New Insights on Chromosome Diversification in Malagasy Chameleons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Molecular Analysis
2.3. Cytogenetic Analysis
3. Results
3.1. Molecular Analysis
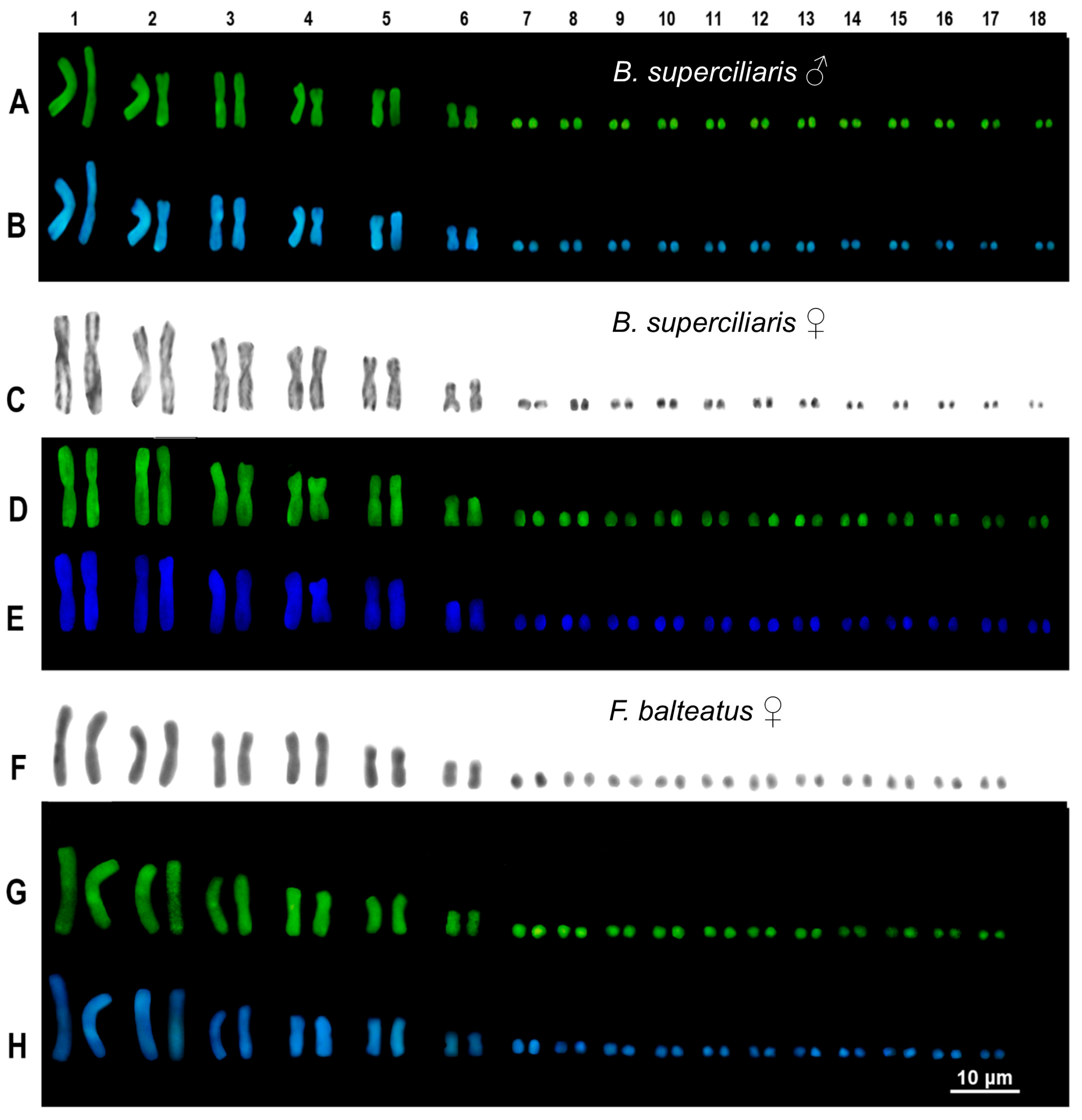
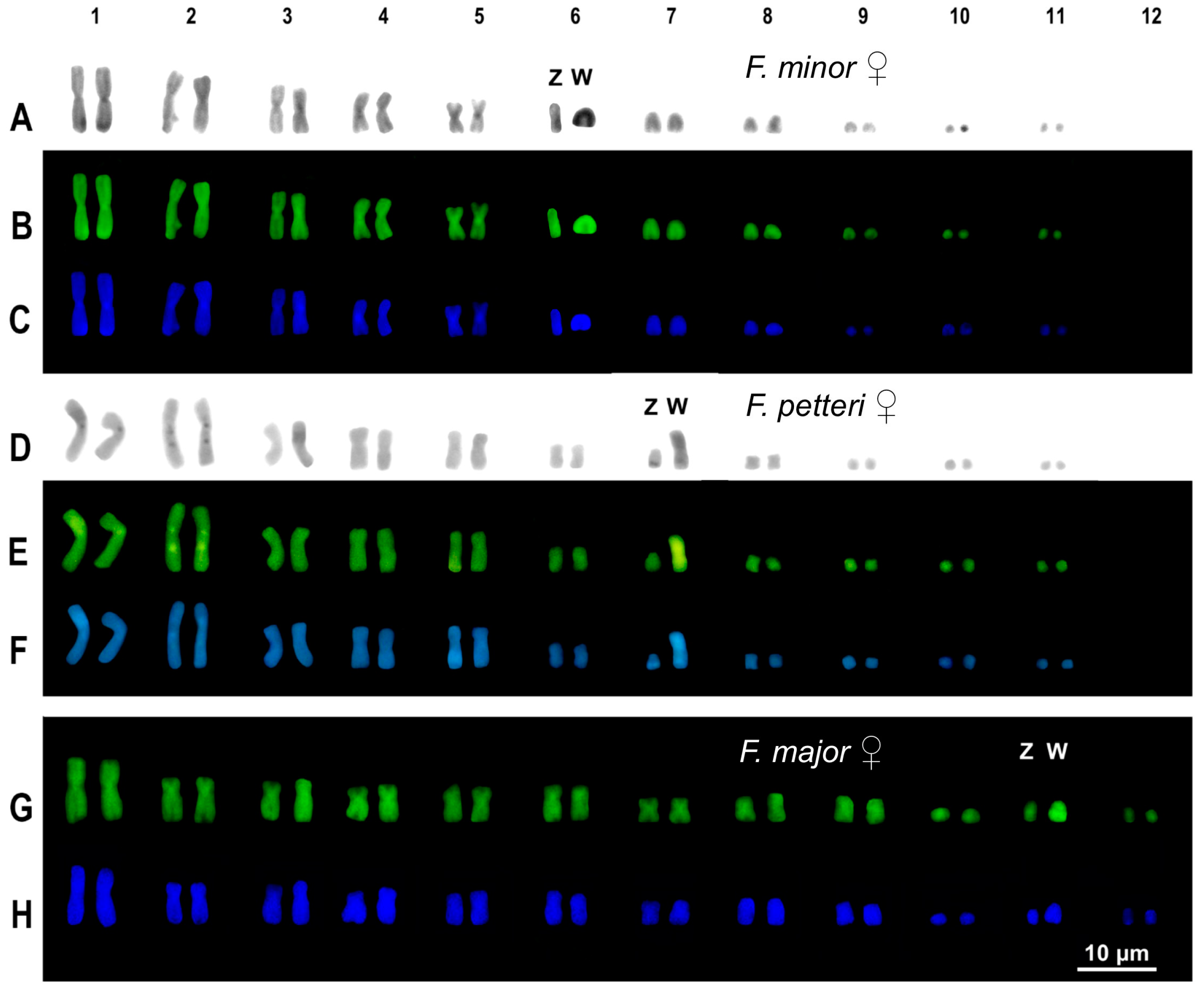
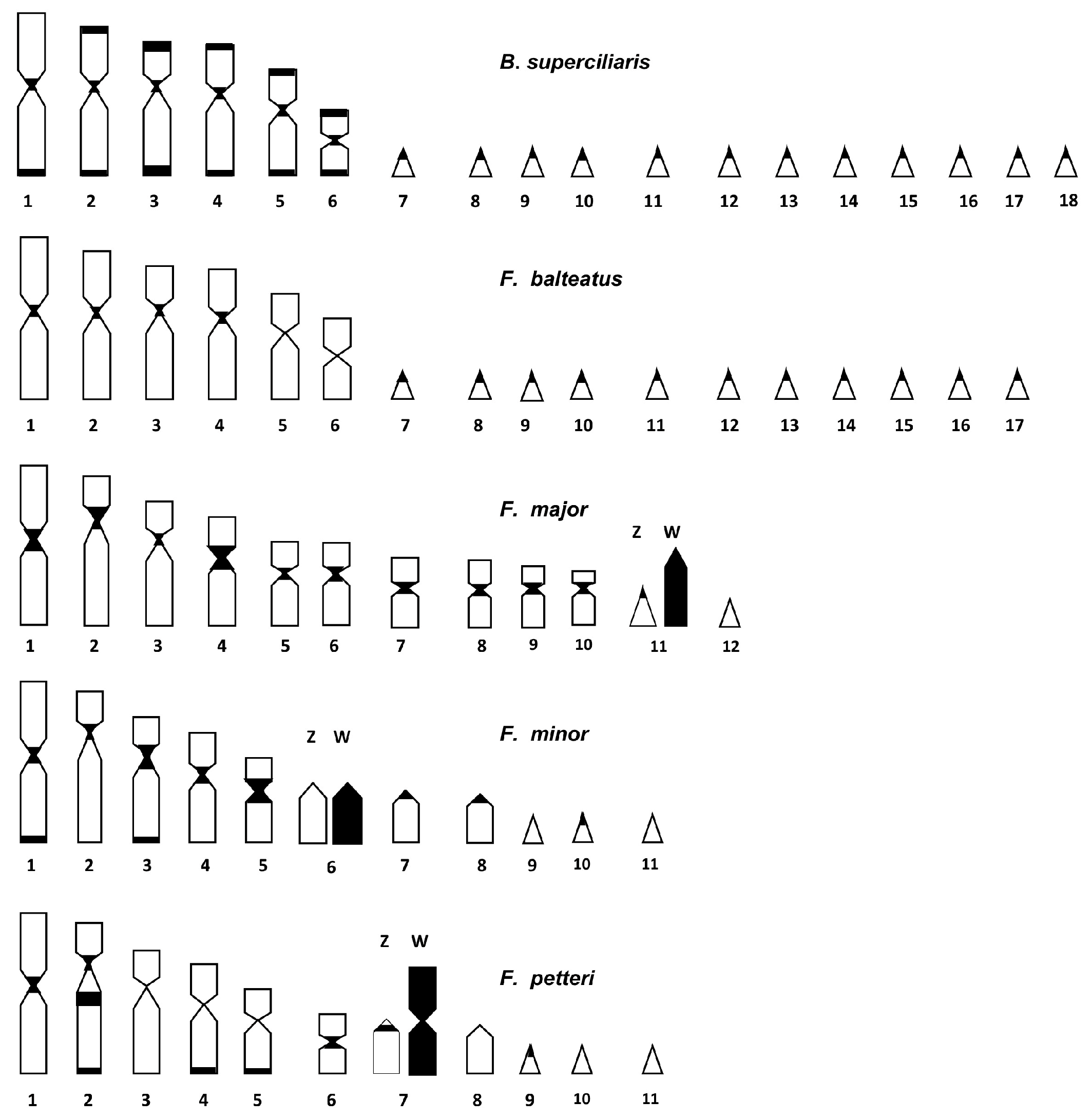
3.2. Cytogenetic Analysis
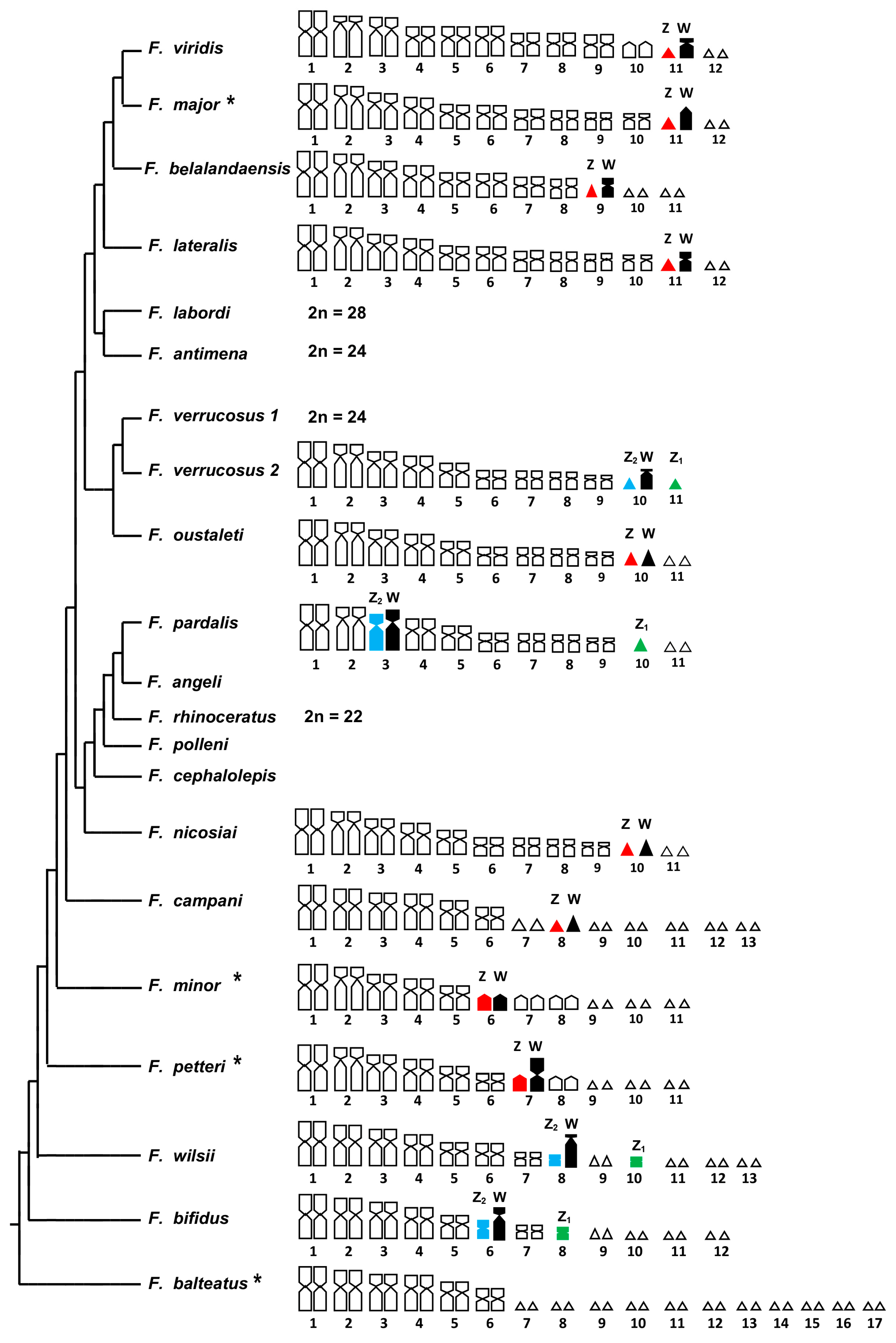
4. Discussion
4.1. Molecular Taxonomic Attribution
4.2. Cytogenetic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rieseberg, L.H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, A.; Vila, R.; Laetsch, D.R.; Hayward, A.; Martin, S.H.; Lohse, K. Chromosome Fissions and Fusions Act as Barriers to Gene Flow between Brenthis Fritillary Butterflies. Mol. Biol. Evol. 2023, 40, msad043. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Kudera, J.; Hošek, J. The Reptile Database. 2023. Available online: http://www.reptile-database.org/ (accessed on 8 August 2024).
- Olmo, E. Trends in the evolution of reptilian chromosomes. Integr. Comp. Biol. 2008, 48, 486–493. [Google Scholar] [CrossRef]
- Viana, P.F.; Ezaz, T.; de Bello Cioffi, M.; Liehr, T.; Al-Rikabi, A.; Goll, L.G.; Rocha, A.M.; Feldberg, E. Landscape of snake’ sex chromosomes evolution spanning 85 MYR reveals ancestry of sequences despite distinct evolutionary trajectories. Sci. Rep. 2020, 10, 12499. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.J.; Gamble, T.; Smith, C.H.; Wilson, M.A. A lizard is never late: Squamate genomics as a recent catalyst for understanding sex chromosome and microchromosome evolution. J. Hered. 2023, 114, 445–458. [Google Scholar] [CrossRef]
- Deakin, J.E.; Ezaz, T. Understanding the evolution of reptile chromosomes through applications of combined cytogenetics and genomics approaches. Cytogenet. Genome Res. 2019, 157, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, M.; Macirella, R.; Odierna, G.; Brunelli, E. Karyotype Diversification and Chromosome Rearrangements in Squamate Reptiles. Genes 2024, 15, 371. [Google Scholar] [CrossRef]
- Rovatsos, M.; Pokorná, M.J.; Altmanová, M.; Kratochvíl, L. Female heterogamety in Madagascar chameleons (Squamata: Chamaeleonidae: Furcifer): Differentiation of sex and neo-sex chromosomes. Sci. Rep. 2015, 5, 13196. [Google Scholar] [CrossRef]
- Rovatsos, M.; Altmanová, M.; Johnson Pokorná, M.; Velenský, P.; Sánchez Baca, A.; Kratochvíl, L. Evolution of karyotypes in chameleons. Genes 2017, 8, 382. [Google Scholar] [CrossRef]
- Rovatsos, M.; Altmanová, M.; Augstenová, B.; Mazzoleni, S.; Velenský, P.; Kratochvíl, L. ZZ/ZW Sex Determination with Multiple Neo-Sex Chromosomes is Common in Madagascan Chameleons of the Genus Furcifer (Reptilia: Chamaeleonidae). Genes 2019, 10, 1020. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Streicher, J.W.; Guarino, F.M.; Jones, M.E.H.; Loader, S.P.; Odierna, G.; Cooper, N. Microchromosome fusions underpin convergent evolution of chameleon karyotypes. Evolution 2023, 77, 1930–1944. [Google Scholar] [CrossRef] [PubMed]
- Vences, M.; Wollenberg, K.C.; Vieites, D.R.; Lees, D.C. Madagascar as a model region of species diversification. Trends Ecol. Evol. 2009, 24, 456–465. [Google Scholar] [CrossRef]
- Hita Garcia, F.; Fisher, B.L. Taxonomy of the hyper-diverse ant genus Tetramorium Mayr in the Malagasy region (Hymenoptera, Formicidae, Myrmicinae)—First record of the T. setigerum species group and additions to the Malagasy species groups with an updated illustrated identification key. ZooKeys 2015, 512, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Smith, R.J.; Perrigo, A.L.; Crottini, A.; Hackel, J.; Testo, W.; Farooq, H.; Jiménez, M.F.T.; Andela, N.; Andermann, T.; et al. Madagascar’s extraordinary biodiversity: Evolution, distribution, and use. Science 2022, 378, eabf0869. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. 2024. Available online: https://www.iucnredlist.org (accessed on 9 August 2024).
- Nagy, Z.T.; Sonet, G.; Glaw, F.; Vences, M. First Large-Scale DNA Barcoding Assessment of Reptiles in the Biodiversity Hotspot of Madagascar, Based on Newly Designed COI Primers. PLoS ONE 2012, 7, e34506. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Lab Press: New York, NY, USA, 1989. [Google Scholar]
- Hall, T.A. BioEdit: A user–friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Mezzasalma, M.; Guarino, F.M.; Aprea, G.; Petraccioli, A.; Crottini, A.; Odierna, G. Karyological evidence for diversification of Italian slow worm populations (Squamata, Anguidae). Comp. Cytogen. 2013, 7, 217–227. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Andreone, F.; Glaw, F.; Petraccioli, A.; Odierna, G.; Guarino, F.M. A karyological study of three typhlopid species with some inferences on chromosome evolution in blindsnakes (Scolecophidia). Zool. Anz. 2016, 264, 34–40. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Belluardo, F.; Quirós, D.D.; Lobón-Rovira, J.; Rosa, G.M.; Rasoazanany, M.; Andreone, F.; Crottini, A. Uncovering the herpetological diversity of small forest fragments in south-eastern Madagascar (Haute Matsiatra). Zoosyst. Evol. 2021, 97, 315–343. [Google Scholar] [CrossRef]
- Porter, C.A.; Hamilton, M.J.; Sites, J.W., Jr.; Baker, R.J. Location of ribosomal DNA in chromosomes of squamate reptiles: Systematic and evolutionary implications. Herpetologica 1991, 47, 271–280. [Google Scholar]
- Viana, P.F.; Ribeiro, L.B.; Souza, G.M.; Chalkidis, H.d.M.; Gross, M.C.; Feldberg, E. Is the Karyotype of Neotropical Boid Snakes Really Conserved? Cytotaxonomy, Chromosomal Rearrangements and Karyotype Organization in the Boidae Family. PLoS ONE 2016, 11, e0160274. [Google Scholar] [CrossRef]
- Rovatsos, M.; Mazzoleni, S.; Augstenová, B.; Altmanová, M.; Velenský, P.; Glaw, F.; Sanchez, A.; Kratochvíl, L. Heteromorphic ZZ/ZW sex chromosomes sharing gene content with mammalian XX/XY are conserved in Madagascan chameleons of the genus Furcifer. Sci. Rep. 2024, 14, 4898. [Google Scholar] [CrossRef]
- Tolley, K.A.; Townsend, T.M.; Vences, M. Large-scale phylogeny of chameleons suggests African origins and Eocene diversification. Proc. Biol. Sci. 2013, 280, 20130184. [Google Scholar] [CrossRef]
- Abbott, J.K.; Nordén, A.K.; Hansson, B. Sex chromosome evolution: Historical insights and future perspectives. Proc. Biol. Sci. 2017, 284, 20162806. [Google Scholar] [CrossRef]
- Alam, S.M.I.; Sarre, S.D.; Gleeson, D.; Georges, A.; Ezaz, T. Did Lizards Follow Unique Pathways in Sex Chromosome Evolution? Genes 2018, 9, 239. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Guarino, F.M.; Odierna, G. Lizards as Model Organisms of Sex Chromosome Evolution: What We Really Know from a Systematic Distribution of Available Data? Genes 2021, 12, 1341. [Google Scholar] [CrossRef]
- Ezaz, T.; Sarre, S.D.; O’Meally, D.; Graves, J.A.M.; Georges, A. Sex chromosome evolution in lizards: Independent origins and rapid transitions. Cytogenet. Genome Res. 2009, 127, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Marchal, J.A.; Giagia-Athanasopoulou, E.; Sánchez, A. Molecular Composition of Heterochromatin and Its Contribution to Chromosome Variation in the Microtus thomasi/Microtus atticus Species Complex. Genes 2021, 12, 807. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Yu, Q.; Ming, R.; Jiang, J. DNA methylation and heterochromatinization in the male-specific region of the primitive Y chromosome of papaya. Genome Res. 2008, 18, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.d.B.; Yano, C.F.; Sember, A.; Bertollo, L.A.C. Chromosomal Evolution in Lower Vertebrates: Sex Chromosomes in Neotropical Fishes. Genes 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L.S.; Metzger, D.C.H.; Darolti, I.; Wright, A.E.; Sandkam, B.A.; Almeida, P.; Shu, J.J.; Mank, J.E. Sex Chromosome Evolution: So Many Exceptions to the Rules. Genome Biol. Evol. 2020, 12, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Payseur, B.A.; Presgraves, D.C.; Filatov, D.A. Introduction: Sex chromosomes and speciation. Mol. Ecol. 2018, 27, 3745–3748. [Google Scholar] [CrossRef]
| Species | Specimen | Locality | Sex |
|---|---|---|---|
| B. superciliaris | GA 199 | Fiherenana | female |
| B. superciliaris | GA A3 | Fiherenana | male |
| F. balteatus | GA 379 | Ranomafana | female |
| F. petteri | FGMV 3015 | Ifaty | female |
| F. minor | GA 489 | Antoetra | female |
| F. major | GA 456 | Belalanda | female |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzasalma, M.; Odierna, G.; Macirella, R.; Brunelli, E. New Insights on Chromosome Diversification in Malagasy Chameleons. Animals 2024, 14, 2818. https://doi.org/10.3390/ani14192818
Mezzasalma M, Odierna G, Macirella R, Brunelli E. New Insights on Chromosome Diversification in Malagasy Chameleons. Animals. 2024; 14(19):2818. https://doi.org/10.3390/ani14192818
Chicago/Turabian StyleMezzasalma, Marcello, Gaetano Odierna, Rachele Macirella, and Elvira Brunelli. 2024. "New Insights on Chromosome Diversification in Malagasy Chameleons" Animals 14, no. 19: 2818. https://doi.org/10.3390/ani14192818








