Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Design
2.3. Growth Performance Parameters
body weight])/time (days)
body weight]/mean initial body weight)
weight + final body weight)/2]/t
2.4. Liver Sample Preparation
2.5. RNA Extraction and Reverse Transcription
2.6. Quantitative Real-Time PCR
2.7. Data Analysis
3. Results
3.1. Effects on Growth Performances
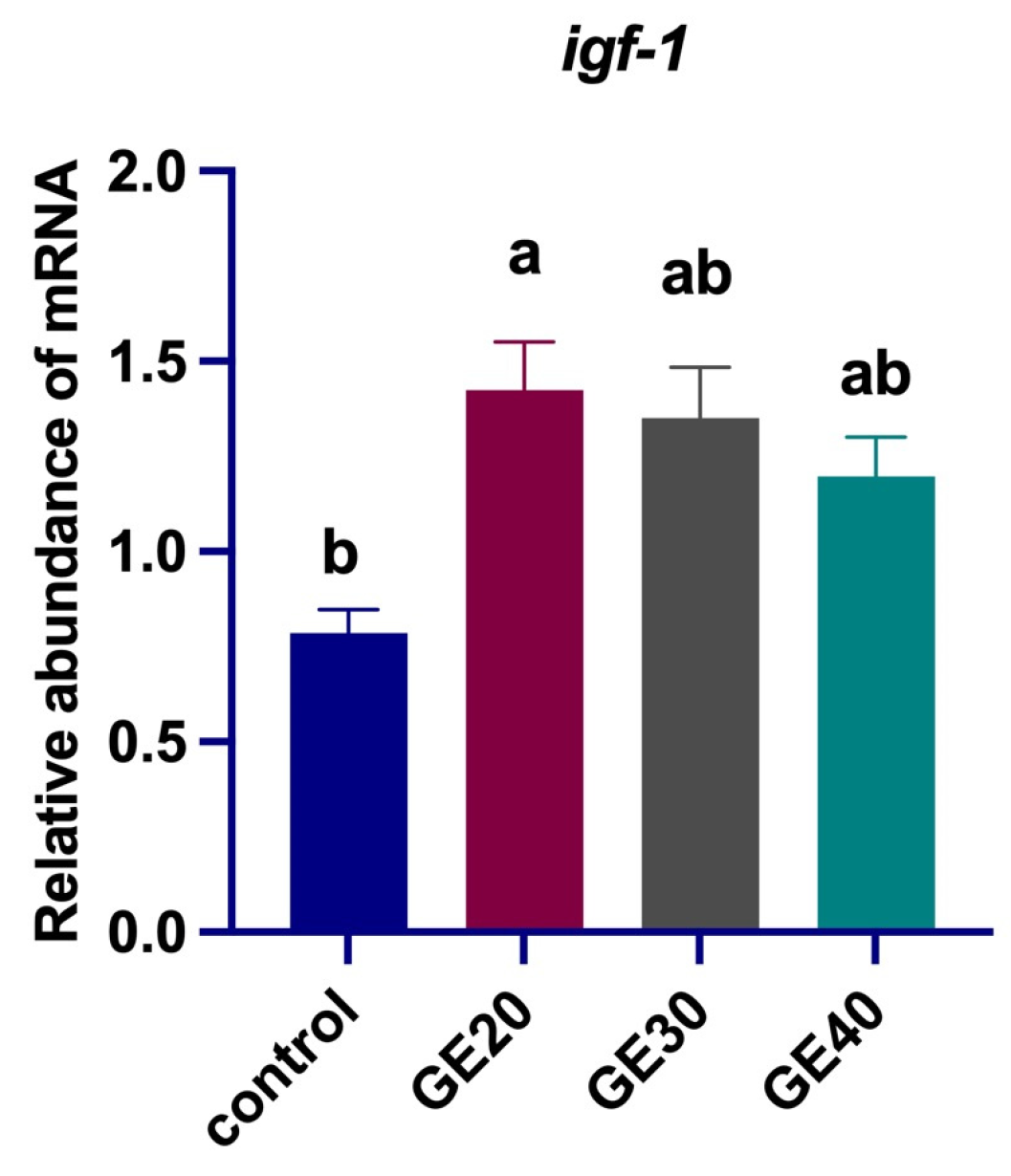
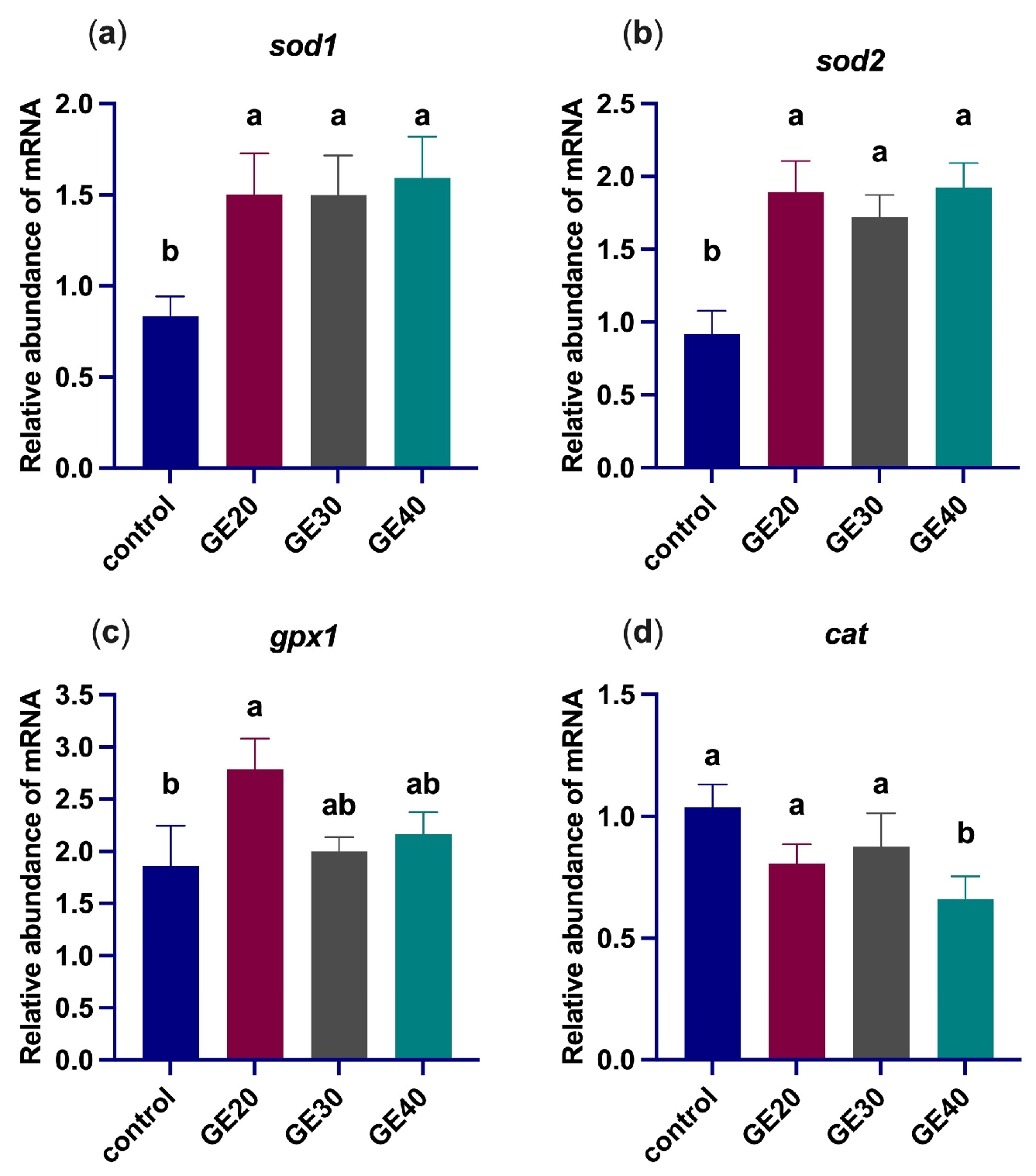
3.2. Effects on Oxidative Stress-Related Genes
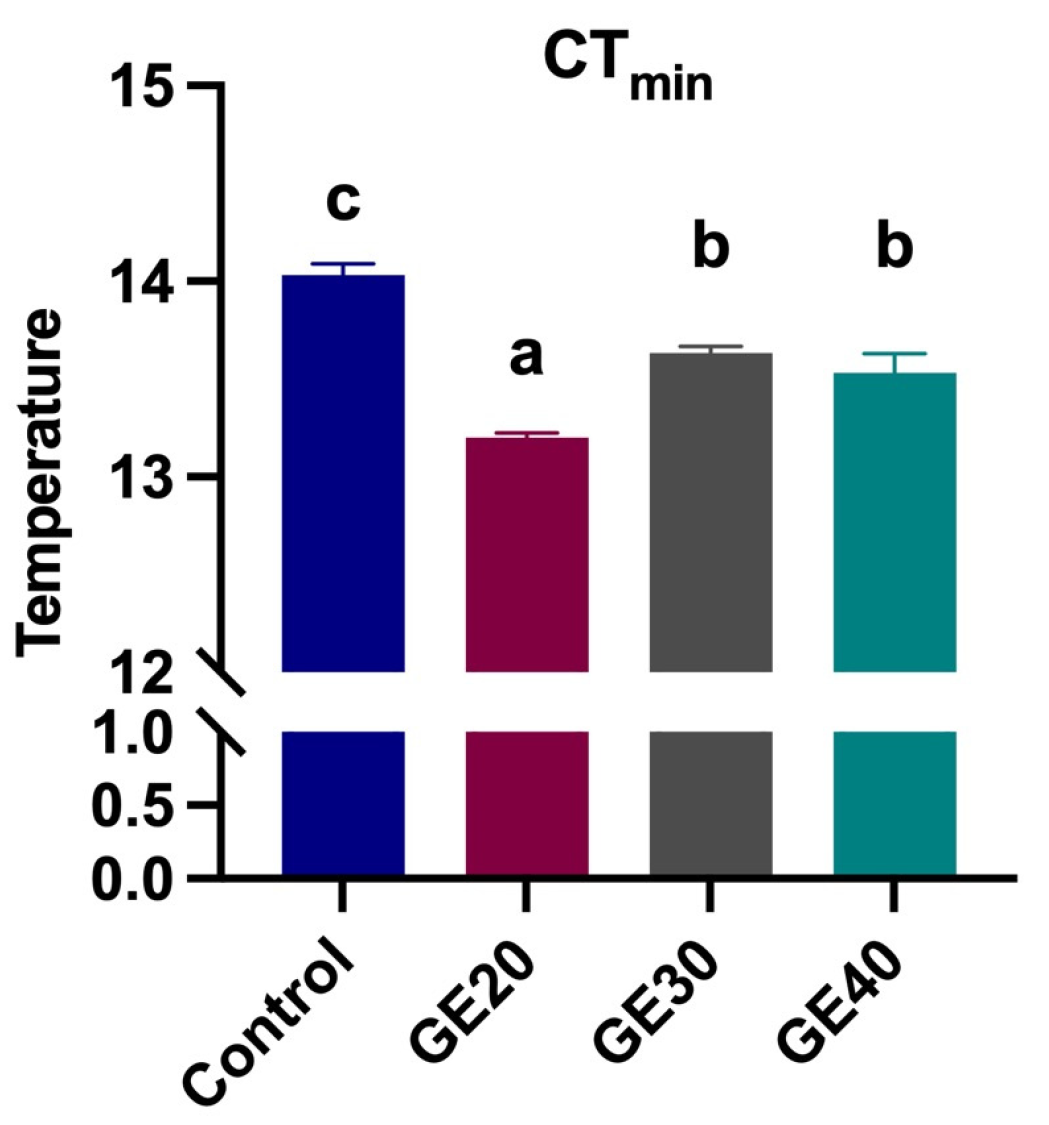
3.3. Effects on Thermal Tolerance
4. Discussion
4.1. Antioxidant Enzyme-Related Genes Expression
4.2. Growth Performances
4.3. Thermal Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoodi, B.; Aberoumand, A.; Ziaei-nejad, S.; Seyyedi, S. Effects of diets containing grape pomace on the growth, nutrition indices, and the quality traits of common carp (Cyprinus carpio). J. Food. Sci. Nutr. 2023, 11, 6660–6669. [Google Scholar] [CrossRef]
- Manam, V.K. Fish feed nutrition and its management in aquaculture. Int. J. Fish. Aquat. Stud. 2023, 11, 58–61. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; González-Aguilar, G.; Siddiqui, M.W. Plant Food by-Products: Industrial Relevance for Food Additives and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O. Antioxidant activity of plant phenols: Chemical mechanisms and biological significance. Curr. Org. Chem. 2012, 16, 692–714. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Imperatore, R.; Orso, G.; Facchiano, S.; Scarano, P.; Hoseinifar, S.H.; Ashouri, G.; Guarino, C.; Paolucci, M. Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 2023, 563, 738878. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Reda, F.M.; Alagawany, M.; Farag, M.R.; El-Naggar, K. The role of dietary chia seed powder in modulating cold stress-related impacts in Nile tilapia, Oreochromis niloticus. Aquaculture 2023, 567, 739246. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Kamangar, B.B.; Zarei, M.A. Effects of diets containing grape seed proanthocyanidin extract on the growth and oxidative capacity of common carp (Cyprinus carpio). Aquaculture 2021, 540, 736689. [Google Scholar] [CrossRef]
- Morante, V.H.P.; Copatti, C.E.; Souza, A.R.L.; da Costa, M.M.; Braga, L.G.T.; Souza, A.M.; de Melo, F.V.S.T.; Camargo, A.C.D.S.; Melo, J.F.B. Assessment the crude grape extract as feed additive for tambaqui (Colossoma macropomum), an omnivorous fish. Aquaculture 2021, 544, 737068. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J Pathol. Rev. Med. Microbiol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Hamidian, G.; Mardani, K.; Oushani, A.K.; Firouzamandi, M.; Esteban, M.Á.; Shohreh, P. Changes in rainbow trout (Oncorhynchus mykiss) growth and mucosal immune parameters after dietary administration of grape (Vitis vinifera) seed extract. Fish Physiol. Biochem. 2021, 47, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Alizadeh-Salteh, S.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Saera-Vila, A.; Calduch-Giner, J.-A.; Kaushik, S.; Pérez-Sánchez, J. Combined replacement of fish meal and oil in practical diets for fast growing juveniles of gilthead sea bream (Sparus aurata L.): Networking of systemic and local components of GH/IGF axis. Aquaculture 2007, 267, 199–212. [Google Scholar] [CrossRef]
- de Almeida Xavier, M.J.M. Improving Growth Performance of Fish Larvae through Early Nutrition. 2021. Available online: https://repositorio-aberto.up.pt/bitstream/10216/138509/2/520563.pdf (accessed on 20 February 2024).
- Chandhini, S.; Trumboo, B.; Jose, S.; Varghese, T.; Rajesh, M.; Kumar, V.J.R. Insulin-like growth factor signalling and its significance as a biomarker in fish and shellfish research. Fish Physiol. Biochem. 2021, 47, 1011–1031. [Google Scholar] [CrossRef]
- Tveteras, R. Global fish production data & analysis. In Proceedings of the Global Outlook for Aquaculture Leadership Conference in Guangzhou, Guangzhou, China, 19–22 September 2016; Available online: https://www.aquaculturealliance.org/wp-content/uploads/2017/06/Day1_RagnarTveteras.pdf (accessed on 21 March 2024).
- Liu, E.L.; Chen, G.R.; Chen, F.M.; Yang, S.D. Temperature causing cold damage to Asian seabass; Fisheries Research Institute, Ministry of Agriculture Taiwan: Keelung, Taiwan, 2021. [Google Scholar]
- Nazir, A.; Chen, T.Y.; Wang, P.L.; Shiao, J.C. Reconstructing habitat use, identifying origin and discrimination of the barramundi (wild and farmed) populations using otolith stable isotope analysis. Estuar. Coast. Shelf Sci. 2023, 285, 108317. [Google Scholar] [CrossRef]
- Williams, K.C.; Barlow, C.G.; Rodgers, L.; Agcopra, C. Dietary composition manipulation to enhance the performance of juvenile barramundi (Lates calcarifer Bloch) reared in cool water. Aquac. Res. 2006, 37, 914–927. [Google Scholar] [CrossRef]
- Jerry, D.R. Biology and Culture of Asian Seabass Lates Calcarifer; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Bardallo, R.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. The FEBS J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef]
- Wang, M.C.; Wang, Y.C.; Peng, H.W.; Hseu, J.R.; Wu, G.C.; Chang, C.F.; Tseng, Y.C. Resveratrol induces expression of metabolic and antioxidant machinery and protects tilapia under cold stress. Int. J. Mol. Sci. 2020, 21, 3338. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Moniruzzaman, M.; Ghosal, I.; Pegu, T.; Das, D.N.; Chakraborty, S.B. Evaluating the role of dietary plant extracts to allow adaptation to thermal stress in a cold stream ornamental fish, Botia rostrata (Günther, 1868). J. Therm. Biol. 2022, 105, 103224. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, C.; Ale, A.; Rossi, A.S.; Karakachoff, M.; Cazenave, J. Effects of cold stress on juvenile Piaractus mesopotamicus and the mitigation by β-carotene. J. Therm. Biol. 2020, 88, 102497. [Google Scholar] [CrossRef] [PubMed]
- Tejaswini, K.; Deo, A.D.; Shamna, N.; Jayant, M.; Aklakur, M.; Annadurai, R. Effect of flavanone rich lemon peel extract on feed intake and growth of Labeo rohita (Hamilton, 1822) fingerlings reared at low temperature recirculatory aquaculture system. Aquaculture 2024, 584, 740450. [Google Scholar] [CrossRef]
- Refaey, M.M.; Mehrim, A.I.; Zenhom, O.A.; Mansour, A.T. Effect of fatty acids manipulation on survival and physiological response of hybrid red tilapia under chronic cold stress. Aquaculture 2022, 561, 738663. [Google Scholar] [CrossRef]
- Ford, T.; Beitinger, T.L. Temperature tolerance in the goldfish, Carassius auratus. J. Therm. Biol. 2005, 30, 147–152. [Google Scholar] [CrossRef]
- Hu, Y.C.; Chu, K.F.; Yang, W.K.; Lee, T.H. Na+, K+-ATPase β1 subunit associates with α1 subunit modulating a “higher-NKA-in-hyposmotic media” response in gills of euryhaline milkfish, Chanos chanos. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 2017, 187, 995–1007. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Lin, C.H.; Lee, T.H. Cholesterol accumulation in livers of Indian medaka, Oryzias dancena, acclimated to fresh water and seawater. Front. Mar. Sci. 2022, 9, 891706. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lange, B.; Currie, K.L.; Howarth, G.S.; Stone, D.A.J. Grape seed extract and dried macroalgae, Ulva lactuca Linnaeus, improve survival of greenlip abalone, Haliotis laevigata Donovan, at high water temperature. Aquaculture 2014, 433, 348–360. [Google Scholar] [CrossRef]
- Ahmadi, A.; Bagheri, D.; Hoseinifar, S.H.; Morshedi, V.; Paolucci, M. Beneficial role of polyphenols as feed additives on growth performances, immune response and antioxidant status of Lates Calcarifer (Bloch, 1790) juveniles. Aquaculture 2022, 552, 737955. [Google Scholar] [CrossRef]
- Rashidah, A.R.; Shariff, M.; Yusoff, F.M.; Ismail, I.S. Dietary supplementation of Polygonum chinense improves the immunity of Asian seabass, Lates calcarifer (Bloch, 1790) against Vibrio harveyi infection. Fish Shellfish Immunol. Rep. 2023, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Sangeetha, P.; Haripriya, D.; Panneerselvam, C. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci. Lett. 2005, 383, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- de Mello Andrade, J.M.; Fasolo, D. Chapter 20–Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 253–265. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2–Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current state of knowledge on the antioxidant effects and mechanisms of action of polyphenolic compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Bashir, N.; Manoharan, V.; Miltonprabu, S. Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J. Nutr. Biochem. 2016, 32, 128–141. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Chi, C.; Gu, Y. Identification and analysis of icCu/Zn-SOD, Mn-SOD and ecCu/Zn-SOD in superoxide dismutase multigene family of Pseudosciaena crocea. Fish Shellfish Immunol. 2015, 43, 491–501. [Google Scholar] [CrossRef]
- Duong, D.N.; Qin, J.G.; Harris, J.O.; Hoang, T.H.; Bansemer, M.S.; Currie, K.L.; Phan-Thien, K.Y.; Dowell, A.; Stone, D.A.J. Effects of dietary grape seed extract, green tea extract, peanut extract and vitamin C supplementation on metabolism and survival of greenlip abalone (Haliotis laevigata Donovan) cultured at high temperature. Aquaculture 2016, 464, 364–373. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Esteban, M.Á.; Abdel-Tawwab, M. Impact of grape pomace flour (GPF) on immunity and immune-antioxidant-anti-inflammatory genes expression in Labeo rohita against Flavobacterium columnaris. Fish Shellfish Immunol. 2021, 111, 69–82. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Wang, G.; Xie, J.; Kaneko, G.; Yu, E. Dietary grape seed proanthocyanidin extract improved the chemical composition, antioxidant capacity, myofiber growth and flesh quality of Nile tilapia muscle. Aquacult. Rep. 2023, 33, 101878. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Spanou, C.; Veskoukis, A.S.; Stagos, D.; Liadaki, K.; Anastasiadi, M.; Haroutounian, S.A.; Tsouka, M.; Tzanakouli, E.; Kouretas, D. Effects of grape extracts on the in vitro activity of enzymes involved in oxidative stress regulation. In Vivo 2011, 25, 657–662. [Google Scholar] [PubMed]
- Arslan, G.; Sönmez, A.Y.; Yan, K.T. Effects of grape Vitis vinifera seed oil supplementation on growth, survival, fatty acid profiles, antioxidant contents and blood parameters in rainbow trout Oncorhynchus mykiss. Aquac. Res. 2018, 49, 2256–2266. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef]
- Chien, A.; Chou, C.Y.; Cheng, Y.C.; Sheen, S.S.; Kirby, R. The optimal dietary level of dry grape extract and its effect on the growth performance and antioxidant activity of the white shrimp Litopenaeus vannamei. Aquacult. Rep. 2023, 29, 101527. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Poolsawat, L.; Rahman, M.M.; Xu, X.; Jiang, X.; Li, X.; Tan, H.; Leng, X. Flavonoid-enriched diets improved the growth and flesh quality of grass carp (Ctenopharyngodon idellus) based on metabolomics. Aquac. Nutr. 2021, 27, 2514–2528. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootecn. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Pascariu, S.; Pop, I.; Simeanu, D.; Pavel, G.; Solcan, C. Effects of wine by-products on growth performance, complete blood count and total antioxidant status in broilers. Braz. J. Poult. Sci. 2017, 19, 191–202. [Google Scholar] [CrossRef]
- Kim, E.Y.; Pai, T.K.; Han, O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 3606–3612. [Google Scholar] [CrossRef]
- Ma, Q.; Kim, E.Y.; Han, O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J. Nutr. 2010, 140, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.W.; Lu, J.J.; Chen, X.H. Effects of dietary grape seed proanthocyanidins on growth performance, some serum biochemical parameters and body composition of tilapia (Oreochromis niloticus) fingerlings. Ital. J. Anim. Sci. 2014, 13, 3357. [Google Scholar] [CrossRef]
- Rosas, V.T.; Mureb, R.A.; Monserrat, J.M.; Wasielesky, W., Jr.; Tesser, M.B. Inclusion of grape bagasse (Vitis sp.) in the diet of white shrimp (Litopenaeus vannamei) and its effects on growth and antioxidant system. Aquac. Res. 2022, 53, 4805–4813. [Google Scholar] [CrossRef]
- Donaldson, M.; Cooke, S.; Patterson, D.; Macdonald, J. Cold shock and fish. J. Fish Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Jin, X.; Wang, Z.; Mao, C.; Guo, S.; Yan, S.; Shi, B. Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs. Animals 2022, 12, 2535. [Google Scholar] [CrossRef]
- Ibarz, A.; Martín-Pérez, M.; Blasco, J.; Bellido, D.; de Oliveira, E.; Fernández-Borràs, J. Gilthead sea bream liver proteome altered at low temperatures by oxidative stress. Proteomics 2010, 10, 963–975. [Google Scholar] [CrossRef]
- Wu, S.M.; Liu, J.H.; Shu, L.H.; Chen, C.H. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, C.; Jiang, L.; Chen, D.; Jiang, P.; Huang, B. Effects of Vitamin E on Immune Response, Antioxidant Capacity, and Liver Tissue Structure of Crucian Carp under Acute Cold Stress. Aquac. Res. 2023, 2023, 2579785. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Sun, S.X.; Zhang, H.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Reduced oxidative stress increases acute cold stress tolerance in zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 235, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective effects and molecular mechanisms of tea polyphenols on cardiovascular diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Niu, M.; Hu, L.; Chen, L. Cold acclimation for enhancing the cold tolerance of zebrafish cells. Front. Physiol. 2022, 12, 813451. [Google Scholar] [CrossRef]
- Chung, D.J.; Schulte, P.M. Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus). J. Exp. Biol. 2015, 218, 1621–1631. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′ to 3′) | Amplicon Size (bp) | Reference Number | |
|---|---|---|---|---|
| igf-1 | F | ACGAGTGCTGCTTCCAAAG | 118 | XM_018697285.1 |
| R | GGTGTTCTCGGCATGTCTG | |||
| sod1 | F | GGTCCCAATGATGCAGAGAG | 108 | XM_018691152.1 |
| R | GGTCCCAATGATGCAGAGAG | |||
| sod2 | F | TGCGGCCAGACTATGTTAAG | 200 | XM_018675982.1 |
| R | GTATCAGTGTTGGTGGTCAGT | |||
| gpx1 | F | GGCTGGGAGTGTTGAAGAG | 177 | XM_018686718.2 |
| R | TTGCTGGAGTAACGAGAGTG | |||
| cat | F | GAGTCTGCATCAGGTGTCTTT | 109 | XM_018675907.1 |
| R | CAAACTGGTTAATGCTGATGGG | |||
| elf1α | F | GTTGCCTTTGTCCCCATCTC | 130 | XM_018699049.1 |
| R | CTTCCAGCAGTGTGGTTCCA |
| Groups | Control | GE20 | GE30 | GE40 |
|---|---|---|---|---|
| Initial weight (g) | 20.1 ± 0.75 | 19.83 ± 0.75 | 20.33 ± 0.816 | 20.33 ± 1.032 |
| Final weight (g) | 80 ± 3.03 b | 93.5 ± 3.67 a | 81.3 ± 4.08 b | 81.66 ± 3.26 b |
| Weight gain (g) | 59.8 ± 3.43 b | 73.6 ± 4.17 a | 61 ± 3.74 b | 61.33 ± 3.26 b |
| SGR% (g/day) | 4.33 ± 0.038 b | 4.48 ± 0.039 a | 4.34 ± 0.04 b | 4.35 ± 0.04 b |
| FI (%/day) | 1.94 ± 0.20 | 1.92 ± 0.10 | 1.91 ± 0.19 | 1.96 ± 0.24 |
| FCR | 1.09 ± 0.04 a | 0.88 ± 0.04 c | 0.92 ± 0.06 ab | 0.96 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, S.; Ranasinghe, N.; Lee, T.-H.; Chou, C.-C. Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed. Animals 2024, 14, 2731. https://doi.org/10.3390/ani14182731
Akram S, Ranasinghe N, Lee T-H, Chou C-C. Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed. Animals. 2024; 14(18):2731. https://doi.org/10.3390/ani14182731
Chicago/Turabian StyleAkram, Salman, Naveen Ranasinghe, Tsung-Han Lee, and Chi-Chung Chou. 2024. "Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed" Animals 14, no. 18: 2731. https://doi.org/10.3390/ani14182731






