossRef]
- Rosenblum, S.L. Inflammation, dysregulated iron metabolism, and cardiovascular disease. Front. Aging 2023, 4, 1124178. [Google Scholar] [CrossRef]
- González, A.S.; Guerrero, D.B.; Soto, M.B.; Díaz, S.P.; Martinez-Olmos, M.; Vidal, O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur. J. Clin. Nutr. 2006, 60, 802–809. [Google Scholar] [CrossRef]
- Barber, M.F.; Elde, N.C. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 2014, 346, 1362–1366. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Ma, R.; Fang, L.; Chen, L.; Wang, X.; Jiang, J.; Gao, L. Ferroptotic stress promotes macrophages against intracellular bacteria. Theranostics 2022, 12, 2266–2289. [Google Scholar] [CrossRef]
- Imada, Y.; Takase, A.; Kikuma, R.; Iwamaru, Y.; Akachi, S.; Hayakawa, Y. Serotyping of 800 strains of erysipelothrix isolated from pigs affected with erysipelas and discrimination of attenuated live vaccine strain by genotyping. J. Clin. Microbiol. 2004, 42, 2121–2126. [Google Scholar] [CrossRef]
- Fu, S.Z.; Xia, W.; Wang, Q.Y.; Rahman, M.M.; Hao, J.W.; Ye, S.G.; Liu, Y.; Li, R.J. Genomic characterization and pathogenicity analysis of the probiotic Vagococcus lutrae strain Vl-18 causing severe skin lesions in warm-blooded animals. Aquaculture 2020, 523, 735166. [Google Scholar] [CrossRef]
- Roman, L.; Real, F.; Sorroza, L.; Padilla, D.; Acosta, B.; Grasso, V.; Bravo, J.; Acosta, F. The in vitro effect of probiotic Vagococcus fluvialis on the innate immune parameters of Sparus aurata and Dicentrarchus labrax. Fish Shellfish. Immunol. 2012, 33, 1071–1075. [Google Scholar] [CrossRef]
- Alves, M.; Nogueira, C.; de Magalhaes-Sant’Ana, A.; Chung, A.P.; Morais, P.V.; da Costa, M.S. Nosocomiicoccus ampullae gen. nov., sp nov., isolated from the surface of bottles of saline solution used in wound cleansing. Int. J. Syst. Evol. Microbiol. 2008, 58, 2939–2944. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Marth, T.; Lagier, J.C.; Angelakis, E.; Raoult, D. Sewage workers with low antibody responses may be colonized successively by several Tropheryma whipplei strains. Int. J. Infect. Dis. 2015, 35, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.L.; Clausen, E.; Nouraie, S.M.; Kingsley, L.; McMahon, D.; Kleerup, E.; Huang, L.; Ghedin, E.; Greenblatt, R.M.; Morris, A. Tropheryma whipplei colonization in HIV-infected individuals is not associated with lung function or inflammation. PLoS ONE 2018, 13, e0205065. [Google Scholar] [CrossRef]
- Li, F.X.; Zhu, P.; Li, Z.H.; Zhao, W.H.; Gao, H.F.; Hong, Q.H.; Song, J.L.; Yang, S.B. Moraxella nasovis sp. nov., isolated from a sheep with respiratory disease. Int. J. Syst. Evol. Microbiol. 2022, 72, 005511. [Google Scholar] [CrossRef]
- Gulmez Saglam, A.G.; Erkilic, E.E.; Buyuk, F.; Kirmizigul, A.H.; Gokce, G.; Balyen, L. Moraxella ovis and Mycoplasma conjunctivae isolation from an ovine infectious keratoconjunctivitis outbreak and fortified treatment approaches. Kafkas Univ. Vet. Fak. 2018, 24, 551–556. [Google Scholar]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part II: Role in immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Hertle, E.; Arts, I.C.W.; van der Kallen, C.J.H.; Feskens, E.J.M.; Schalkwijk, C.G.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J. Classical pathway of complement activation: Longitudinal associations of C1q and C1-inh with cardiovascular outcomes: The codam study (cohort on diabetes and atherosclerosis maastricht) brief report. Arterioscl. Throm. Vas. 2018, 38, 1242–1244. [Google Scholar] [CrossRef]
- Heurich, M.; Martínez-Barricarte, R.; Francis, N.J.; Roberts, D.L.; de Córdoba, S.R.; Morgan, B.P.; Harris, C.L. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl. Acad. Sci. USA 2011, 108, 8761–8766. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beltran, J.F.; Brito, I.L. Host-microbiome protein-protein interactions capture disease-relevant pathways. Genome Biol. 2022, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Dieudonne-Vatran, A.; Krentz, S.; Blom, A.M.; Meri, S.; Henriques-Normark, B.; Riesbeck, K.; Albiger, B. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor cob-binding protein in a pspc allele-dependent fashion. J. Immunol. 2009, 182, 7865–7877. [Google Scholar] [CrossRef] [PubMed]
- Jarva, H.; Ram, S.; Vogel, U.; Blom, A.M.; Meri, S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J. Immunol. 2005, 174, 6299–6307. [Google Scholar] [CrossRef] [PubMed]
- Laabei, M.; Colineau, L.; Bettoni, S.; Marziarz, K.; Ermert, D.; Riesbeck, K.; Ram, S.; Blom, A. Novel antibacterial fusion proteins enhance Moraxella catarrhalis killing. Front. Immunol. 2020, 11, 2122. [Google Scholar] [CrossRef]
- Hallstrom, T.; Nordstrom, T.; Tan, T.T.; Manolov, T.; Lambris, J.D.; Isenman, D.E.; Zipfel, P.F.; Blom, A.M.; Riesbeck, K. Immune evasion of Moraxella catarrhalis involves ubiquitous surface protein a-dependent C3d binding. J. Immunol. 2011, 186, 3120–3129. [Google Scholar] [CrossRef]
- Bicoll, P.S.; Goyal, A.; Blatt, N.B.; Freij, B.J. Eculizumab-associated Moraxella lacunata bacteremia and systemic inflammatory response syndrome in a toddler with atypical hemolytic uremic syndrome. Clin. Med. Insights Pediatr. 2021, 15, 1179556521992367. [Google Scholar] [CrossRef]
- Mueller-Ortiz, S.L.; Calame, D.G.; Shenoi, N.; Li, Y.D.; Wetsel, R.A. The complement anaphylatoxins C5a and C3a suppress IFN-beta production in response to Listeria monocytogenes by inhibition of the cyclic dinucleotide-activated cytosolic surveillance pathway. J. Immunol. 2017, 198, 3237–3244. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lie, Y.; He, T.; Yang, T.T.; Wu, J.; Cianflone, K.; Lu, H.L. Anaphylatoxin C5a induces inflammation and reduces insulin sensitivity by activating Tlr4/Nf-kappa B/Pi3k signaling pathway in 3t3-L1 adipocytes. Biomed. Pharmacother. 2018, 103, 955–964. [Google Scholar] [CrossRef]
- Karasu, E.; Demmelmaier, J.; Kellermann, S.; Holzmann, K.; Kohl, J.; Schmidt, C.Q.; Kalbitz, M.; Gebhard, F.; Huber-Lang, M.S.; Halbgebauer, R. Complement C5a induces pro-inflammatory microvesicle shedding in severely injured patients. Front. Immunol. 2020, 11, 1789. [Google Scholar] [CrossRef]
- Gong, D.S.; Chi, X.M.; Wei, J.H.; Zhou, G.W.; Huang, G.X.Y.; Zhang, L.; Wang, R.W.; Lei, J.L.; Chen, S.R.W.; Yan, N. Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature 2019, 572, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.L.; Agrawal, R.S.; Connolly, S.F.; McCaffrey, R.L.; Schlomann, J.; Kusner, D.J. MHC class II levels and intracellular localization in human dendritic cells are regulated by calmodulin kinase II. J. Leukoc. Biol. 2007, 82, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Kozik, A.; Golda, A.; Mak, P.; Suder, P.; Silberring, J.; Barbasz, A.; Rapala Kozik, M. Myeloperoxidase-catalyzed oxidative inactivation of human kininogens: The impairment of kinin-precursor and prekallikrein-binding functions. Biol. Chem. 2011, 392, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Lora, I. Kinin-mediated inflammation in neurodegenerative disorders. Neurochem. Int. 2012, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bekassy, Z.; Fagerstrom, I.L.; Bader, M.; Karpman, D. Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation. Nat. Rev. Immunol. 2022, 22, 411–428. [Google Scholar] [CrossRef]
- Smeets, R.L.; Joosten, L.A.B.; Arntz, O.J.; Bennink, M.B.; Takahashi, N.; Carlsen, H.; Martin, M.U.; van den Berg, W.B.; van de Loo, F.A. Soluble interleukin-1 receptor accessory protein ameliorates collagen-induced arthritis by a different mode of action from that of interleukin-1 receptor antagonist. Arthritis Rheum. 2005, 52, 2202–2211. [Google Scholar] [CrossRef]


| Protein Accession | Protein Description | Peptides | Unique Peptides | MW (kDa) | Score | Coverage (%) | Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|
| XP_036752253.1 | ceruloplasmin | 17 | 2 | 124.9 | 54.99 | 19 | 0.57 | 0.011 |
| XP_036739216.1 | lactotransferrin | 63 | 12 | 76.8 | 552.35 | 66 | 0.57 | 0.011 |
| XP_036778791.1 | ferritin | 2 | 2 | 34.9 | 1.96 | 5 | 0.57 | 0.011 |
| XP_036738762.1 | serotransferrin | 52 | 12 | 110.7 | 298.14 | 40 | 0.57 | 0.011 |
| XP_036731391.1 | complement factor B | 20 | 2 | 88.1 | 87.86 | 27 | 0.5 | 0.052 |
| XP_036746741.1 | complement C3 | 57 | 10 | 186.1 | 184.87 | 34 | 0.5 | 0.052 |
| XP_036771376.1 | complement C5-like | 2 | 1 | 188.4 | 4.31 | 1 | 0.5 | 0.052 |
| XP_036750838.1 | properdin | 2 | 2 | 50.8 | 7.06 | 5 | 0.5 | 0.052 |
| Protein Accession | Protein Description | Peptides | Unique Peptides | MW (kDa) | Score | Coverage (%) | Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|
| XP_036748218.1 | calmodulin-3-like | 3 | 3 | 16.5 | 16.06 | 36 | 0.6 | 0.030 |
| XP_036730991.1 | kininogen-1 | 12 | 3 | 47.7 | 33.9 | 28 | 0.6 | 0.030 |
| XP_036731091.1 | IL-1 receptor accessory protein | 2 | 2 | 78.1 | 5.15 | 2 | 0.6 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Yu, Y.; Sun, H.; Zhang, X.; Liu, P.; Deng, J.; Hu, X.; Chen, J. Proteomics and Microbiota Conjoint Analysis in the Nasal Mucus: Revelation of Differences in Immunological Function in Manis javanica and Manis pentadactyla. Animals 2024, 14, 2683. https://doi.org/10.3390/ani14182683
Han Q, Yu Y, Sun H, Zhang X, Liu P, Deng J, Hu X, Chen J. Proteomics and Microbiota Conjoint Analysis in the Nasal Mucus: Revelation of Differences in Immunological Function in Manis javanica and Manis pentadactyla. Animals. 2024; 14(18):2683. https://doi.org/10.3390/ani14182683
Chicago/Turabian StyleHan, Qing, Yepin Yu, Hongbin Sun, Xiujuan Zhang, Ping Liu, Jianfeng Deng, Xinyuan Hu, and Jinping Chen. 2024. "Proteomics and Microbiota Conjoint Analysis in the Nasal Mucus: Revelation of Differences in Immunological Function in Manis javanica and Manis pentadactyla" Animals 14, no. 18: 2683. https://doi.org/10.3390/ani14182683






