Effects of the Interaction between Dietary Vitamin D3 and Vitamin K3 on Growth, Skeletal Anomalies, and Expression of Bone and Calcium Metabolism-Related Genes in Juvenile Gilthead Seabream (Sparus aurata)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Proximate Composition
2.2. Juvenile Rearing
2.3. Sample Collection and Analyses
2.4. Growth Performance
2.5. Serum Calcitriol
2.6. Vertebral Mineral Composition
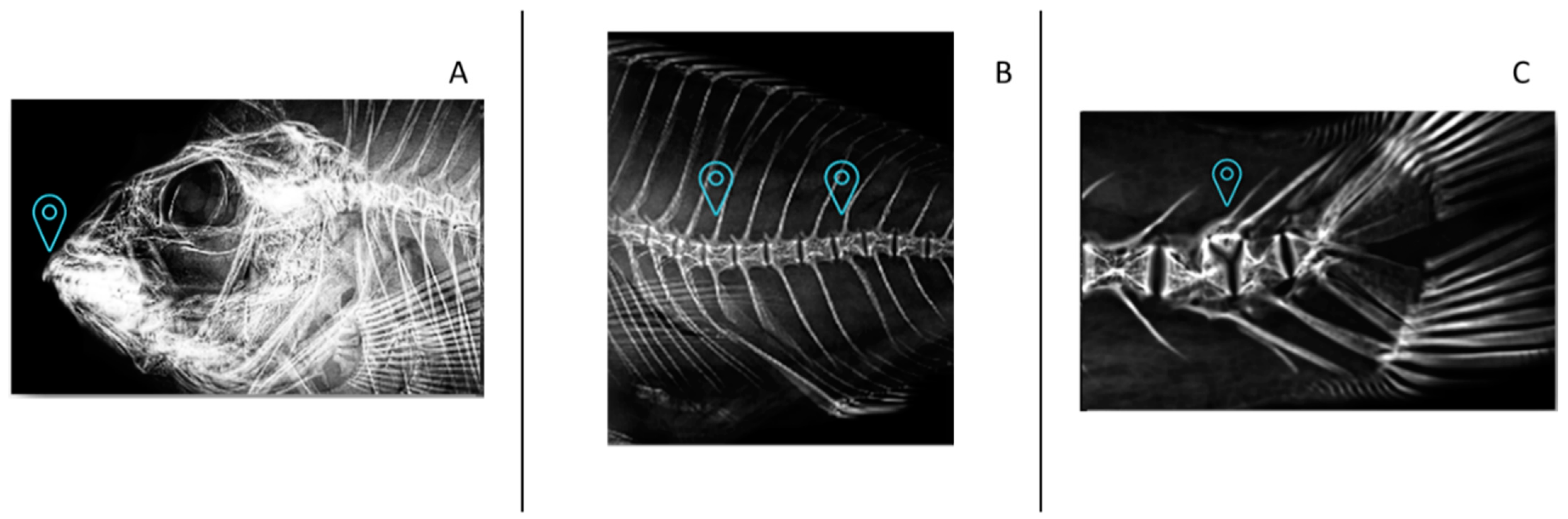
2.7. Skeletal Anomalies
2.8. Morphometric Analysis
2.9. Gene Expression
2.10. Statistical Analysis
3. Results
3.1. Experimental Diets and Proximate Composition
3.2. Growth Performance
3.3. Serum Calcitriol
3.4. Vertebral Mineral Composition
3.5. Skeletal Anomalies
3.6. Morphometric Analysis
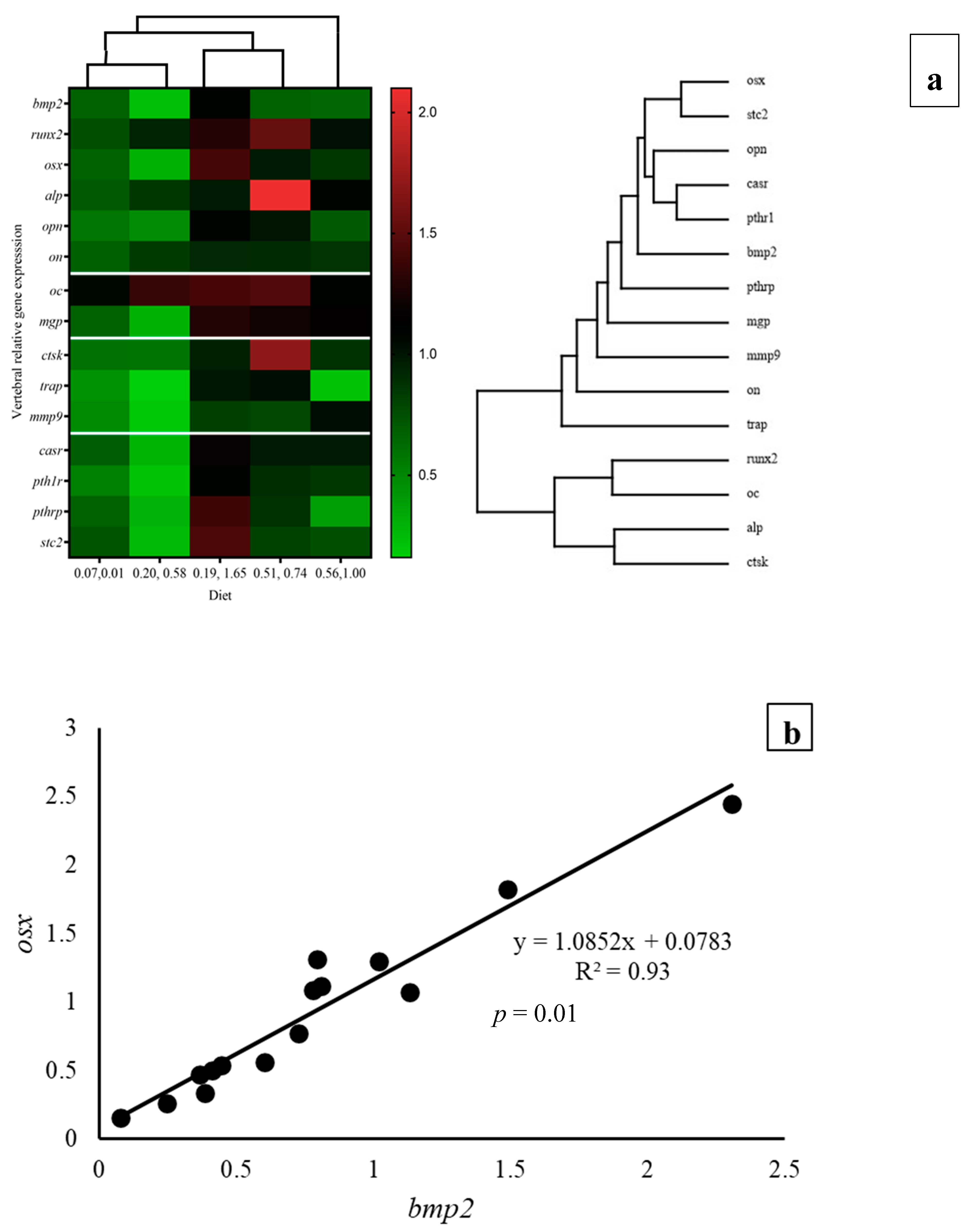
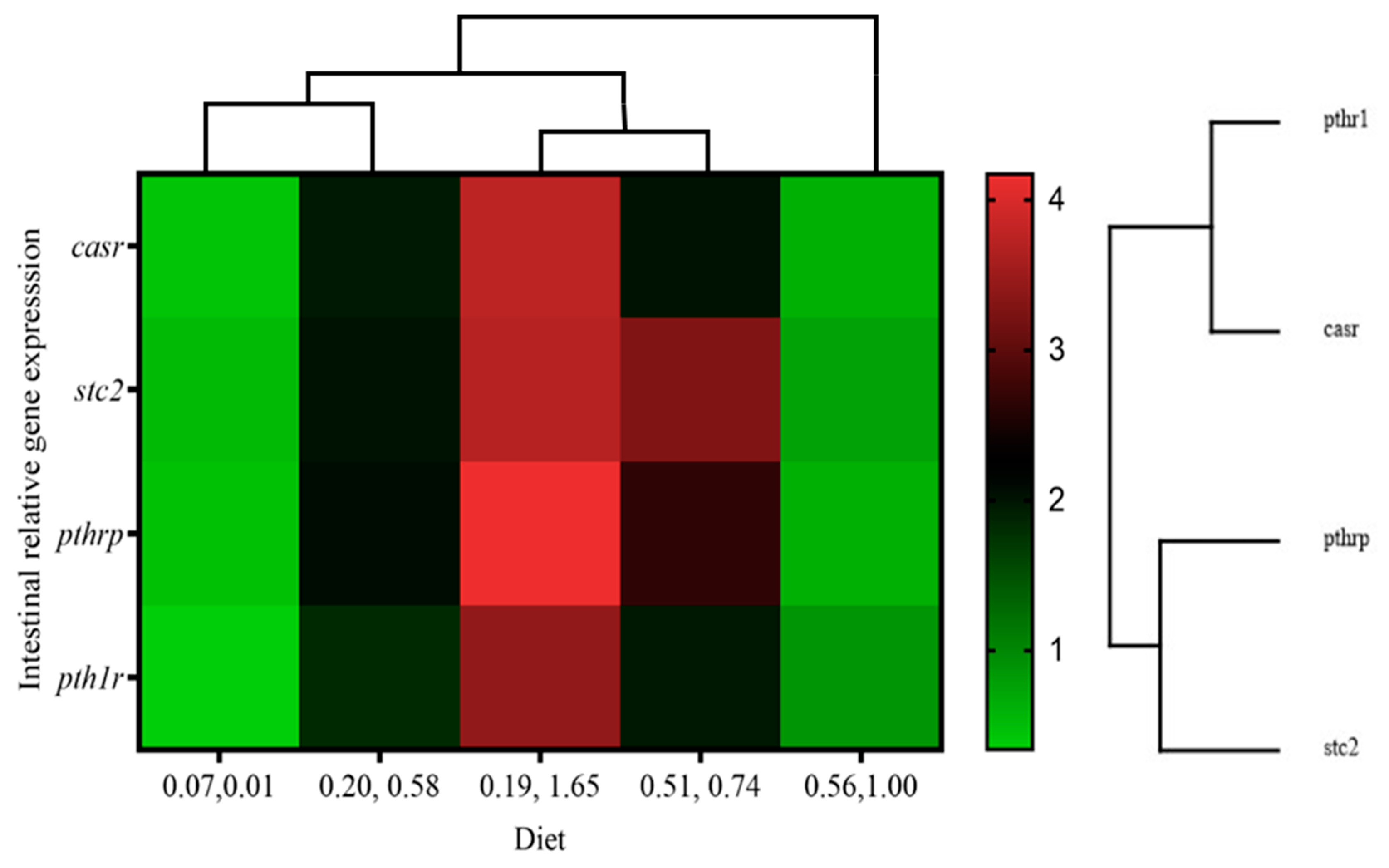
3.7. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang, X.; Liu, C.; Guo, X.; Li, K.; Deng, Q.; Li, D. The Combination Effect of Vitamin K and Vitamin D on Human Bone Quality: A Meta-Analysis of Randomized Controlled Trials. Food Funct. 2020, 11, 3280–3297. [Google Scholar] [CrossRef] [PubMed]
- Ballegooijen; Cepelis, A.; Visser, M.; Brouwer, I.A.; Van Schoor, N.M.; Beulens, J.W. Joint Association of Low Vitamin D and Vitamin K Status with Blood Pressure and Hypertension. Hypertension 2017, 69, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.J.; WaagbØ, R.; Wendelaar Bonga, S.; Flik, G. The Significance of Vitamin D for Fish: A Review. Aquac. Nutr. 2010, 16, 100–116. [Google Scholar] [CrossRef]
- Pierens, S.L.; Fraser, D.R. The origin and metabolism of vitamin D in rainbow trout. J. Steroid Biochem. Mol. Biol. 2015, 145, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, D.; Castro, P.; Lall, S.; Montero, D.; Zamorano, M.J.; Fontanillas, R.; Izquierdo, M. Effects of Menadione Sodium Bisulphite (Vitamin K3) Supplementation of the Diets Based on Plant Feed Ingredients on Growth and Bone Health of Gilthead Seabream (Sparus aurata) Fingerlings. Aquac. Nutr. 2022, 2022, 1613030. [Google Scholar] [CrossRef]
- Takeuchi, A.; Tanda, M. Possible origin of extremely high contents of vitamin D3 in some kinds of fish liver. Comp. Biochem. Physiol. A Physiol. 1991, 100, 483–487. [Google Scholar]
- Norman, A.W. Minireview: Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Haga, Y.; Takeuchi, T.; Murayama, Y.; Ohta, K. Vitamin D3 compounds induce hypermelanosis on the blind side and vertebral deformity in juvenile Japanese flounder Paralichthys olivaceus. Fish. Sci. 2004, 70, 59–67. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D in the Context of Evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef]
- Kollitz, E.M.; Hawkins, M.B.; Whitfield, G.K.; Kullman, S.W. Functional Diversification of Vitamin D Receptor Paralogs in Teleost Fish after a Whole Genome Duplication Event. Endocrinology 2014, 155, 4641–4654. [Google Scholar] [CrossRef]
- Kidd, P.M. Vitamins D and K as Pleiotropic Nutrients: Clinical Importance to the Skeletal and Cardiovascular Systems and Preliminary Evidence for Synergy. Altern. Med. Rev. 2010, 15, 199–222. [Google Scholar] [PubMed]
- Darias, M.J.; Lan Chow Wing, O.; Cahu, C.; Zambonino-Infante, J.L.; Mazurais, D. Double Staining Protocol for Developing European Sea Bass (Dicentrarchus labrax) Larvae. J. Appl. Ichthyol. 2010, 26, 280–285. [Google Scholar] [CrossRef]
- Fleming, A.; Sato, M.; Goldsmith, P. High-Throughput in Vivo Screening for Bone Anabolic Compounds with Zebrafish. J. Biomol. Screen. 2005, 10, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Parra, E.; Rojas-Rivera, J.; Tuñón, J.; Praga, M.; Ortiz, A.; Egido, J. Vitamin D Receptor Activation and Cardiovascular Disease. Nephrol. Dial. Transplant. 2012, 27, 17–21. [Google Scholar] [CrossRef]
- Bouillon, R.; Gielen, E.; Vanderschueren, D. Vitamin D Receptor and Vitamin D Action in Muscle. Endocrinology 2014, 155, 3210–3213. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Sitrin, M.D. Vitamin D’s Role in Cell Proliferation and Differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef]
- Yuan, J.; Guo, X.; Liu, Z.; Zhao, X.; Feng, Y.; Song, S.; Cui, C.; Jiang, P. Vitamin D Receptor Activation Influences the ERK Pathway and Protects against Neurological Deficits and Neuronal Death. Int. J. Mol. Med. 2018, 41, 364–372. [Google Scholar] [CrossRef]
- Sivagurunathan, U.; Srivastava, P.P.; Gupta, S.; Krishna, G. Responses of Corpuscles of Stannius to Intra-Peritoneal Vitamin-D3 Administration in Teleost Labeo Rohita (Hamilton, 1822) Reared in Water with Two Different Levels of Calcium Concentration. Saudi J. Biol. Sci. 2020, 27, 3593–3600. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Wang, Y.; Wang, C.; Li, J. Effects of the Supplementation of Vitamin D3 on the Growth and Vitamin D Metabolites in Juvenile Siberian Sturgeon (Acipenser baerii). Fish Physiol. Biochem. 2017, 43, 901–909. [Google Scholar] [CrossRef]
- Pierson, P.M.; Lamers, A.; Flik, G.; Mayer-Gostan, N. The Stress Axis, Stanniocalcin, and Ion Balance in Rainbow Trout. Gen. Comp. Endocrinol. 2004, 137, 263–271. [Google Scholar] [CrossRef]
- Seale, A.P.; Fiess, J.C.; Hirano, T.; Cooke, I.M.; Grau, E.G. Disparate Release of Prolactin and Growth Hormone from the Tilapia Pituitary in Response to Osmotic Stimulation. Gen. Comp. Endocrinol. 2006, 145, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Abbink, W.; Flik, G. Parathyroid Hormone-Related Protein in Teleost Fish. Gen. Comp. Endocrinol. 2007, 152, 243–251. [Google Scholar] [CrossRef]
- Guerreiro, P.M.; Renfro, J.L.; Power, D.M.; Canario, A.V.M. The Parathyroid Hormone Family of Peptides: Structure, Tissue Distribution, Regulation, and Potential Functional Roles in Calcium and Phosphate Balance in Fish. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R679–R696. [Google Scholar] [CrossRef] [PubMed]
- Swarup, K.; Das, V.K.; Norman, A.W. Dose-Dependent Vitamin D3 and 1, 25-Dihydrxyvitamin D3 Induced Hypercalcemia and Hyperphosphatemia in Male Cyprinoid Cyprinus Carpio. Comp. Biochem. Physiol. A 1991, 100, 445–447. [Google Scholar] [CrossRef]
- Rao, D.S.; Raghuramulu, N. Lack of vitamin D3 synthesis in Tilapia mossambica from cholesterol and acetate. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1996, 114, 21–25. [Google Scholar]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Eryalçın, K.M.; Roo, J.; Boglione, C.; Prabhu, P.A.J.; Izquierdo, M. Effects of dietary vitamin D3 levels on survival, mineralization, and skeletal development of gilthead seabream (Sparus aurata) larvae. Aquaculture 2022, 560, 738505. [Google Scholar] [CrossRef]
- Sundell, K.; Norman, A.W.; Björnsson, B.T. 1,25(OH)2 Vitamin D3 Increases Ionized Plasma Calcium Concentrations in the Immature Atlantic Cod Gadus morhua. Gen. Comp. Endocrinol. 1993, 91, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Liu, J.; Lan, Y.; Liao, X.; Zhang, J.; Xu, W.; Mai, K.; Ai, Q.; Wan, M. Vitamin D impacts on the intestinal health, immune status and metabolism in turbot (Scophthalmus maximus L.). Br. J. Nutr. 2022, 128, 2083–2096. [Google Scholar] [CrossRef]
- Rao, D.S.; Raghuramulu, N. Vitamin D3 and Its Metabolites Have No Role in Calcium and Phosphorus Metabolism in Tilapia Mossambica. J. Nutr. Sci. Vitaminol. 1999, 45, 9–19. [Google Scholar] [CrossRef]
- Vielma, J.; Lall, S.P.; Koskela, J. Effects of Dietary Phytase and Cholecalciferol on Phosphorus Bioavailability in Rainbow Trout ž Oncorhynchus Mykiss. Aquaculture 1998, 163, 309–323. [Google Scholar] [CrossRef]
- Sundell, K.; Bishop, J.E.; Björnsson, B.T.; Norman, A.W. 1, 25-Dihydroxyvitamin D3 in the Atlantic Cod: Plasma Levels, a Plasma Binding Component, and Organ Distribution of a High Affinity Receptor. Endocrinology 1992, 131, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Larsson, D.; Sundell, K. A Specific Binding Moiety for 1,25-Dihydroxyvitamin D3 in Basal Lateral Membranes of Carp Enterocytes. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E614–E621. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.; Björnsson, B.T.; Sundell, K. Physiological Concentrations of 24, 25-Dihydroxyvitamin D3 Rapidly Decrease the in Vitro Intestinal Calcium Uptake in the Atlantic Cod, Gadus morhua. Gen. Comp. Endocrinol. 1995, 100, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Flik, G. Drinking in (Very Young) Fish. Osmoregul. Drink. Vertebr. 2002, 41, 22–30. [Google Scholar]
- Hwang, P.-P.; Lee, T.-H. New Insights into Fish Ion Regulation and Mitochondrion-Rich Cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.A.; Sommer, S.; Sussman, C.R.; Grande, J.P.; Kumar, R. Expression and Regulation of the Vitamin D Receptor in the Zebrafish, Danio Rerio. J. Bone Miner. Res. 2008, 23, 1486–1496. [Google Scholar] [CrossRef]
- He, S.; Ding, M.; Watson Ray, G.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. Effect of Dietary Vitamin D Levels on Growth, Serum Biochemical Parameters, Lipid Metabolism Enzyme Activities, Fatty Acid Synthase and Hepatic Lipase MRNA Expression for Orange-Spotted Grouper (Epinephelus coioides) in Growth Mid-Stage. Aquac. Nutr. 2021, 27, 655–665. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Glynatsi, N.; Christodoulopoulou, S.; Huelvan, C.; Desbruyeres, E.; Le Gall, M.M.; Quazuguel, P.; Cahu, C.L. Dietary Vitamin D3 Affects Digestive System Ontogenesis and Ossification in European Sea Bass (Dicentrachus labrax, Linnaeus, 1758). Aquaculture 2010, 298, 300–307. [Google Scholar] [CrossRef]
- Tan, Q.; He, R.; Xie, S.; Xie, C.; Zhang, S. Effect of Dietary Supplementation of Vitamins A, D3, E, and C on Yearling Rice Field Eel, Monopterus albus: Serum Indices, Gonad Development, and Metabolism of Calcium and Phosphorus. J. World Aquac. Soc. 2007, 38, 146–153. [Google Scholar] [CrossRef]
- Takahashi, H.; Suzuki, N.; Takagi, C.; Ikegame, M.; Yamamoto, T.; Takahashi, A.; Moriyama, S.; Hattori, A.; Sakamoto, T.; Takahashi, H.; et al. Prolactin Inhibits Osteoclastic Activity in the Goldfish Scale: A Novel Direct Action of Prolactin in Teleosts. Zool. Sci. 2008, 25, 739–745. [Google Scholar] [CrossRef]
- Dominguez, D.; Montero, D.; Zamorano, M.J.; Castro, P.; Fontanillas, R.; Antony Jesu Prabhu, P.; Izquierdo, M. Effects of Vitamin D3 Supplementation in Gilthead Seabream (Sparus aurata) Juveniles Fed Diets High in Plant Based Feedstuffs. Aquaculture 2021, 543, 736991. [Google Scholar] [CrossRef]
- Redruello, B.; Estêvão, M.D.; Rotllant, J.; Guerreiro, P.M.; Anjos, L.I.; Canário, A.V.M.; Power, D.M. Isolation and Characterization of Piscine Osteonectin and Downregulation of Its Expression by PTH-related Protein. J. Bone Miner. Res. 2005, 20, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, U.; Srivastava, P.P. Characterization and Partial Sequencing of Calcium Regulating Hormone Stanniocalcin Gene in Freshwater Fish, Labeo rohita (Hamilton, 1822). Natl. Acad. Sci. Lett. 2020, 44, 299–301. [Google Scholar] [CrossRef]
- NRC Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011; ISBN 0309216192.
- Miao, H.; Ge, X.; Xie, J.; Liu, B.; Wang, K.; Zhu, J.; Ren, M.; Zhou, Q.; Pan, L.; Chen, R. Dietary Vitamin D3 Requirement of Wuchang Bream (Megalobrama amblycephala). Aquaculture 2015, 436, 104–109. [Google Scholar]
- Shiau, S.-Y.; Hwang, J.-Y. Vitamin D Requirements of Juvenile Hybrid Tilapia Oreochromis Niloticus* O. Aureus. Nippon. Suisan Gakkaishi Jpn. Ed. 1993, 59, 553–558. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, Q.; Chan, J.; Chen, P.; Wang, C. The Effects of Concurrent Supplementation of Dietary Phytase, Citric Acid and Vitamin D3 on Growth and Mineral Utilization in Juvenile Yellow Cat fish Pelteobagrus fulvidraco. Aquaculture 2015, 436, 143–150. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Simes, D.C.; Laizé, V.; Williamson, M.K.; Price, P.A.; Cancela, M.L. Gla-Rich Protein (GRP), a New Vitamin K-Dependent Protein Identified from Sturgeon Cartilage and Highly Conserved in Vertebrates. J. Biol. Chem. 2008, 283, 36655–36664. [Google Scholar] [CrossRef]
- Oldenburg, J.; Marinova, M.; Müller-Reible, C.; Watzka, M. The Vitamin K Cycle. Vitam. Horm. 2008, 78, 35–62. [Google Scholar]
- Krossøy, C.; Waagbø, R.; Ørnsrud, R. Vitamin K in Fish Nutrition. Aquac. Nutr. 2011, 17, 585–594. [Google Scholar] [CrossRef]
- Alonso, N.; Meinitzer, A.; Fritz-Petrin, E.; Enko, D.; Herrmann, M. Role of Vitamin K in Bone and Muscle Metabolism. Calcif. Tissue Int. 2023, 112, 178–196. [Google Scholar] [CrossRef]
- Emekli-Alturfan, E.; Alturfan, A.A. The Emerging Relationship between Vitamin K and Neurodegenerative Diseases: A Review of Current Evidence. Mol. Biol. Rep. 2023, 50, 815–828. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the Safety and Efficacy of Vitamin K3 (Menadione Sodium Bisulphite and Menadione Nicotinamide Bisulphite) as a Feed Additive for All Animal Species. EFSA J. 2014, 12, 3532. [Google Scholar]
- Krossøy, C.; Ørnsrud, R.; Wargelius, A. Differential Gene Expression of bgp and mgp in Trabecular and Compact Bone of Atlantic Salmon (Salmo salar L.). Vertebrae. J. Anat. 2009, 215, 663–672. [Google Scholar] [CrossRef]
- Graff, I.E.; Krossøy, C.; Gjerdevik, K.; Julshamn, K. Influence of Dietary Menadione Nicotinamide Bisulphite (Vitamin K3) and Phylloquinone (Vitamin K1) on Atlantic Salmon (Salmo salar L.) Tissue Levels, Determined by High-Performance Liquid Chromatography with Fluorescence Detection. Aquac. Nutr. 2010, 16, 637–647. [Google Scholar] [CrossRef]
- Fernández, I.; Gavaia, P.; Darias, M.J.; Gisbert, E. Fat-Soluble Vitamins in Fish: A Transcriptional Tissue-Specific Crosstalk That Remains to Be Unveiled and Characterized; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319732442. [Google Scholar]
- Elshaikh, A.O.; Shah, L.; Joy Mathew, C.; Lee, R.; Jose, M.T.; Cancarevic, I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus 2020, 12, e10816. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Li, W.; Zjawiony, J.K.; Zmijewski, M.A.; Minh, N.; Sweatman, T.; Miller, D.; Slominski, A. NIH Public Access 2009, 275, 2585–2596.
- KrossØy, C.; WaagbØ, R.; Fjelldal, P.G.; Wargelius, A.; Lock, E.J.; Graff, I.E.; Ørnsrud, R. Dietary Menadione Nicotinamide Bisulphite (Vitamin K3) Does Not Affect Growth or Bone Health in First-Feeding Fry of Atlantic Salmon (Salmo salar L.). Aquac. Nutr. 2009, 15, 638–649. [Google Scholar] [CrossRef]
- Beato, S.; Toledo-Solís, F.J.; Fernández, I. Vitamin k in Vertebrates’ Reproduction: Further Puzzling Pieces of Evidence from Teleost Fish Species. Biomolecules 2020, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, M. Studies on Distribution and Physiological Function of Vitamin K in Fish. Bull. Fish. Res. Agen. 2006, 18, 1–40. [Google Scholar]
- Udagawa, M. The Effect of Parental Vitamin K Deficiency on Bone Structure in Mummichog Fundulus heteroclitus. J. World Aquac. Soc. 2004, 35, 366–371. [Google Scholar] [CrossRef]
- Graff, I.E.; Waagbø, R.; Fivelstad, S.; Vermeer, C.; Lie; Lundebye, A.K. A Multivariate Study on the Effects of Dietary Vitamin K, Vitamin D3 and Calcium, and Dissolved Carbon Dioxide on Growth, Bone Minerals, Vitamin Status and Health Performance in Smolting Atlantic Salmon Salmo Salar L. J. Fish Dis. 2002, 25, 599–614. [Google Scholar] [CrossRef]
- Roy, P.K.; Lall, S.P. Vitamin K Deficiency Inhibits Mineralization and Enhances Deformity in Vertebrae of Haddock (Melanogrammus aeglefinus L.). Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2007, 148, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L.; Collaborators, O.R. and Vitamin K–Sources, Physiological Role, Kinetics, Deficiency, Detection, Therapeutic Use, and Toxicity. Nutr. Rev. 2022, 80, 677–698. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Newman, P. Recent Trends in the Metabolism and Cell Biology of Vitamin K with Special Reference to Vitamin K Cycling and MK-4 Biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Zamorano, M.J.; Prabhu, A.J.; Izquierdo, M. Deficiency and Excess in Dietary Vitamin K3 Negatively Affect Gilthead Seabream (Sparus aurata) Larvae Performance and Bone Health. Aquaculture 2023, 574, 739646. [Google Scholar] [CrossRef]
- O’Connor, E.; Moølgaard, C.; Michaelsen, K.F.; Jakobsen, J.; Cashman, K.D. Vitamin D-Vitamin K Interaction: Effect of Vitamin D Supplementation on Serum Percentage Undercarboxylated Osteocalcin, a Sensitive Measure of Vitamin K Status, in Danish Girls. Br. J. Nutr. 2010, 104, 1091–1095. [Google Scholar] [CrossRef]
- Janus, S.E.; Durieux, J.C.; Hajjari, J.; Carneiro, H.; McComsey, G.A. Inflammation-Mediated Vitamin K and Vitamin D Effects on Vascular Calcifications in People with HIV on Active Antiretroviral Therapy. Aids 2022, 36, 647–655. [Google Scholar] [CrossRef]
- Ziemińska, M.; Sieklucka, B.; Pawlak, K. Vitamin k and d Supplementation and Bone Health in Chronic Kidney Disease—Apart or Together? Nutrients 2021, 13, 809. [Google Scholar] [CrossRef]
- Miyake, N.; Hoshi, K.; Sano, Y.; Kikuchi, K.; Tadano, K.; Koshihara, Y. 1, 25-Dihydroxyvitamin D3 Promotes Vitamin K2 Metabolism in Human Osteoblasts. Osteoporos. Int. 2001, 12, 680–687. [Google Scholar] [CrossRef]
- Je, S.H.; Joo, N.-S.; Choi, B.; Kim, K.-M.; Kim, B.-T.; Park, S.-B.; Cho, D.-Y.; Kim, K.-N.; Lee, D.-J. Vitamin K Supplement along with Vitamin D and Calcium Reduced Serum Concentration of Undercarboxylated Osteocalcin While Increasing Bone Mineral Density in Korean Postmenopausal Women over Sixty-Years-Old. J. Korean Med. Sci. 2011, 26, 1093–1098. [Google Scholar] [CrossRef]
- Sanguineti, R.; Monacelli, F.; Parodi, A.; Furfaro, A.L.; Borghi, R.; Pacini, D.; Pronzato, M.A.; Odetti, P.; Molfetta, L.; Traverso, N. Vitamins D3 and K2 May Partially Counterbalance the Detrimental Effects of Pentosidine in Ex Vivo Human Osteoblasts. J. Biol. Regul. Homeost. Agents 2016, 30, 713–726. [Google Scholar]
- Mayer Jr, O.; Seidlerová, J.; Wohlfahrt, P.; Filipovský, J.; Cífková, R.; Černá, V.; Kučerová, A.; Pešta, M.; Fuchsová, R.; Topolčan, O. Synergistic Effect of Low K and D Vitamin Status on Arterial Stiffness in a General Population. J. Nutr. Biochem. 2017, 46, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Zamorano, M.J.; Philip, A.J.P.; Izquierdo, M. Interaction between Dietary Vitamin D3 and Vitamin K3 in Gilthead Seabream Larvae (Sparus aurata) in Relation to Growth and Expression of Bone Development-Related Genes. Aquac. Nutr. 2023, 2023, 3061649. [Google Scholar] [CrossRef]
- FAO Sparus Aurata. Cultured Aquatic Species Information Programme. Text by Colloca, F.; Cerasi, S. Fisheries and Aquaculture Division [Online]. Rome. Updated 2005-05-17. Available online: https://www.fao.org/fishery/en/culturedspecies/sparus_aurata?lang=en (accessed on 4 March 2024).
- Rydmark, H. Replacement of Fish Protein in Fish Feed. 2023. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-s-18590 (accessed on 5 November 2023).
- Saleh, R.; Betancor, M.B.; Roo, J.; Benítez-Santana, T.; Zamorano, M.J.; Izquierdo, M. Biomarkers of Bone Development and Oxidative Stress in Gilthead Sea Bream Larvae Fed Microdiets with Several Levels of Polar Lipids and α-Tocopherol. Aquac. Nutr. 2015, 21, 341–354. [Google Scholar] [CrossRef]
- Riera-Heredia, N.; Vélez, E.J.; Gutiérrez, J.; Navarro, I.; Capilla, E. Gene Expression Analyses in Malformed Skeletal Structures of Gilthead Sea Bream (Sparus aurata). J. Fish Dis. 2019, 42, 1169–1180. [Google Scholar] [CrossRef]
- Riera-Heredia, N.; Martins, R.; Mateus, A.P.; Costa, R.A.; Gisbert, E.; Navarro, I.; Gutiérrez, J.; Power, D.M.; Capilla, E. Temperature Responsiveness of Gilthead Sea Bream Bone; an in Vitro and in Vivo Approach. Sci. Rep. 2018, 8, 11211. [Google Scholar] [CrossRef] [PubMed]
- Rafael, M.S.; Laizé, V.; Cancela, M.L. Identification of Sparus Aurata Bone Morphogenetic Protein 2: Molecular Cloning, Gene Expression and in Silico Analysis of Protein Conserved Features in Vertebrates. Bone 2006, 39, 1373–1381. [Google Scholar] [CrossRef]
- Marques, C.L.; Cancela, M.L.; Laizé, V. Transcriptional Regulation of Gilthead Seabream Bone Morphogenetic Protein (BMP) 2 Gene by Bone- and Cartilage-Related Transcription Factors. Gene 2016, 576, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, X.; Hao, R.; Wang, Q.; Deng, Y.; Sun, R. Effect of Vitamin D3 on Immunity and Antioxidant Capacity of Pearl Oyster Pinctada fucata Martensii after Transplantation: Insights from LC–MS-Based Metabolomics Analysis. Fish Shellfish Immunol. 2019, 94, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Kim, B.S.; Kim, C.S.; Im, G. Il Effects of BMP-2 and Vitamin D3 on the Osteogenic Differentiation of Adipose Stem Cells. Biochem. Biophys. Res. Commun. 2011, 408, 126–131. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Q.Y.; Xu, H.; Wang, C.A.; Shi, L.Y. Effects of Dietary Vitamin D3 Supplementation on Body Composition and Activity of Alkaline Phosphatase in the Serum of Juvenile Mirror Carp (C. Carpio Songpu Mirror Carp). Acta Agric. Boreali-Sin. 2011, 26, 258–263. [Google Scholar]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.M.; Fuentes, J.; Power, D.M.; Ingleton, P.M.; Flik, G.; Canario, A.V.M. Parathyroid Hormone-Related Protein: A Calcium Regulatory Factor in Sea Bream (Sparus aurata L.) Larvae. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.M.; Fuentes, J.; Canario, A.V.M.; Power, D.M. Calcium balance in sea bream (Sparus aurata): The effect of oestradiol-17 beta. J. Endocrinol. 2002, 173, 377–385. [Google Scholar] [CrossRef]
- Guerreiro; Fuentes, J.; Flik, G.; Rotllant, J.; Power, D.M.; Canario, A.V.M. Water Calcium Concentration Modifies Whole-Body Calcium Uptake in Sea Bream Larvae during Short-Term Adaptation to Altered Salinities. J. Exp. Biol. 2004, 207, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D.; Hendy, G.N. The Calcium-Sensing Receptor in Bone—Mechanistic and Therapeutic Insights. Nat. Rev. Endocrinol. 2015, 11, 298–307. [Google Scholar] [CrossRef]
- Boisen, I.M.; Nielsen, J.E.; Verlinden, L.; Lorenzen, M.; Holt, R.; Pinborg, A.; Andreassen, C.H.; Juul, A.; Lanske, B.; Carmeliet, G. Calcium Transport in Male Reproduction Is Possibly Influenced by Vitamin D and CaSR. J. Endocrinol. 2021, 251, 213–228. [Google Scholar] [CrossRef]
- Abbink, W.; Hang, X.M.; Guerreiro, P.M.; Spanings, F.A.T.; Ross, H.A.; Canario, A.V.M.; Flik, G. Parathyroid Hormone-Related Protein and Calcium Regulation in Vitamin D-Deficient Sea Bream (Sparus auratus). J. Endocrinol. 2007, 193, 473–480. [Google Scholar] [CrossRef]
- Dukes, J.P.; Deaville, R.; Gottelli, D.; Neigel, J.E.; Bruford, M.W.; Jordan, W.C. Isolation and Characterisation of Main Olfactory and Vomeronasal Receptor Gene Families from the Atlantic Salmon (Salmo salar). Gene 2006, 371, 257–267. [Google Scholar] [CrossRef]
- Loretz, C.A. Extracellular Calcium-Sensing Receptors in Fishes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 225–245. [Google Scholar] [CrossRef]
- Greenwood, M.P.; Flik, G.; Wagner, G.F.; Balment, R.J. The Corpuscles of Stannius, Calcium-Sensing Receptor, and Stanniocalcin: Responses to Calcimimetics and Physiological Challenges. Endocrinology 2009, 150, 3002–3010. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Yang, L.; Wu, Y.; Zhou, Y.; Cui, Y.; Yang, G.; Hong, Y. Stanniocalcin 2 Improved Osteoblast Differentiation via Phosphorylation of ERK. Mol. Med. Rep. 2016, 14, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D. New Insights into Physiological and Pathophysiological Functions of Stanniocalcin 2. Front. Endocrinol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- McCudden, C.R.; Kogon, M.R.; DiMattia, G.E.; Wagner, G.F. Novel Expression of the Stanniocalcin Gene in Fish. J. Endocrinol. 2001, 171, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and Intestinal Calcium Absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Vitamin D: Historical Overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar]
- Leurs, N.; Martinand-Mari, C.; Ventéo, S.; Haitina, T.; Debiais-Thibaud, M. Evolution of Matrix Gla and Bone Gla Protein Genes in Jawed Vertebrates. Front. Genet. 2021, 12, 620659. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meijden, K.; Lips, P.; Van Driel, M.; Heijboer, A.C.; Schulten, E.A.J.M.; Den Heijer, M.; Bravenboer, N. Primary Human Osteoblasts in Response to 25-Hydroxyvitamin D3, 1,25-Dihydroxyvitamin D3 and 24R,25-Dihydroxyvitamin D3. PLoS ONE 2014, 9, e110283. [Google Scholar] [CrossRef] [PubMed]
- Woeckel, V.J.; Alves, R.D.A.M.; Swagemakers, S.M.A.; Eijken, M.; Chiba, H.; Van Der Eerden, B.C.J.; Van Leeuwen, J.P.T.M. 1α,25-(OH)2D3 Acts in the Early Phase of Osteoblast Differentiation to Enhance Mineralization via Accelerated Production of Mature Matrix Vesicles. J. Cell. Physiol. 2010, 225, 593–600. [Google Scholar] [CrossRef]
- Wu, C.; Lu, B.; Wang, Y.; Jin, C.; Zhang, Y.; Ye, J. Effects of Dietary Vitamin D3 on Growth Performance, Antioxidant Capacities and Innate Immune Responses in Juvenile Black Carp Mylopharyngodon piceus. Fish Physiol. Biochem. 2020, 46, 2243–2256. [Google Scholar] [CrossRef]
- Keller, J.; Schinke, T. The Role of the Gastrointestinal Tract in Calcium Homeostasis and Bone Remodeling. Osteoporos. Int. 2013, 24, 2737–2748. [Google Scholar] [CrossRef]
- Christakos, S.; Veldurthy, V.; Patel, N.; Wei, R. Intestinal Regulation of Calcium: Vitamin D and Bone Physiology. Underst. Gut-Bone Signal. Axis Mech. Ther. Implic. 2017, 1033, 3–12. [Google Scholar]
- Fleet, J.C.; Schoch, R.D. Molecular Mechanisms for Regulation of Intestinal Calcium Absorption by Vitamin D and Other Factors. Crit. Rev. Clin. Lab. Sci. 2010, 47, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.M.; Power, D.; Flik, G.; Balment, R.J. Measurement of PTHrP, PTHR1, and CaSR Expression Levels in Tissues of Sea Bream (Sparus aurata) Using Quantitative PCR. Ann. N. Y. Acad. Sci. 2005, 1040, 340–344. [Google Scholar] [CrossRef]
- Lall, S.P.; Lewis-McCrea, L.M. Role of Nutrients in Skeletal Metabolism and Pathology in Fish—An Overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Gregório, S.F.; Fuentes, J. Regulation of Bicarbonate Secretion in Marine Fish Intestine by the Calcium-Sensing Receptor. Int. J. Mol. Sci. 2018, 19, 1072. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Figueiredo, J.; Power, D.M.; Canário, A.V.M. Parathyroid Hormone-Related Protein Regulates Intestinal Calcium Transport in Sea Bream (Sparus aurata). Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R1499–R1506. [Google Scholar] [CrossRef]
- Cheng, K.; Huang, Y.; Wang, C.; Ali, W.; Karrow, N.A. Physiological Function of Vitamin D3 in Fish. Rev. Aquac. 2023, 15, 1732–1748. [Google Scholar] [CrossRef]
- Ishibashi, K.; Imai, M. Prospect of a Stanniocalcin Endocrine/Paracrine System in Mammals. Am. J. Physiol. Physiol. 2002, 282, F367–F375. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Recent Advances in the Renal–Skeletal–Gut Axis That Controls Phosphate Homeostasis. Lab. Investig. 2009, 89, 7–14. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pandey, A.C. Endocrinology of Calcium Homeostasis in Fishes. J. Exp. Zool. India 2013, 16, 381–403. [Google Scholar]
- Guerreiro, P.M.; Fuentes, J. Control of Calcium Balance in Fish. Fish Osmoregul. 2019, 427–495. [Google Scholar] [CrossRef]
- Bevelander, G.S.; Pinto, E.S.L.; Canario, A.V.M.; Spanings, T.; Flik, G. CYP27A1 Expression in Gilthead Sea Bream (Sparus auratus, L.): Effects of Calcitriol and Parathyroid Hormone-Related Protein. J. Endocrinol. 2008, 196, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Lieben, L.; Carmeliet, G.; Masuyama, R. Calcemic Actions of Vitamin D: Effects on the Intestine, Kidney and Bone. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E.; Flik, G. Calcium Regulation in Fish. Aquac. Fundam. Appl. Res. 1993, 43, 47–59. [Google Scholar]
- Blaine, J.; Chonchol, M.; Levi, M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Abbink, W.; Bevelander, G.S.; Rotllant, J.; Canario, A.V.M.; Flik, G. Calcium Handling in Sparus auratus: Effects of Water and Dietary Calcium Levels on Mineral Composition, Cortisol and PTHrP Levels. J. Exp. Biol. 2004, 207, 4077–4084. [Google Scholar] [CrossRef]
- Swarup, K.; Srivastav, S.P. Vitamin D3-Induced Hypercalcaemia in Male Catfish, Clarias batrachus. Gen. Comp. Endocrinol. 1982, 46, 271–274. [Google Scholar] [CrossRef]
- Lopez, E.; Mac Intyre, I.; Martelly, E.; Lallier, F.; Vidal, B. Paradoxical Effect of 1.25 Dihydroxycholecalciferol on Osteoblastic and Osteoclastic Activity in the Skeleton of the Eel Anguilla anguilla L. Calcif. Tissue Int. 1980, 32, 83–87. [Google Scholar] [CrossRef]
- Fragkoulis, S.; Koumoundouros, G. Simple Morphometrics for Predicting Lordosis-Induced Deviations of Body-Shape in Reared Gilthead Seabream (Sparus aurata L.). J. Fish Dis. 2021, 44, 1265–1267. [Google Scholar]
- Beato, S.; Marques, C.; Laizé, V.; Gavaia, P.J.; Fernández, I. New Insights on Vitamin K Metabolism in Senegalese Sole (Solea senegalensis) Based on Ontogenetic and Tissue-Specific Vitamin K Epoxide Reductase Molecular Data. Int. J. Mol. Sci. 2020, 21, 3489. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, W.; Lin, S.; Xu, W.; Mai, K. Effects of Dietary Vitamin K on Growth Performances, Blood Coagulation Time and Menaquinone-4 (MK-4) Concentration in Tissues of Juvenile Large Yellow Croaker Pseudosciaena crocea. Aquac. Res. 2015, 46, 1269–1275. [Google Scholar] [CrossRef]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval Performance and Skeletal Deformities in Farmed Gilthead Sea Bream (Sparus aurata) Fed with Graded Levels of Vitamin A Enriched Rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Arlington, VA, USA, 2000.
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- CEN Foodstuffs—Determination of Vitamin D by High Performance Liquid Chromatography-Measurement of Cholecalciferol (D3) or Ergocalciferol (D2); CEN 12821:2009(Main): Brussels, Belgium, 2009.
- Julshamn, K.; Måge, A.; Norli, H.S.; Grobecker, K.H.; Jorhem, L.; Fecher, P. Determination of Arsenic, Cadmium, Mercury and Lead by ICP-MS in Foods after Pressure Digestion: NMKL Collaborative Study. J. AOAC Int. 2007, 90, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Boglione, C.; Gagliardi, F.; Scardi, M.; Cataudella, S. Skeletal Descriptors and Quality Assessment in Larvae and Post-Larvae of Wild-Caught and Hatchery-Reared Gilthead Sea Bream (Sparus aurata L. 1758). Aquaculture 2001, 192, 1–22. [Google Scholar] [CrossRef]
- Navarro, A.; Lee-Montero, I.; Santana, D.; Henríquez, P.; Ferrer, M.A.; Morales, A.; Soula, M.; Badilla, R.; Negrín-Báez, D.; Zamorano, M.J.; et al. IMAFISH_ML: A Fully-Automated Image Analysis Software for Assessing Fish Morphometric Traits on Gilthead Seabream (Sparus aurata L.), Meagre (Argyrosomus regius) and Red Porgy (Pagrus pagrus). Comput. Electron. Agric. 2016, 121, 66–73. [Google Scholar] [CrossRef]



| Ingredients (%) | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) |
|---|---|---|---|---|---|
| Fishmeal | 10 | 10 | 10 | 10 | 10 |
| Defatted squid meal | 1 | 1 | 1 | 1 | 1 |
| Squid meal | 2 | 2 | 2 | 2 | 2 |
| Casein | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Soy protein concentrate | 16 | 16 | 16 | 16 | 16 |
| Wheat gluten meal | 12.9 | 12.9 | 12.9 | 12.9 | 12.9 |
| Corn gluten meal | 12.9 | 12.9 | 12.9 | 12.9 | 12.9 |
| Soybean meal | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Wheat, whole | 9 | 9 | 9 | 9 | 9 |
| Rapeseed oil | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 |
| Fish oil | 6 | 6 | 6 | 6 | 6 |
| Linseed oil | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 |
| Soybean lecithin | 2 | 2 | 2 | 2 | 2 |
| L-Lysine | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| L-Methionine | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 |
| L-Threonine | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| CaHPO4.2H2O | 1 | 1 | 1 | 1 | 1 |
| Carboxymethyl cellulose | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Mineral premix * | 2 | 2 | 2 | 2 | 2 |
| Vitamin premix ** | 2 | 2 | 2 | 2 | 2 |
| Vitamin D3 (mg/kg) | 0 | 0.04 | 0.04 | 0.5 | 0.5 |
| Vitamin K3 (mg/kg) | 0 | 6 | 12 | 6 | 12 |
| Diet proximate composition (%) | |||||
| Moisture | 6.42 ± 0.13 | 6.28 ± 0.03 | 6.29 ± 0.04 | 6.41 ± 0.08 | 7.40 ± 0.05 |
| Crude protein | 44.81 ± 0.14 | 44.32 ± 0.37 | 44.60 ± 0.36 | 44.51 ± 0.02 | 44.34 ± 0.07 |
| Crude lipid | 21.64 ± 0.59 | 23.33 ± 0.46 | 22.74 ± 0.16 | 23.03 ± 1.15 | 21.57 ± 0.33 |
| Ash | 6.46 ± 0.08 | 6.20 ± 0.01 | 5.96 ± 0.24 | 6.10 ± 0.09 | 6.04 ± 0.02 |
| Analyzed dietary vitamins | |||||
| Vitamin D3 (mg/kg) | 0.07 ± 0.03 | 0.20 ± 0.04 | 0.19 ± 0.04 | 0.51 ± 0.10 | 0.56 ± 0.05 |
| Vitamin K3 (mg/kg) | 0.01 ± 0.00 | 0.58 ± 0.04 | 1.65 ± 0.05 | 0.74 ± 0.04 | 1.00 ± 0.06 |
| Supplemented dietary vitamins | |||||
| Vitamin D3 (mg/kg) | 0 | 0.04 | 0.04 | 0.5 | 0.5 |
| Vitamin K3 (mg/kg) | 0 | 6 | 12 | 6 | 12 |
| Gene | Forward Primer | Reverse Primer | Annealing Temperature Tm | Accession Number |
|---|---|---|---|---|
| House Keeping Gene | ||||
| β-actin2 (Beta actin 2) | TCTGTCTGGATCGGAGGCTC | AAGCATTTGCGGTGGACG | 58.1 | X89920 |
| ef1α (Elongation factor 1 alpha) | CTTCAACGCTCAGGTCATCAT | GCACAGCGAAACGACCAAGGGGA | 60 | AF184170 |
| rpl27 (Ribosomal protein L27) | AAGAGGAACACAACTCACTGCCCCAC | GCTTGCCTTTGCCCAGAACTTTGTAG | 68 | AY188520 |
| Bone Biomarker | ||||
| bmp2 (Bone morphogenetic protein 2) | GTGGCTTCCATCGTATCAACATTTT | GCTCCCCGCCATGAGT | 60 | JF261172.1 |
| runx2 (Runt-related transcription factor 2) | GCCTGTCGCCTTTAAGGTGGTTGC | TCGTCGTTGCCCGCCATAGCTG | 61 | AJ619023 |
| (osx) Osterix | CAGTCAGGGATTCAGCAACA | GGTGAAGGAGCCAGTGTAGG | 60 | ERR22591_isotig06993 |
| alp (Alkaline phosphatase) | AGAACG CCCTGACGC TGCAA | TTCAGTATACGAGCA GCCGTCAC | 61 | AY266359 |
| opn (Osteopontin) | AAGATGGCCTACGACATGACAGAC | CCTGAAGAGCCTTGTACACCTGC | 61 | AY651247 |
| on (Osteonectin) | AAAATGATCGAGCCCTGCATGGAC | TACAGAGTCACCAGGACGTT | 61 | AY239014 |
| oc (osteocalcin) | AGCCCAAAGCACGTAAGCAAGCTA | TTTCATCACGCTACTCTACGGGTT | 58.1 | AF048703 |
| Calcium Regulating Gene | ||||
| vdrβ (Vitamin D receptor β) | TGACGACTCCTACTCTGACT | CTGCTGCCCTGCTCTTGGTA | 62.3 | XM_030420365.1 |
| cyp27a1 (Cytochrome P450 family 27 subfamily A member 1) | TGGCTCTACAAGTTTGGCTTTGA | TGAACCGCAGCG TGTCTTT | 60 | AM885865 |
| stc2 (Stanniocalcin 2) | CTGGAGCAAGTAGTGGGAT | CCTGTAGCCCTCGTATCTCG | 62 | XM_030395344 |
| casr (Calcium sensing receptor) | GCTTCTCCAGCTCGCTCATC | AGGCGGGCTGGCGTAA | 60 | AJ289717 |
| pthrp (Parathyroid hormone related protein) | GAGGCAAATGAA TGGAACAG | TGGCCAGCTCAA AACTTGT | 60 | AF197904 |
| pth1r (Parathyroid hormone 1 receptor) | GAACCTGCCCGG CTACGTGAAG | GCTCCTGTCCCG ACGAGGGTAT | 60 | AJ619024 |
| mgp (Matrix gla protein) | CGCCCGAAATACACCTCAGA | GACGGACGGATACTAGGAGTCTA | 60 | AY065652 |
| mmp9 (Matrix metallopeptidase 9) | ATTCAGAAGGTGGAGGGAGCG | CATTGGGGACACCACCGAAGA | 60 | AM905938 |
| ctsk (Cathepsin K) | AGCGAGCAGAACCTGGTGGAC | GCAGAGTTGTAGTTGGGGTCGTAG | 60 | DQ875329 |
| trap (Tartrate-resistant acid phosphatase) | CTTAATCGTTGCCATCCCTGTG | CTCCCATCTGCTCTGCTACTTTG | 60 | FM147928 |
| Growth Parameters | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | Two-Way ANOVA (p Value) | ||
|---|---|---|---|---|---|---|---|---|
| VD | VK | VD × VK | ||||||
| FBW (g/fish) | 148.36 ± 6.34 | 147.99 ± 2.26 | 150.54 ± 3.45 | 141.64 ± 9.35 | 151.61 ± 5.78 | 0.99 | 0.23 | 0.72 |
| WG (%) | 103.32 ± 8.23 | 103.64 ± 3.72 | 108.28 ± 4.71 | 102.66 ± 0.86 | 109.13 ± 9.28 | 0.99 | 0.20 | 0.82 |
| SGR (%) | 0.68 ± 0.04 | 0.68 ± 0.02 | 0.70 ± 0.04 | 0.68 ± 0.01 | 0.70 ± 0.04 | 0.97 | 0.26 | 0.90 |
| FCR | 1.55 ± 0.08 | 1.56 ± 0.06 | 1.51 ± 0.05 | 1.54 ± 0.04 | 1.49 ± 0.09 | 0.64 | 0.26 | 1.00 |
| PER | 1.05 ± 0.05 | 1.05 ± 0.04 | 1.07 ± 0.04 | 1.06 ± 0.04 | 1.10 ± 0.06 | 0.60 | 0.27 | 0.64 |
| FI (g/fish) | 159.97 ± 5.29 | 161.47 ± 2.20 | 163.62 ± 3.81 | 154.85 ± 6.13 | 161.92 ± 4.55 | 0.34 | 0.27 | 0.74 |
| CF | 1.69 ± 0.01 | 1.71 ± 0.02 | 1.69 ± 0.04 | 1.69 ± 0.03 | 1.71 ± 0.04 | 0.80 | 0.80 | 0.35 |
| Serum Calcitriol and Bone Mineral Composition | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | Two-Way ANOVA (p Value) | ||
|---|---|---|---|---|---|---|---|---|
| VD | VK | VD × VK | ||||||
| Serum calcitriol (pg/mL) | 5.26 ± 0.41 | 5.71 ± 0.07 | 5.61 ± 0.16 | 4.78 ± 0.76 | 5.16 ± 0.22 | 0.05 | 0.64 | 0.44 |
| Bone calcium (mg/g) | 113.33 ± 5.77 | 109.00 ± 11.53 | 106.33 ± 11.85 | 113.33 ± 5.77 | 110.00 ± 0.00 | 0.42 | 0.54 | 0.95 |
| Bone phosphorus (mg/g) | 57.33 ± 3.06 | 58.00 ± 6.25 | 55.67 ± 5.51 | 57.67 ± 2.52 | 57.67 ± 1.53 | 0.74 | 0.64 | 0.64 |
| Skeletal Anomaly Frequency (%) | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | Kruskal-Wallis Test |
|---|---|---|---|---|---|---|
| Haemal Lordosis | 0.00 ± 0.00 | 2.77 ± 4.79 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.33 ± 14.43 | 0.58 |
| Haemal vertebral fusion | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.77 ± 4.79 | 0.45 |
| Caudal vertebral anomaly | 3.03 ± 5.25 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.57 ± 9.64 | 0.58 |
| Anomalous maxillary and/or pre-maxillary | 6.07 ± 10.51 | 11.13 ± 9.64 | 2.77 ± 4.79 | 8.35 ± 11.81 | 8.33 ± 8.35 | 0.86 |
| Anomalous dentary | 2.77 ± 4.79 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.35 ± 11.81 | 0.00 ± 0.00 | 0.36 |
| Caudal fin supernumerary bone | 8.60 ± 8.36 | 2.77 ± 4.79 | 8.90 ± 8.40 | 4.55 ± 6.43 | 2.77 ± 4.79 | 0.51 |
| Cranium | 8.83 ± 9.11 | 11.13 ± 9.64 | 2.77 ± 4.79 | 16.65 ± 23.55 | 8.33 ± 8.35 | 0.80 |
| Haemal vertebrae | 0.00 ± 0.00 | 2.77 ± 4.79 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 19.23 | 0.58 |
| Caudal vertebrae | 3.03 ± 5.25 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.57 ± 9.64 | 0.58 |
| Total severe anomaly | 11.87 ± 14.00 | 13.90 ± 12.73 | 2.77 ± 4.79 | 16.65 ± 23.55 | 25.00 ± 22.05 | 0.64 |
| Total anomaly | 20.47 ± 18.62 | 16.67 ± 16.65 | 11.67 ± 12.58 | 21.20 ± 17.11 | 27.77 ± 25.46 | 0.87 |
| Fish Morphometric Traits | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | Two-Way ANOVA (p Value) | ||
|---|---|---|---|---|---|---|---|---|
| VD | VK | VD × VK | ||||||
| Total lateral area (cm2) | 98.47 ± 4.70 | 97.13 ± 2.44 | 96.22 ± 1.46 | 95.47 ± 5.08 | 97.45 ± 2.60 | 0.92 | 0.80 | 0.50 |
| Total lateral length (cm) | 20.48 ± 0.18 | 20.32 ± 0.30 | 20.17 ± 0.11 | 20.08 ± 0.57 | 20.20 ± 0.31 | 0.58 | 0.95 | 0.51 |
| Fish maximum height (cm) | 7.15 ± 0.13 | 7.16 ± 0.17 | 7.06 ± 0.02 | 7.05 ± 0.26 | 7.12 ± 0.10 | 0.79 | 0.84 | 0.35 |
| Caudal peduncle height (cm) | 2.34 ± 0.48 | 2.34 ± 0.34 | 2.13 ± 0.04 | 2.14 ± 0.17 | 2.21 ± 0.11 | 0.71 | 0.65 | 0.41 |
| Fork length (cm) | 20.29 ± 0.19 | 20.09 ± 0.29 | 19.91 ± 0.12 | 19.85 ± 0.58 | 20.02 ± 0.30 | 0.74 | 0.98 | 0.39 |
| Head height (cm) | 5.96 ± 0.09 | 6.11 ± 0.28 | 6.20 ± 0.12 | 6.00 ± 0.14 | 6.20 ± 0.23 | 0.59 | 0.20 | 0.61 |
| Fillet area (cm2) | 67.65 ± 2.55 | 65.68 ± 1.18 | 63.91 ± 0.66 | 64.21 ± 4.28 | 64.72 ± 1.02 | 0.81 | 0.65 | 0.42 |
| Fillet area percentage (%) | 0.69 ± 0.02 | 0.68 ± 0.01 | 0.66 ± 0.02 | 0.67 ± 0.01 | 0.66 ± 0.01 | 0.81 | 0.11 | 0.81 |
| Standard length (cm) | 17.61 ± 0.27 | 17.42 ± 0.22 | 17.34 ± 0.06 | 17.27 ± 0.42 | 17.37 ± 0.30 | 0.73 | 0.95 | 0.60 |
| Fillet maximum length (cm) | 11.60 ± 0.21 | 11.32 ± 0.08 | 11.08 ± 0.11 | 11.20 ± 0.38 | 11.15 ± 0.12 | 0.85 | 0.27 | 0.45 |
| Fish eccentricity | 0.89 ± 0.00 | 0.89 ± 0.00 | 0.89 ± 0.00 | 0.89 ± 0.01 | 0.89 ± 0.00 | 0.29 | 0.29 | 0.29 |
| Head eccentricity | 0.65 ± 0.01 | 0.66 ± 0.04 | 0.69 ± 0.02 | 0.66 ± 0.03 | 0.68 ± 0.02 | 0.74 | 0.12 | 0.91 |
| Tail-excluded length (cm) | 15.51 ± 0.21 | 15.40 ± 0.28 | 15.31 ± 0.03 | 15.23 ± 0.38 | 15.35 ± 0.21 | 0.66 | 0.90 | 0.48 |
| Equidistant fish height A | 2.89 ± 0.28 | 2.86 ± 0.23 | 2.86 ± 0.12 | 2.90 ± 0.09 | 2.95 ± 0.02 | 0.52 | 0.77 | 0.80 |
| Equidistant fish height B | 4.26 ± 0.27 | 4.25 ± 0.02 | 4.27 ± 0.13 | 4.24 ± 0.17 | 4.35 ± 0.12 | 0.71 | 0.52 | 0.68 |
| Equidistant fish height C | 6.40 ± 0.16 | 6.36 ± 0.06 | 6.34 ± 0.08 | 6.26 ± 0.22 | 6.34 ± 0.11 | 0.55 | 0.72 | 0.52 |
| Equidistant fish height D | 7.10 ± 0.14 | 7.07 ± 0.11 | 7.02 ± 0.03 | 7.00 ± 0.27 | 7.08 ± 0.10 | 0.93 | 0.90 | 0.46 |
| Equidistant fish height E | 5.68 ± 0.09 | 5.67 ± 0.06 | 5.59 ± 0.03 | 5.58 ± 0.20 | 5.62 ± 0.07 | 0.62 | 0.73 | 0.31 |
| Relative Gene Expression—Vertebra | Two-Way ANOVA (p Value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | VD | VK | VD × VK |
| Bone Biomarkers (Osteoblast-Related Genes) | ||||||||
| bmp2 | 0.67 ± 0.09 ab | 0.23 ± 0.15 a | 1.11 ± 0.44 b | 0.67 ± 0.22 ab | 0.64 ± 0.42 ab | 0.94 | 0.00 | 0.00 |
| runx2 | 0.75 ± 0.25 | 0.94 ± 0.29 | 1.30 ± 0.91 | 1.54 ± 0.64 | 1.02 ± 0.70 | 0.57 | 0.76 | 0.12 |
| osx | 0.67 ± 0.19 a | 0.30 ± 0.16 a | 1.41 ± 0.39 b | 0.98 ± 0.56 ab | 0.86 ± 0.41 ab | 0.69 | 0.01 | 0.00 |
| alp | 0.70 ± 0.22 a | 0.86 ± 0.42 a | 0.98 ± 0.43 a | 2.10 ± 0.42 b | 1.08 ± 0.56 a | 0.00 | 0.02 | 0.00 |
| opn | 0.58 ± 0.08 a | 0.47 ± 0.25 a | 1.08 ± 0.30 b | 1.00 ± 0.54 ab | 0.70 ± 0.52 a | 0.66 | 0.34 | 0.01 |
| on | 0.68 ± 0.26 | 0.84 ± 0.33 | 0.92 ± 0.35 | 0.91 ± 0.27 | 0.87 ± 0.29 | 0.92 | 0.87 | 0.61 |
| oc | 1.05 ± 0.33 | 1.36 ± 1.18 | 1.42 ± 0.49 | 1.46 ± 0.50 | 1.10 ± 0.57 | 0.74 | 0.64 | 0.51 |
| Bone Biomarkers (Osteoclast-Related Genes) | ||||||||
| mgp | 0.67 ± 0.09 ab | 0.30 ± 0.11 a | 1.28 ± 0.34 c | 1.22 ± 0.14 c | 1.14 ± 0.38 bc | 0.00 | 0.00 | 0.00 |
| ctsk | 0.60 ± 0.10 a | 0.59 ± 0.12 a | 0.96 ± 0.53 a | 1.70 ± 0.92 b | 0.88 ± 0.34 a | 0.06 | 0.39 | 0.03 |
| trap | 0.44 ± 0.06 a | 0.16 ± 0.04 a | 0.99 ± 0.64 b | 1.03 ± 0.83 b | 0.21 ± 0.13 a | 0.84 | 0.98 | 0.00 |
| mmp9 | 0.48 ± 0.10 | 0.19 ± 0.11 | 0.82 ± 0.36 | 0.78 ± 0.50 | 1.03 ± 0.85 | 0.07 | 0.05 | 0.37 |
| Calcium Regulators | ||||||||
| casr | 0.69 ± 0.18 ab | 0.28 ± 0.15 a | 1.18 ± 0.28 c | 0.98 ± 0.12 bc | 0.98 ± 0.36 bc | 0.03 | 0.00 | 0.00 |
| pth1r | 0.53 ± 0.12 ab | 0.21 ± 0.09 a | 1.10 ± 0.35 b | 0.90 ± 0.23 b | 0.86 ± 0.50 b | 0.10 | 0.01 | 0.00 |
| pthrp | 0.67 ± 0.25 ab | 0.29 ± 0.21 a | 1.39 ± 0.36 c | 0.88 ± 0.34 bc | 0.39 ± 0.07 ab | 0.10 | 0.02 | 0.00 |
| stc2 | 0.73 ± 0.13 a | 0.25 ± 0.10 a | 1.44 ± 0.64 b | 0.81 ± 0.35 ab | 0.75 ± 0.30 a | 0.68 | 0.00 | 0.00 |
| Relative Gene Expression—Intestine | Two-Way ANOVA (p Value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | VD | VK | VD × VK |
| casr | 0.43 ± 0.11 a | 1.96 ± 0.85 b | 3.75 ± 1.31 c | 2.01 ± 0.41 b | 0.62 ± 0.1 ab | 0.01 | 0.53 | 0.00 |
| pth1r | 0.34 ± 0.04 a | 1.83 ± 1.04 b | 3.42 ± 0.65 c | 1.98 ± 0.80 b | 0.87 ± 0.36 ab | 0.16 | 0.80 | 0.01 |
| pthrp | 0.46 ± 0.09 a | 2.07 ± 1.43 ab | 4.17 ± 1.13 b | 2.66 ± 1.48 ab | 0.62 ± 0.15 a | 0.30 | 0.26 | 0.01 |
| stc2 | 0.52 ± 0.25 a | 2.02 ± 1.22 ab | 3.71 ± 1.42 b | 3.28 ± 1.09 b | 0.76 ± 0.27 a | 0.44 | 0.23 | 0.00 |
| pxr | 0.50 ± 0.32 | 1.95 ± 0.69 | 2.30 ± 1.42 | 1.92 ± 0.20 | 1.97 ± 3.05 | 0.85 | 0.84 | 0.88 |
| Relative Gene Expression—Kidney | Two-Way ANOVA (p Value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | VD | VK | VD × VK |
| vdrβ | 0.72 ± 0.29 ab | 0.51 ± 0.34 a | 0.42 ± 0.20 a | 0.87 ± 0.41 b | 0.94 ± 0.37 c | 0.02 | 0.79 | 0.34 |
| stc2 | 0.55 ± 0.14 ab | 0.22 ± 0.13 a | 3.54 ± 0.37 d | 1.07 ± 0.22 b | 1.96 ± 0.51 c | 0.14 | 0.00 | 0.00 |
| Relative Gene Expression—Liver | Two-Way ANOVA (p Value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | VD | VK | VD × VK |
| casr | 1.14 ± 0.73 | 1.54 ± 0.81 | 0.58 ± 0.27 | 1.73 ± 1.39 | 2.57 ± 1.65 | 0.35 | 0.23 | 0.55 |
| pth1r | 1.12 ± 0.67 | 2.70 ± 1.59 | 0.71 ± 0.12 | 1.75 ± 1.04 | 2.24 ± 0.83 | 0.48 | 0.19 | 0.34 |
| pthrp | 0.86 ± 0.19 | 1.52 ± 0.76 | 0.72 ± 0.31 | 1.65 ±1.20 | 1.70 ± 0.31 | 0.21 | 0.40 | 0.34 |
| stc2 | 0.06 ± 0.05 | 0.05 ± 0.03 | 0.46 ±0.49 | 0.30 ± 0.39 | 0.13 ± 0.08 | 0.26 | 0.57 | 0.74 |
| cyp27a1 | 1.04 ± 0.33 | 1.21 ± 0.49 | 0.85 ± 0.35 | 2.27 ± 1.64 | 2.38 ± 0.92 | 0.05 | 0.73 | 0.77 |
| Relative Gene Expression—Gill | Two-Way ANOVA (p Value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | (0.07/0.01) | (0.20/0.58) | (0.19/1.65) | (0.51/0.74) | (0.56/1.00) | VD | VK | VD × VK |
| casr | 1.80 ± 1.01 b | 0.58 ± 0.16 a | 0.31 ± 0.20 a | 0.30 ± 0.36 a | 0.15 ± 0.07 a | 0.24 | 0.03 | 0.65 |
| pth1r | 1.89 ± 0.71 b | 0.51 ± 0.33 a | 0.21 ± 0.08 a | 0.92 ± 0.64 a | 0.28 ± 0.13 a | 0.74 | 0.01 | 0.94 |
| pthrp | 2.40 ± 1.00 | 0.33 ± 0.06 | 0.27 ± 0.16 | 0.36 ± 0.33 | 0.19 ± 0.09 | 0.88 | 0.11 | 0.51 |
| stc2 | 1.91 ± 1.05 b | 0.51 ± 0.25 a | 0.39 ± 0.21 a | 0.32 ± 0.20 a | 0.21 ± 0.08 a | 0.27 | 0.03 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivagurunathan, U.; Izquierdo, M.; Tseng, Y.; Prabhu, P.A.J.; Zamorano, M.J.; Robaina, L.; Domínguez, D. Effects of the Interaction between Dietary Vitamin D3 and Vitamin K3 on Growth, Skeletal Anomalies, and Expression of Bone and Calcium Metabolism-Related Genes in Juvenile Gilthead Seabream (Sparus aurata). Animals 2024, 14, 2808. https://doi.org/10.3390/ani14192808
Sivagurunathan U, Izquierdo M, Tseng Y, Prabhu PAJ, Zamorano MJ, Robaina L, Domínguez D. Effects of the Interaction between Dietary Vitamin D3 and Vitamin K3 on Growth, Skeletal Anomalies, and Expression of Bone and Calcium Metabolism-Related Genes in Juvenile Gilthead Seabream (Sparus aurata). Animals. 2024; 14(19):2808. https://doi.org/10.3390/ani14192808
Chicago/Turabian StyleSivagurunathan, Ulaganathan, Marisol Izquierdo, Yiyen Tseng, Philip Antony Jesu Prabhu, María Jesús Zamorano, Lidia Robaina, and David Domínguez. 2024. "Effects of the Interaction between Dietary Vitamin D3 and Vitamin K3 on Growth, Skeletal Anomalies, and Expression of Bone and Calcium Metabolism-Related Genes in Juvenile Gilthead Seabream (Sparus aurata)" Animals 14, no. 19: 2808. https://doi.org/10.3390/ani14192808







