The Effects of Onychectomy (Declawing) on Forearm and Leg Myology in a Kinkajou (Potos flavus)
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Myological Effects of Onychectomies
1.2. The Kinkajou
1.3. Kinkajous as Exotic Pets
1.4. A Case Study: The Myological Effects of Onychectomy in a Kinkajou
2. Hypotheses
3. Materials and Methods
3.1. Sample
3.2. Data Collection
3.3. Architectural Variables Studied
4. Results
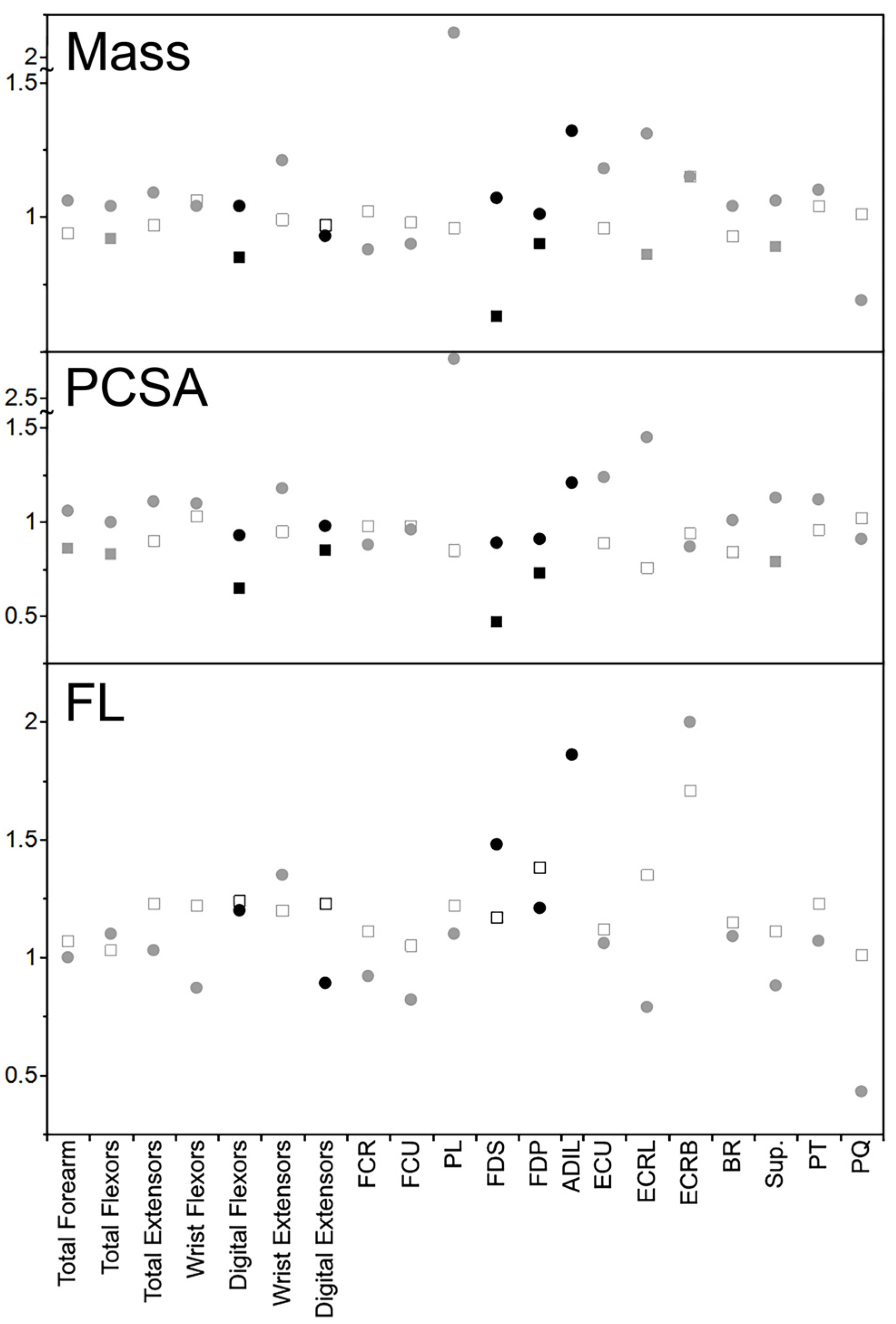
4.1. Forearm Muscle Differences (Muscle Mass, Physiological Cross-Sectional Area, and Fascicle Length)
4.2. Comparison to Felids
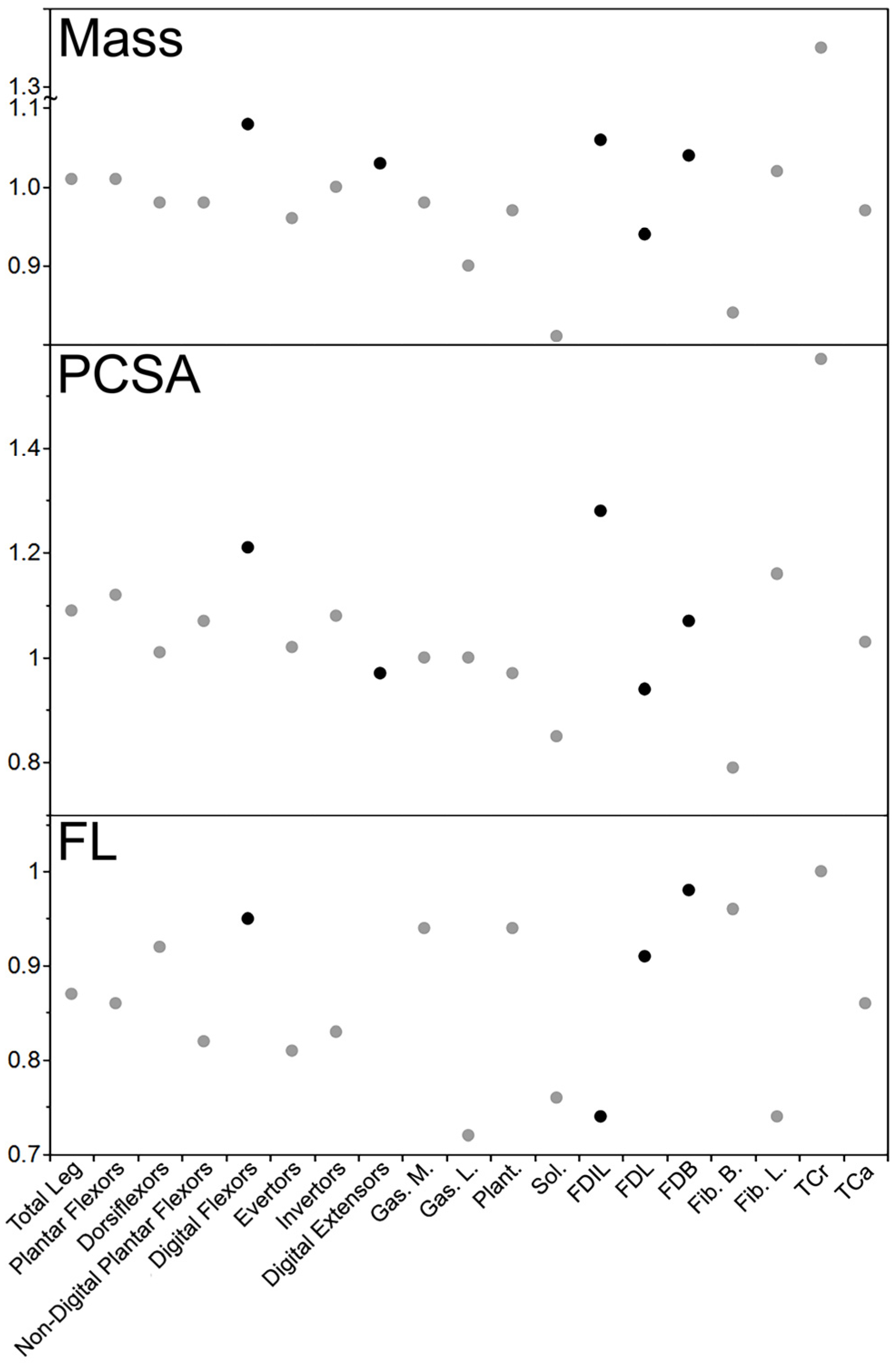
4.3. Leg Muscle Differences (Muscle Mass, Physiological Cross-Sectional Area, and Fascicle Length)
4.4. Forearm Compared to Leg
5. Discussion
5.1. Forearm Muscle Differences
5.2. Comparison to Felids
5.3. Leg Muscle Differences and Comparison of Onychectomy Effects in the Forearm and Leg
5.4. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.E.; Mittermeier, R.A.; Ruff, S.; Martinez-Vilalta, A.; Hoyo, J.D. Handbook of the Mammals of the World, 1st ed.; Lynx Edicions: Barcelona, Spain, 2009; Volume 1, p. 8496553493. [Google Scholar]
- Kays, R.W. Food preferences of kinkajous (Potos flavus): A frugivorous carnivore. J. Mammal. 1999, 80, 589–599. [Google Scholar] [CrossRef]
- Youlatos, D. Osteological correlates of tail prehensility in carnivorans. J. Zool. 2003, 259, 423–430. [Google Scholar] [CrossRef]
- McClearn, D. Locomotion, posture, and feeding behavior of kinkajous, coatis, and raccoons. J. Mammal. 1992, 73, 245–261. [Google Scholar] [CrossRef]
- Marsh, S.F.; Manfredi, K.; Smith, H.F. Myological and osteological correlates of hindfoot reversal in the kinkajou (Potos flavus). J. Mamm. Evol. 2021, 28, 813–830. [Google Scholar] [CrossRef]
- Perdomo-Cárdenas, V.; Patiño-Holguín, C.; Vélez-García, J.F. Evolutionary and terminological analysis of the flexor digitorum superficialis, interflexorii and palmaris longus muscles in kinkajou (Potos flavus) and crab-eating racoon (Procyon cancrivorus). Anat. Histol. Embryol. 2021, 50, 520–533. [Google Scholar] [CrossRef]
- Organ, J.M.; Teaford, M.F.; Taylor, A.B. Functional correlates of fiber architecture of the lateral caudal musculature in prehensile and nonprehensile tails of the Platyrrhini (Primates) and Procyonidae (Carnivora). Anat. Rec. Adv. Integr. Anat. Evol. Biol. Adv. Integr. Anat. Evol. Biol. 2009, 292, 827–841. [Google Scholar] [CrossRef]
- Bush, E.R.; Baker, S.E.; MacDonald, D.W. Global trade in exotic pets 2006–2012. Conserv. Biol. 2014, 28, 663–676. [Google Scholar] [CrossRef]
- Sinclair, J.S.; Stringham, O.C.; Udell, B.; Mandrak, N.E.; Leung, B.; Romagosa, C.M.; Lockwood, J.L. The international vertebrate pet trade network and insights from US imports of exotic pets. BioScience 2021, 71, 977–990. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. Literature Review on the Welfare Implications of Declawing of Domestic Cats; American Veterinary Medical Association: Schaumburg, IL, USA, 2019; pp. 1–11. [Google Scholar]
- Swinderski, J. Onychectomy and its alternatives in the feline patient. Clin. Tech. Small Anim. Pract. 2002, 17, 158–161. [Google Scholar] [CrossRef]
- Clark, K.; Bailey, T.; Rist, P.; Matthews, A. Comparison of 3 methods of onychectomy. Can. Vet. J. La Rev. Vet. Can. 2014, 55, 255–262. [Google Scholar]
- Fowler, M.E.; McDonald, S.E. Untoward effects of onychectomy in wild felids and ursids. J. Am. Vet. Med. Assoc. 1982, 181, 1242–1245. [Google Scholar] [PubMed]
- Martens, L.L.; Piersanti, S.J.; Berger, A.; Kida, N.A.; Deutsch, A.R.; Bertok, K.; Humphries, L.; Lassiter, A.; Hartstone-Rose, A. The effects of onychectomy (declawing) on antebrachial myology across the full body size range of exotic species of Felidae. Animals 2023, 13, 2462. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Bain, M.; DePorter, T.; Beck, A.; Grassi, V.; Landsberg, G. Owner observations regarding cat scratching behavior: An internet-based survey. J. Feline Med. Surg. 2016, 18, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Divers, S.M. Exotic felid medicine. In Proceedings of the American Association of Zoo Veterinarians Conference, Los Angeles, CA, USA, 11–17 January 2008. [Google Scholar]
- Becker, C.; Raadschelders, J. Ohio’s Dangerous Wild Animal Act of 2012: Enactment, Implementation and Evaluation; Columbia University Press: New York, NY, USA, 2014. [Google Scholar]
- City the Kitty. Declawing a Cat, Dog, or Kinkajou Is Always an Amputation Surgery. Available online: https://citythekitty.org/declawing-a-cat-dog-or-kinkajou-is-always-an-amputation-surgery/#:~:text=The%20owner%20said%20in%20a,would%20%E2%80%9Cinadvertently%E2%80%9D%20claw%20her. (accessed on 9 June 2024).
- Carolina Tiger Rescue. Kinkajous. Available online: https://carolinatigerrescue.org/newsroom/kinkajous/ (accessed on 9 June 2024).
- Martell-Moran, N.K.; Solano, M.; Townsend, H.G. Pain and adverse behavior in declawed cats. J. Feline Med. Surg. 2018, 20, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Fritscher, S.J.; Ha, J. Declawing has no effect on biting behavior but does affect adoption outcomes for domestic cats in an animal shelter. Appl. Anim. Behav. Sci. 2016, 180, 107–113. [Google Scholar] [CrossRef]
- Conrad, J.; Wendelburg, K.; Santinelli, S.; Park, A. Deleterious effects of onychectomy (Declawing) in exotic felids and a reparative surgical technique: A preliminary report. In Proceedings of the 35th Conference of the American Association of Zoo Veterinarians, Milwaukee, WI, USA, 5–10 October 2002; pp. 5–10. [Google Scholar]
- Cisneros, A.; Litwin, D.; Niel, L.; Stellato, A.C. Unwanted scratching behavior in cats: Influence of management strategies and cat and owner characteristics. Animals 2022, 12, 2551. [Google Scholar] [CrossRef]
- PETA. Cat-Friendly Cities, States, and Countries where Declawing Is Illegal. Available online: https://www.peta.org/blog/where-declawing-is-illegal/#:~:text=U.S.%20States%20With%20Declawing%20Bans,and%20New%20York%20(2019) (accessed on 25 November 2023).
- Waite, C.J. Starting from scratch: A study in claws and clauses in cat declaw legislation. Drake L. Rev. 2021, 69, 675. [Google Scholar]
- Conrad, J. Milestones—The Paw Project’s Role Advocating Anti-Declawing Legislation. 2014. Available online: https://pawproject.org/legislation/ (accessed on 3 December 2023).
- Wang, M.; Song, Y.; Baker, J.S.; Fekete, G.; Ugbolue, U.C.; Li, S.; Gu, Y. The biomechanical characteristics of a feline distal forelimb: A finite element analysis study. Comput. Biol. Med. 2021, 129, 104174. [Google Scholar] [CrossRef]
- Honeycutt, C.F.; Nichols, T.R. The mechanical actions of muscles predict the direction of muscle activation during postural perturbations in the cat hindlimb. J. Neurophysiol. 2014, 111, 900–907. [Google Scholar] [CrossRef]
- Hubbard, C.; Naples, V.; Ross, E.; Carlon, B. Comparative analysis of paw pad structure in the clouded leopard (Neofelis nebulosa) and domestic cat (Felis catus). Anat. Rec. 2009, 292, 1213–1228. [Google Scholar] [CrossRef]
- Gonyea, W.; Ashworth, R. The form and function of retractile claws in the Felidae and other representative carnivorans. J Morphol. 1975, 145, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J. Bassariscus and probassariscus (Mammalia, Carnivora, Procyonidae) from the early barstovian (middle miocene). J. Vertebr. Paleontol. 2004, 24, 709–720. [Google Scholar] [CrossRef]
- Decker, D.M.; Wozencraft, W.C. Phylogenetic analysis of recent procyonid genera. J. Mammal. 1991, 72, 42–55. [Google Scholar] [CrossRef]
- Koepfli, K.-P.; Gompper, M.E.; Eizirik, E.; Ho, C.-C.; Linden, L.; Maldonado, J.E.; Wayne, R.K. Phylogeny of the Procyonidae (Mammalia: Carnivora): Molecules, morphology and the great american interchange. Mol. Phylogenetics Evol. 2007, 43, 1076–1095. [Google Scholar] [CrossRef] [PubMed]
- Julien-Laferrière, D. Frugivory and seed dispersal by Kinkajous. In Nouragues: Dynamics and Plant-Animal Interactions in a Neotropical Rainforest; Bongers, F., Charles-Dominique, P., Forget, P.-M., Théry, M., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 217–226. [Google Scholar]
- Lemelin, P.; Cartmill, M. The effect of substrate size on the locomotion and gait patterns of the kinkajou (Potos flavus). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 157–168. [Google Scholar] [CrossRef]
- Jenkins, F.A., Jr.; McClearn, D. Mechanisms of hind foot reversal in climbing mammals. J. Morphol. 1984, 182, 197–219. [Google Scholar] [CrossRef]
- Sustaita, D.; Pouydebat, E.; Manzano, A.; Abdala, V.; Hertel, F.; Herrel, A. Getting a grip on tetrapod grasping: Form, function, and evolution. Biol. Rev. 2013, 88, 380–405. [Google Scholar] [CrossRef]
- Convention on International Trade in Endangered Species of Wild Fauna and Flora. Potos flavus. Available online: https://cites.org/eng/taxonomy/term/591 (accessed on 9 June 2024).
- Drouet, M. Detailed Discussion of Exotic Pet Laws Update. Available online: https://www.animallaw.info/article/detailed-discussion-exotic-pet-laws-update#id-2 (accessed on 9 June 2024).
- Harrington, L.A. International commercial trade in live carnivores and primates 2006–2012: Response to Bush et al. 2014. Conserv. Biol. 2015, 29, 293–296. [Google Scholar] [CrossRef]
- World Population Review. Kinkajous Legality by State 2024. Available online: https://worldpopulationreview.com/state-rankings/kinkajous-legality-by-state (accessed on 5 May 2024).
- McCleod, L. Should You Keep a Kinkajou as a Pet? Available online: https://www.thesprucepets.com/kinkajous-as-pets-1237227 (accessed on 20 January 2024).
- Hess, L. Exotic animals: Appropriately owned pets or inappropriately kept problems? J. Avian Med. Surg. 2011, 25, 50–56, 57. [Google Scholar] [CrossRef]
- Kinkatopia. Interested in a Kink? Available online: https://www.kinkatopia.org/kinksarenotpets (accessed on 20 January 2024).
- Noah, P. Kinkajou Potos flavus. Available online: https://www.projectnoah.org/spottings/8937379 (accessed on 25 November 2023).
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria. 2017, Sixth Edition. Available online: https://www.vetmed.uni-leipzig.de/fileadmin/Fakult%C3%A4t_VMF/Institut_Veterin%C3%A4r-Anatomisches/Dokumente/NAV_6th-Edition-2017.pdf (accessed on 19 August 2024).
- Facer, T.; Townsend, K.E.B.; Adrian, B. Anatomy of the Forelimb of the Kinkajou (Potos flavus): A Functional and Comparative Overview across Procyonidae and Other Arboreal Mammals; Midwestern University: Glendale, Arizona, 2017. [Google Scholar]
- Fields, S.; Smith, H.F. Hindlimb myology of the kinkajou (Potos flavus). FASEB J. 2019, 33, 615.3. [Google Scholar] [CrossRef]
- Leonard, K.C.; Worden, N.; Boettcher, M.L.; Dickinson, E.; Hartstone-Rose, A. Effects of freezing and short-term fixation on muscle mass, volume and density. Anat. Rec. 2022, 305, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hartstone-Rose, A.; Perry, J.M.G.; Morrow, C.J. Bite force estimation and the fiber architecture of felid masticatory muscles. Anat. Rec. 2012, 295, 1336–1351. [Google Scholar] [CrossRef] [PubMed]
- Leonard, K.C.; Worden, N.; Boettcher, M.L.; Dickinson, E.; Omstead, K.M.; Burrows, A.M.; Hartstone-Rose, A. Anatomical and ontogenetic influences on muscle density. Sci. Rep. 2021, 11, 2114. [Google Scholar] [CrossRef] [PubMed]
- Hartstone-Rose, A.; Dickinson, E.; Deutsch, A.R.; Worden, N.; Hirschkorn, G.A. Masticatory muscle architectural correlates of dietary diversity in Canidae, Ursidae, and across the order Carnivora. Anat. Rec. 2021, 305, 477–497. [Google Scholar] [CrossRef]
- Hartstone-Rose, A.; Hertzig, I.; Dickinson, E. Bite force and masticatory muscle architecture adaptations in the dietarily diverse Musteloidea (Carnivora). Anat. Rec. 2019, 302, 2287–2299. [Google Scholar] [CrossRef]
- Boettcher, M.L.; Leonard, K.C.; Dickinson, E.; Aujard, F.; Herrel, A.; Hartstone-Rose, A. The forearm musculature of the gray mouse lemur (Microcebus murinus): An ontogenetic study. Anat. Rec. 2020, 303, 1354–1363. [Google Scholar] [CrossRef]
- Herrel, A.; De Smet, A.; Aguirre, L.F.; Aerts, P. Morphological and mechanical determinants of bite force in bats: Do muscles matter? J. Exp. Biol. 2008, 211, 86–91. [Google Scholar] [CrossRef]
- Boettcher, M.L.; Leonard, K.C.; Dickinson, E.; Herrel, A.; Hartstone-Rose, A. Extraordinary grip strength and specialized myology in the hyper-derived hand of Perodicticus potto? J. Anat. 2019, 235, 931–939. [Google Scholar] [CrossRef]
- Schumacher, G.-H. Funktionelle Morphologie der Kaumuskulatur; G. Fischer: Schaffhausen. Switzerland, 1961; Volume 262. [Google Scholar]
- Dickinson, E.; Boettcher, M.L.; Smith, M.R.; Worden, N.A.; Swindell, S.R.; Seelye, J.S.; Pastor, F.; Hartstone-Rose, A. Myological variation in the forearm anatomy of Callitrichidae and Lemuridae. J. Anat. 2021, 239, 669–681. [Google Scholar] [CrossRef]
- Leischner, C.L.; Crouch, M.; Allen, K.L.; Marchi, D.; Pastor, F.; Hartstone-Rose, A. Scaling of primate forearm muscle architecture as it relates to locomotion and posture. Anat. Rec. 2018, 301, 484–495. [Google Scholar] [CrossRef]
- Perry, J.M.G.; Hartstone-Rose, A.; Wall, C.E. The jaw adductors of strepsirrhines in relation to body size, diet, and ingested food size. Anat. Rec. 2011, 294, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A.R.; Dickinson, E.; Leonard, K.C.; Pastor, F.; Muchlinski, M.N.; Hartstone-Rose, A. Scaling of anatomically derived maximal bite force in primates. Anat. Rec. 2020, 303, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Rasch, P.J. The problem of muscle hypertrophy: A review. J. Am. Osteopath Assoc. 1955, 54, 525–528. [Google Scholar] [PubMed]
- Erskine, R.M.; Fletcher, G.; Folland, J.P. The contribution of muscle hypertrophy to strength changes following resistance training. Eur. J. Appl. Physiol. 2014, 114, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Ahtiainen, J.P.; Walker, S.; Peltonen, H.; Holviala, J.; Sillanpää, E.; Karavirta, L.; Sallinen, J.; Mikkola, J.; Valkeinen, H.; Mero, A.; et al. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age 2016, 38, 10. [Google Scholar] [CrossRef]
- Deutsch, A.R.; Berger, A.; Martens, L.L.; Witt, B.R.; Smith, R.L.J.; Hartstone-Rose, A. Myological and osteological approaches to gape and bite force reconstruction in Smilodon fatalis. Anat. Rec. 2024, 1–18. [Google Scholar] [CrossRef]
- Hudson, P.E.; Corr, S.A.; Payne-Davis, R.C.; Clancy, S.N.; Lane, E.; Wilson, A.M. Functional anatomy of the cheetah (Acinonyx jubatus) forelimb. J. Anat. 2011, 218, 375–385. [Google Scholar] [CrossRef]
- Goto, M.; Kawai, M.; Nakata, M.; Itamoto, K.; Miyata, H.; Ikebe, Y.; Tajima, T.; Wada, N. Distribution of muscle fibers in skeletal muscles of the cheetah (Acinonyx jubatus). Mamm. Biol. 2013, 78, 127–133. [Google Scholar] [CrossRef]
- Meachen-Samuels, J.; Van Valkenburgh, B. Forelimb indicators of prey-size preference in the Felidae. J. Morphol. 2009, 270, 729–744. [Google Scholar] [CrossRef]
- Bryant, H.N.; Russell, A.P.; Laroiya, R.; Powell, G.L. Claw retraction and protraction in the Carnivora: Skeletal microvariation in the phalanges of the Felidae. J. Morphol. 1996, 229, 289–308. [Google Scholar] [CrossRef]
- Russell, A.P.; Bryant, H.N. Claw retraction and protraction in the Carnivora: The cheetah (Acinonyx jubatus) as an atypical felid. J. Zool. 2001, 254, 67–76. [Google Scholar] [CrossRef]
- Cartmill, M. Climbing. In Functional Vertebrate Morphology; Harvard University Press: Cambridge, MA, USA; London, UK, 1985; pp. 73–88. [Google Scholar]
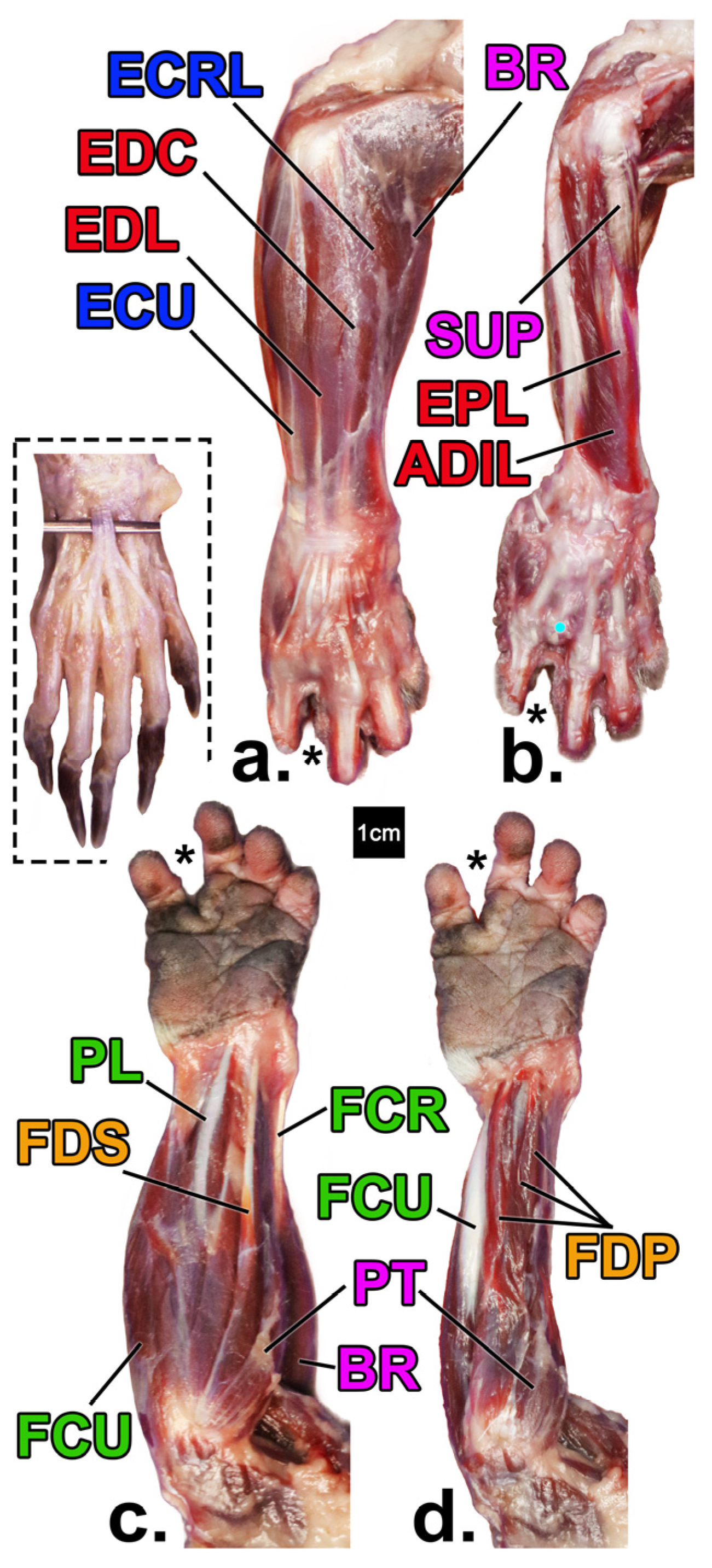
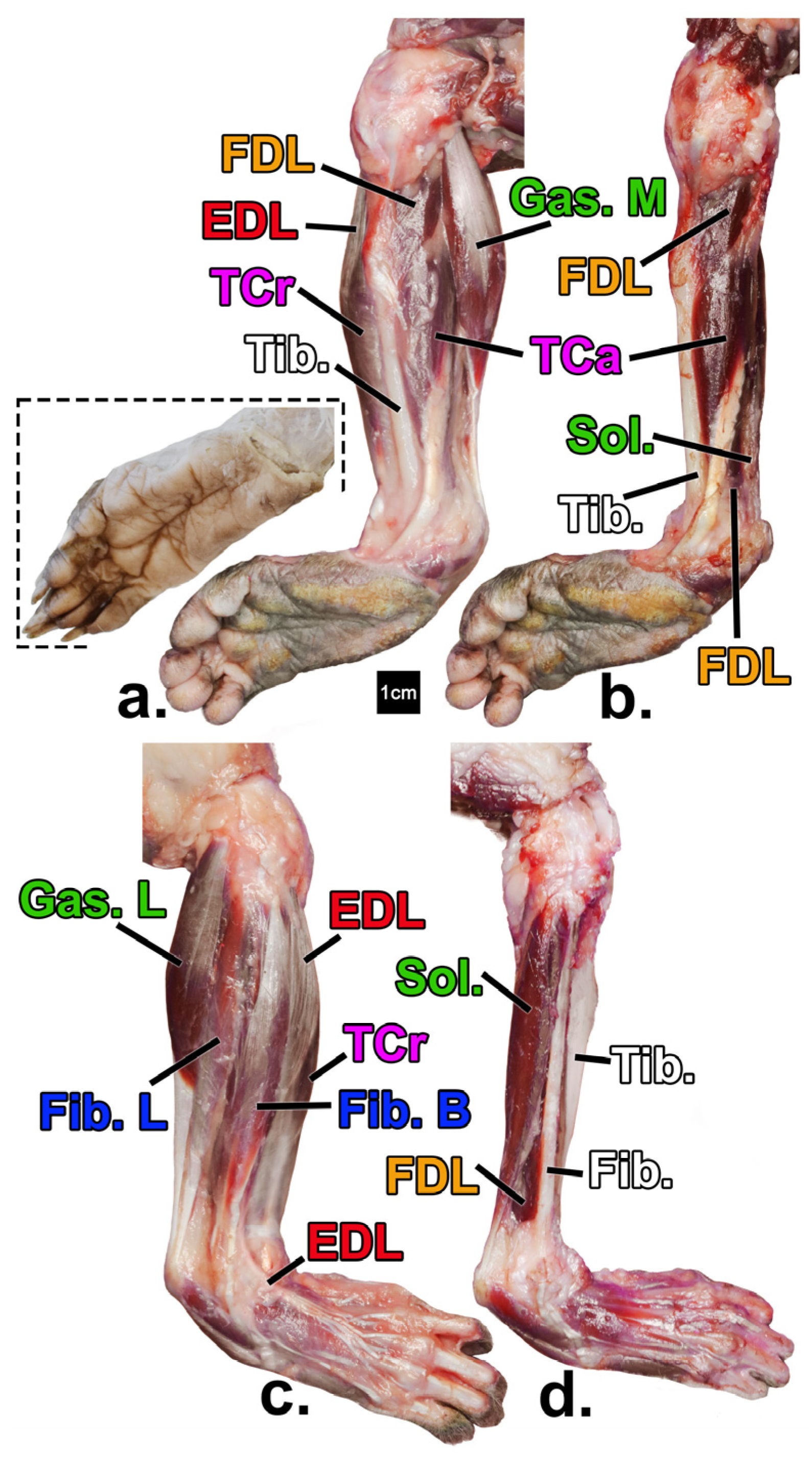
| Limb Segment | Broad Functional Group | Functional Subgroup | Muscle Name | Muscle Abbreviation |
|---|---|---|---|---|
| Forearm | Flexors (Forearm) | Wrist Flexors | Flexor carpi radialis | FCR |
| Flexor carpi ulnaris | FCU | |||
| Palmaris longus | PL | |||
| Digital Flexors | Flexor digitorum superficialis | FDS | ||
| Flexor digitorum profundus | FDP | |||
| Abductor digiti I longus | ADIL | |||
| Extensors (Forearm) | Wrist Extensors | Extensor carpi ulnaris | ECU | |
| Extensor carpi radialis longus | ECRL | |||
| Extensor carpi radialis brevis | ECRB | |||
| Digital Extensors a | a | a | ||
| Other | Brachioradialis | BR | ||
| Supinator | Sup. | |||
| Pronator teres | PT | |||
| Pronator quadratus | PQ | |||
| Leg | Plantar Flexors | Non-Digital Plantar Flexors | Gastrocnemius medialis | Gas. M |
| Gastrocnemius lateralis | Gas. L | |||
| Plantaris | Plant. | |||
| Soleus | Sol. | |||
| Digital Flexors | Flexor digiti I longus | FDIL | ||
| Flexor digitorum longus | FDL | |||
| Flexor digitorum brevis | FDB | |||
| Evertors | Fibularis brevis | Fib. B | ||
| Fibularis longus | Fib. L | |||
| Invertors | Tibialis caudalis | TCa | ||
| Dorsiflexors | Tibialis cranialis | TCr | ||
| Digital Extensors a | a | a |
| Relative MM | Relative PCSA | Relative FL | ||||
|---|---|---|---|---|---|---|
| Kinkajous | Felids | Kinkajous | Felids | Kinkajous | Felids | |
| Total Forearm | 1.06 | 0.94 | 1.06 | 0.86 * | 1.00 | 1.07 |
| Total Flexors | 1.04 | 0.92 * | 1.00 | 0.83 * | 1.10 | 1.03 |
| Total Extensors | 1.09 | 0.97 | 1.11 | 0.90 | 1.03 | 1.23 |
| Wrist Flexors | 1.04 | 1.06 | 1.10 | 1.03 | 0.87 | 1.22 |
| Digital Flexors | 1.04 | 0.85 * | 0.93 | 0.65 * | 1.20 | 1.24 |
| Wrist Extensors | 1.21 | 0.99 | 1.18 | 0.95 | 1.35 | 1.20 |
| Digital Extensors | 0.93 | 0.97 | 0.98 | 0.85 * | 0.89 | 1.23 |
| FCR | 0.88 | 1.02 | 0.88 | 0.98 | 0.92 | 1.11 |
| FCU | 0.90 | 0.98 | 0.96 | 0.98 | 0.82 | 1.05 |
| PL | 2.09 | 0.96 | 2.71 | 0.85 | 1.10 | 1.22 |
| FDS | 1.07 | 0.63 * | 0.89 | 0.47 * | 1.48 | 1.17 |
| FDP | 1.01 | 0.90 * | 0.91 | 0.73 * | 1.21 | 1.38 |
| ADIL | 1.32 | b | 1.21 | b | 1.00 | b |
| ECU | 1.18 | 0.96 | 1.24 | 0.89 | 1.06 | 1.12 |
| ECRL | 1.31 | 0.86 * | 1.45 | 0.76 | 0.79 | 1.35 |
| ECRB | 1.15 | 1.15 | 0.87 | 0.94 | 2.00 | 1.71 |
| BR | 1.04 | 0.93 | 1.01 | 0.84 | 1.09 | 1.15 |
| Sup. | 1.06 | 0.89 * | 1.13 | 0.79 * | 0.88 | 1.11 |
| PT | 1.10 | 1.04 | 1.12 | 0.96 | 1.07 | 1.23 |
| PQ | 0.69 | 1.01 | 0.91 | 1.02 | 0.43 | 1.01 |
| Relative MM | Relative PCSA | Relative FL | |
|---|---|---|---|
| Total Leg | 1.01 | 1.09 | 0.87 |
| Plantar Flexors | 1.01 | 1.12 | 0.86 |
| Dorsiflexors | 0.98 | 1.01 | 0.92 |
| Non-Digital Plantar Flexors | 0.98 | 1.07 | 0.82 |
| Digital Flexors | 1.08 | 1.21 | 0.95 |
| Evertor | 0.96 | 1.02 | 0.81 |
| Invertors | 1.00 | 1.08 | 0.83 |
| Digital Extensors | 1.03 | 0.97 | 1.17 |
| Gas. M | 0.98 | 1.00 | 0.94 |
| Gas. L | 0.90 | 1.00 | 0.72 |
| Plant. | 0.97 | 0.97 | 0.94 |
| Sol. | 0.81 | 0.85 | 0.76 |
| FDIL | 1.06 | 1.28 | 0.74 |
| FDL | 0.94 | 0.94 | 0.91 |
| FDB | 1.04 | 1.07 | 0.98 |
| Fib. B | 0.84 | 0.79 | 0.96 |
| Fib. L | 1.02 | 1.16 | 0.74 |
| TCa | 1.35 | 1.57 | 1.00 |
| TCr | 0.97 | 1.03 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, L.L.; Brown, R.A.; Faillace, A.C.L.; Berger, A.; Smith, R.L.J.; Bertok, K.; Humphries, L.; Lassiter, A.; Hartstone-Rose, A. The Effects of Onychectomy (Declawing) on Forearm and Leg Myology in a Kinkajou (Potos flavus). Animals 2024, 14, 2774. https://doi.org/10.3390/ani14192774
Martens LL, Brown RA, Faillace ACL, Berger A, Smith RLJ, Bertok K, Humphries L, Lassiter A, Hartstone-Rose A. The Effects of Onychectomy (Declawing) on Forearm and Leg Myology in a Kinkajou (Potos flavus). Animals. 2024; 14(19):2774. https://doi.org/10.3390/ani14192774
Chicago/Turabian StyleMartens, Lara L., Reece A. Brown, Ana Carolina Lourenço Faillace, Arin Berger, Rachel L. J. Smith, Kathryn Bertok, Lauren Humphries, Angela Lassiter, and Adam Hartstone-Rose. 2024. "The Effects of Onychectomy (Declawing) on Forearm and Leg Myology in a Kinkajou (Potos flavus)" Animals 14, no. 19: 2774. https://doi.org/10.3390/ani14192774
APA StyleMartens, L. L., Brown, R. A., Faillace, A. C. L., Berger, A., Smith, R. L. J., Bertok, K., Humphries, L., Lassiter, A., & Hartstone-Rose, A. (2024). The Effects of Onychectomy (Declawing) on Forearm and Leg Myology in a Kinkajou (Potos flavus). Animals, 14(19), 2774. https://doi.org/10.3390/ani14192774





