Establishing Joint Orientation Angles of the Limbs in Korean Raccoon Dogs (Nyctereutes procyonoides koreensis) Using Computed Tomographic Imaging
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CT Technique
2.3. Measurements
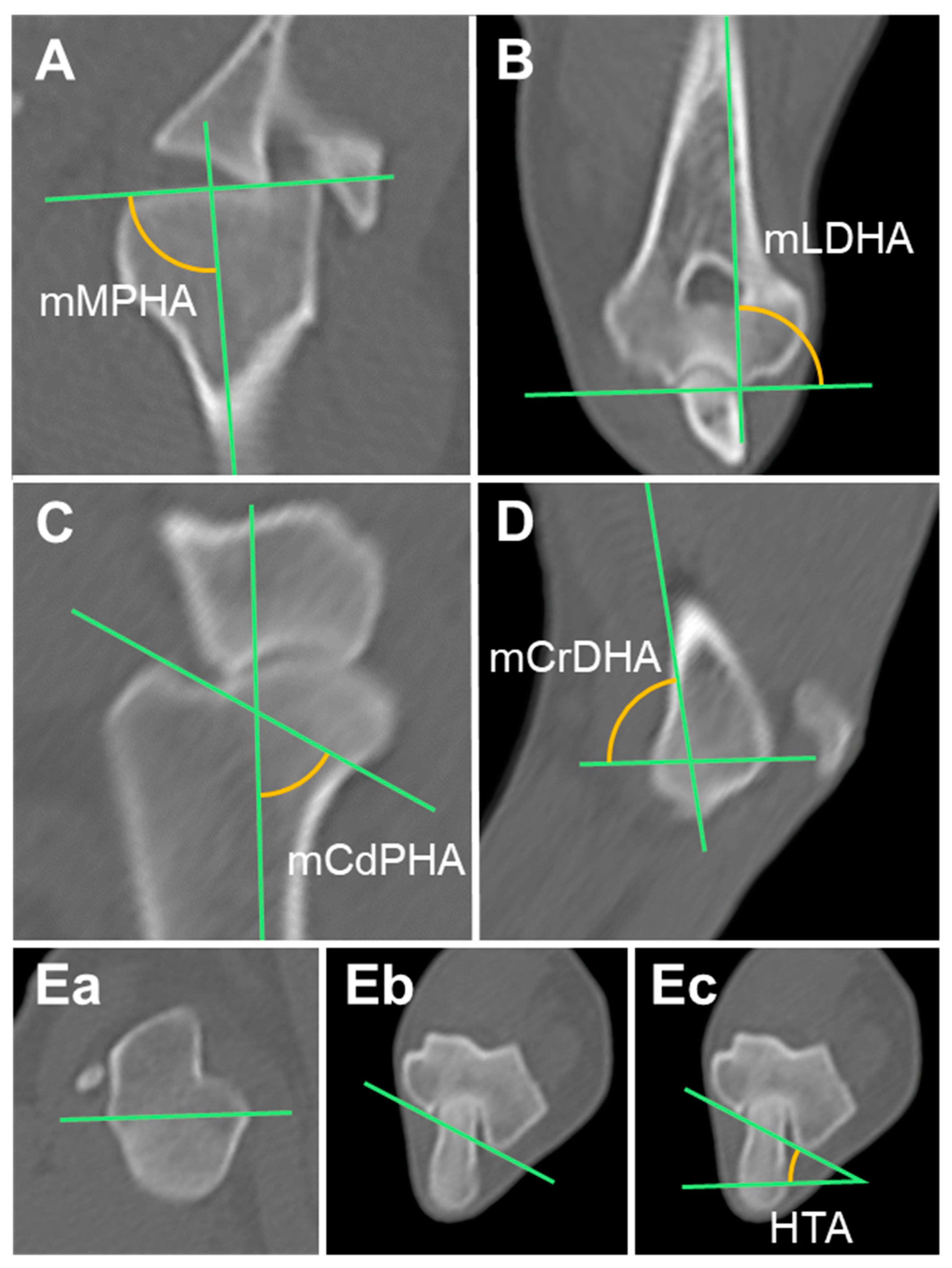
2.3.1. Mechanical Medial Proximal Humeral Angle (mMPHA) and Mechanical Lateral Distal Humeral Angle (mLDHA)
2.3.2. Mechanical Caudal Proximal Humeral Angle (mCdPHA) and Mechanical Cranial Distal Humeral Angle (mCrDHA)
2.3.3. Humeral Torsional Angle (HTA)
2.3.4. Anatomical Medial Proximal Radial Angle (aMPRA) and Anatomical Lateral Distal Radial Angle (aLDRA)
2.3.5. Anatomical Cranial Proximal Radial Angle (aCrPRA) and Anatomical Caudal Distal Radial Angle (aCdDRA)
2.3.6. Radial Torsional Angle (RTA)
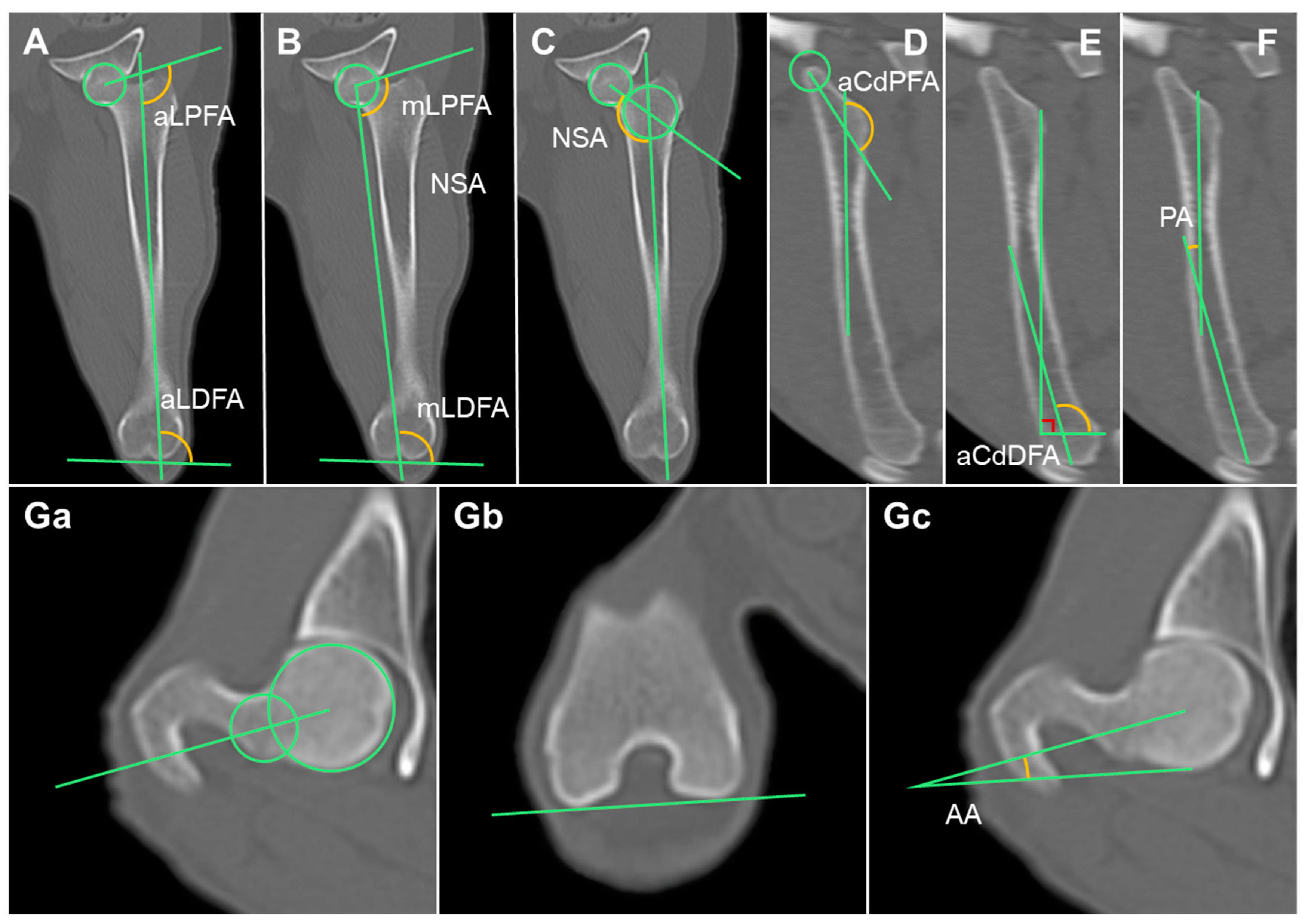
2.3.7. Anatomical Lateral Proximal Femoral Angle (aLPFA) and Anatomical Lateral Distal Femoral Angle (aLDFA)
2.3.8. Mechanical Lateral Proximal Femoral Angle (mLPFA) and Mechanical Lateral Distal Femoral Angle (mLDFA)
2.3.9. Neck Shaft Angle
2.3.10. Anatomical Caudal Proximal Femoral Angle (aCdPFA), Anatomical Caudal Distal Femoral Angle (aCdDFA), and Procurvation Angle (PA)
2.3.11. Anteversion Angle (AA)
2.3.12. Mechanical Medial Proximal Tibial Angle (mMPTA) and Mechanical Medial Distal Tibial Angle (mMDTA)
2.3.13. Mechanical Caudal Proximal Tibial Angle (mCdPTA), Mechanical Cranial Distal Tibial Angle (mCrDTA), and Tibial Plateau Angle (TPA)
2.3.14. Tibial Torsional Angle (TTA)
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef]
- Hyun, B.-H.; Lee, K.-K.; Kim, I.-J.; Lee, K.-W.; Park, H.-J.; Lee, O.-S.; An, S.-H.; Lee, J.-B. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res. 2005, 114, 113–125. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, K.-S.; Lee, H.; Min, M.-S. Population genetic study of the raccoon dog (Nyctereutes procyonoides) in South Korea using newly developed 12 microsatellite markers. Genes Genet. Syst. 2013, 88, 69–76. [Google Scholar] [CrossRef]
- Kim, S.I.; Park, S.K.; Lee, H.; Oshida, T.; Kimura, J.; Kim, Y.J.; Nguyen, S.; Sashika, M.; Min, M.S. Phylogeography of Korean raccoon dogs: Implications of peripheral isolation of a forest mammal in E ast A sia. J. Zool. 2013, 290, 225–235. [Google Scholar] [CrossRef]
- Han, Y.-J.; Park, J.; Lee, Y.-S.; Chae, J.-s.; Yu, D.-H.; Park, B.-K.; Kim, H.-C.; Choi, K.-S. Molecular identification of selected tick-borne pathogens in wild deer and raccoon dogs from the Republic of Korea. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 25–31. [Google Scholar] [CrossRef]
- Oem, J.-K.; Kim, S.-H.; Kim, Y.-H.; Lee, M.-H.; Lee, K.-K. Complete genome sequences of three rabies viruses isolated from rabid raccoon dogs and a cow in Korea. Virus Genes 2013, 47, 563–568. [Google Scholar] [CrossRef]
- Sesoko, N.F.; Rahal, S.C.; Bortolini, Z.; De Souza, L.P.; Vulcano, L.C.; Monteiro, F.O.B.; Teixeira, C.R. Skeletal morphology of the forelimb of Myrmecophaga tridactyla. J. Zoo Wildl. Med. 2015, 46, 713–722. [Google Scholar] [CrossRef]
- Kirberger, R.M.; du Plessis, W.M.; Turner, P.H. Radiologic anatomy of the normal appendicular skeleton of the lion (Panthera leo). Part 1: Thoracic limb. J. Zoo Wildl. Med. 2005, 36, 21–28. [Google Scholar] [CrossRef]
- Van Staden, S.L. The thoracic limb of the suricate (Suricata suricatta): Osteology, radiologic anatomy, and functional morphologic changes. J. Zoo Wildl. Med. 2014, 45, 476–486. [Google Scholar] [CrossRef]
- Kamioka, M.; Sasaki, M.; Yamada, K.; Endo, H.; Oishi, M.; Yuhara, K.; Tomikawa, S.; Sugimoto, M.; Oshida, T.; Kondoh, D. Mobility of the forearm in the raccoon (Procyon lotor), raccoon dog (Nyctereutes procyonoides) and red panda (Ailurus fulgens). J. Vet. Med. Sci. 2017, 79, 224–229. [Google Scholar] [CrossRef]
- Lee, E.; Jang, Y.-J.; Kim, I.-S.; Tae, H.-J.; Sim, J.; Ahn, D. Morphology of the aortic arch branching pattern in raccoon dogs (Nyctereutes procyonoides, Gray, 1834). J. Vet. Sci. 2024, 25, e32. [Google Scholar] [CrossRef]
- Hidaka, S.; Matsumoto, M.; Hiji, H.; Ohsako, S.; Nishinakagawa, H. Morphology and morphometry of skulls of raccoon dogs, Nyctereutes procyonoides and badgers, Meles meles. J. Vet. Med. Sci. 1998, 60, 161–167. [Google Scholar] [CrossRef][Green Version]
- Wood, M.C.; Fox, D.B.; Tomlinson, J.L. Determination of the mechanical axis and joint orientation lines in the canine humerus: A radiographic cadaveric study. Vet. Surg. 2014, 43, 414–417. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, E.; Jeong, Y.; Jeong, S.M.; Lee, H.B.; Kwon, Y. Measurement of Thoracic Limb Joint Reference Angles in Purebred Shih-Tzu Dogs by Computed Tomography. J. Vet. Clin. 2020, 37, 169–174. [Google Scholar] [CrossRef]
- Fox, D.B.; Tomlinson, J.L.; Cook, J.L.; Breshears, L.M. Principles of uniapical and biapical radial deformity correction using dome osteotomies and the center of rotation of angulation methodology in dogs. Vet. Surg. 2006, 35, 67–77. [Google Scholar] [CrossRef]
- Kroner, K.; Cooley, K.; Hoey, S.; Hetzel, S.J.; Bleedorn, J.A. Assessment of radial torsion using computed tomography in dogs with and without antebrachial limb deformity. Vet. Surg. 2017, 46, 24–31. [Google Scholar] [CrossRef]
- Barnes, D.M.; Anderson, A.A.; Frost, C.; Barnes, J. Repeatability and reproducibility of measurements of femoral and tibial alignment using computed tomography multiplanar reconstructions. Vet. Surg. 2015, 44, 85–93. [Google Scholar] [CrossRef]
- Lusetti, F.; Bonardi, A.; Eid, C.; De Bellesini, A.B.; Martini, F.M. Pelvic limb alignment measured by computed tomography in purebred English Bulldogs with medial patellar luxation. Vet. Comp. Orthop. Traumatol. 2017, 30, 200–208. [Google Scholar] [CrossRef]
- Yasukawa, S.; Edamura, K.; Tanegashima, K.; Seki, M.; Teshima, K.; Asano, K.; Nakayama, T.; Hayashi, K. Evaluation of bone deformities of the femur, tibia, and patella in Toy Poodles with medial patellar luxation using computed tomography. Vet. Comp. Orthop. Traumatol. 2016, 29, 29–38. [Google Scholar] [CrossRef]
- Towle, H.A.; Griffon, D.J.; Thomas, M.W.; Siegel, A.M.; Dunning, D.; Johnson, A. Pre-and postoperative radiographic and computed tomographic evaluation of dogs with medial patellar luxation. Vet. Surg. 2005, 34, 265–272. [Google Scholar] [CrossRef]
- Apelt, D.; Kowaleski, M.P.; Dyce, J. Comparison of computed tomographic and standard radiographic determination of tibial torsion in the dog. Vet. Surg. 2005, 34, 457–462. [Google Scholar] [CrossRef]
- Aper, R.; Kowaleski, M.P.; Apelt, D.; Tod Drost, W.; Dyce, J. Computed tomographic determination of tibial torsion in the dog. Vet. Radiol. Ultrasound 2005, 46, 187–191. [Google Scholar] [CrossRef]
- Kwon, M.; Kwon, D.; Lee, J.; Lee, K.; Yoon, H. Evaluation of the radial procurvatum using the center of rotation of angulation methodology in chondrodystrophic dogs. Front. Vet. Sci. 2022, 8, 774993. [Google Scholar] [CrossRef]
- Paley, D. Principles of Deformity Correction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Lindblad-Toh, K.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas III, E.J.; Zody, M.C. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005, 438, 803–819. [Google Scholar] [CrossRef]
- Nyakatura, K.; Bininda-Emonds, O.R. Updating the evolutionary history of Carnivora (Mammalia): A new species-level supertree complete with divergence time estimates. BMC Biol. 2012, 10, 12. [Google Scholar] [CrossRef]
- Grant, R.A.; Montrose, V.T.; Wills, A.P. ExNOTic: Should we be keeping exotic pets? Animals 2017, 7, 47. [Google Scholar] [CrossRef]
- Rowden, P.; Steinhardt, D.; Sheehan, M. Road crashes involving animals in Australia. Accid. Anal. Prev. 2008, 40, 1865–1871. [Google Scholar] [CrossRef]
- Cserkesz, T.; Farkas, J. Annual trends in the number of wildlife-vehicle collisions on the main linear. North-West. J. Zool. 2014, 11, 141707. [Google Scholar]
- Sanchez, J.A.; Berta, A. Comparative anatomy and evolution of the odontocete forelimb. Mar. Mammal Sci. 2010, 26, 140–160. [Google Scholar] [CrossRef]
- Aversi-Ferreira, T.A. Comparative Anatomical Description of Forearm and Hand Arteries of Cebus libidinosus. Int. J. Morphol. 2009, 27, 219–226. [Google Scholar] [CrossRef][Green Version]


| Humeral Joint Orientation Angles (°) | Measurements | |||
|---|---|---|---|---|
| Mean | SD | Min | Max | |
| mMPHA (°) | 90.49 | 3.65 | 93.00 | 97.70 |
| mLDHA (°) | 87.58 | 4.67 | 80.90 | 101.00 |
| mCdPHA (°) | 54.23 | 7.87 | 37.10 | 66.70 |
| mCrDHA (°) | 104.12 | 10.98 | 81.00 | 123.60 |
| HTA (°) | 22.24 | 5.55 | 13.70 | 34.30 |
| Radial Joint Orientation Angles (°) | Measurements | |||
| Mean | SD | Min | Max | |
| aMPRA (°) | 88.12 | 5.99 | 74.60 | 100.60 |
| aLDRA (°) | 89.70 | 5.15 | 77.00 | 98.40 |
| aCrPRA (°) | 87.30 | 4.72 | 72.90 | 92.50 |
| aCdDRA (°) | 85.36 | 4.59 | 75.40 | 95.40 |
| RTA (°) | 1.17 | 6.81 | −11.50 | 8.50 |
| Femoral Joint Orientation Angles (°) | Measurements | |||
|---|---|---|---|---|
| Mean | SD | Min | Max | |
| aLPFA (°) | 108.37 | 5.81 | 97.40 | 123.30 |
| aLDFA (°) | 92.94 | 4.02 | 85.40 | 101.80 |
| NSA (°) | 132.16 | 8.13 | 117.40 | 144.20 |
| mLPFA (°) | 103.43 | 5.76 | 91.30 | 117.10 |
| mLDFA (°) | 97.93 | 3.78 | 90.00 | 107.00 |
| aCdPFA (°) | 141.18 | 13.72 | 109.30 | 166.10 |
| aCdDFA (°) | 104.46 | 2.80 | 101.00 | 109.50 |
| PA (°) | 9.26 | 3.99 | 3.90 | 20.70 |
| AA (°) | 25.15 | 6.03 | 14.80 | 35.20 |
| Tibial Joint Orientation Angles (°) | Measurements | |||
| Mean | SD | Min | Max | |
| mMPTA (°) | 91.40 | 2.77 | 87.10 | 96.00 |
| mMDTA (°) | 91.43 | 3.27 | 87.30 | 98.90 |
| mCdPTA (°) | 57.76 | 5.74 | 47.50 | 71.50 |
| mCrDTA (°) | 90.51 | 4.10 | 82.10 | 96.90 |
| TPA (°) | 31.75 | 4.88 | 23.70 | 42.50 |
| TTA (°) | 0.49 | 5.79 | −11.40 | 8.40 |
| Raccoon Dogs | Shih Tzus [14] | Canine Cadavers [13] | Poodles [23] | 10 Normal Dogs [16] | |
|---|---|---|---|---|---|
| CT | CT | Radiograph | Radiograph | CT | |
| Humerus (°) | |||||
| mMPHA (°) | 90.49 ± 3.65 | 84.74 ± 3.95 | - | - | - |
| mLDHA (°) | 87.58 ± 4.67 | 85.04 ± 2.57 | 86.92 ± 1.24 | - | - |
| mCdPHA (°) | 54.23 ± 7.87 | 46.75 ± 2.20 | 43.28 ± 5.44 | - | - |
| mCrDHA (°) | 104.12 ± 10.98 | 79.47 ± 1.97 | 71.86 ± 3.97 | - | - |
| HTA (°) | 22.24 ± 5.55 | 19.16 ± 2.38 | - | - | 27.4 |
| Radius (°) | |||||
| aMPRA (°) | 88.12 ± 5.99 | 85.04 ± 1.58 | - | 77.91 ± 3.44 | - |
| aLDRA (°) | 89.70 ± 5.15 | 87.59 ± 1.37 | - | 89.60 ± 2.03 | - |
| aCrPRA (°) | 87.30 ± 4.72 | 84.60 ± 1.16 | - | 88.13 ± 3.91 | - |
| aCdDRA (°) | 85.36 ± 4.59 | 84.27 ± 1.79 | - | 71.11 ± 3.80 | - |
| RTA (°) | 1.17 ± 6.81 | 20.91 ± 3.00 | - | - | 2.7 |
| Raccoon Dogs | English Bulldogs [18] | Poodles [19] | ||
|---|---|---|---|---|
| CT | CT | Radiograph | CT | |
| Femur (°) | ||||
| aLPFA (°) | 108.37 ± 5.81 | 111.75 ± 6.66 | 106.6 ± 8.7 | 119.5 ± 5.7 |
| aLDFA (°) | 92.94 ± 4.02 | 92.33 ± 4.75 | 94.4 ± 4.1 | 90.3 ± 2.8 |
| NSA (°) | 132.16 ± 8.13 | 129.11 ± 8.03 | 127.7 ± 6.3 | 116.8 ± 6.1 |
| mLPFA (°) | 103.43 ± 5.76 | 111.02 ± 6.90 | 102.1 ± 8.8 | 113.6 ± 6.1 |
| mLDFA (°) | 97.93 ± 3.78 | 101.56 ± 2.43 | 99.1 ± 3.1 | 96.2 ± 2.5 |
| aCdPFA (°) | 141.18 ± 13.72 | - | 157.3 ± 7.7 | 153.3 ± 5.1 |
| aCdDFA (°) | 104.46 ± 2.80 | - | 104.3 ± 2.1 | 102.9 ± 3.2 |
| PA (°) | 9.26 ± 3.99 | - | 12.7 ± 4.1 | 11.2 ± 5.2 |
| AA (°) | 25.15 ± 6.03 | - | - | 20.8 ± 4.1 |
| Tibia (°) | ||||
| mMPTA (°) | 91.40 ± 2.77 | 91.98 ± 4.28 | 94.4 ± 3.8 | 92.8 ± 2.1 |
| mMDTA (°) | 91.43 ± 3.27 | 91.34 ± 2.98 | 96.5 ± 2.3 | 96.5 ± 4.1 |
| mCdPTA (°) | 57.76 ± 5.74 | 63.25 ± 6.15 | - | - |
| mCrDTA (°) | 90.51 ± 4.10 | 86.73 ± 3.52 | 91.0 ± 4.6 | 98.5 ± 3.8 |
| TPA (°) | 31.75 ± 4.88 | - | 27.6 ± 4.7 | 21.3 ± 3.3 |
| TTA (°) | 0.49 ± 5.79 | 4.00 ± 8.82 | - | 11.3 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.; Ahn, S.; Kwak, H.-H.; Woo, H.-M.; Kim, J. Establishing Joint Orientation Angles of the Limbs in Korean Raccoon Dogs (Nyctereutes procyonoides koreensis) Using Computed Tomographic Imaging. Animals 2024, 14, 2827. https://doi.org/10.3390/ani14192827
Ko S, Ahn S, Kwak H-H, Woo H-M, Kim J. Establishing Joint Orientation Angles of the Limbs in Korean Raccoon Dogs (Nyctereutes procyonoides koreensis) Using Computed Tomographic Imaging. Animals. 2024; 14(19):2827. https://doi.org/10.3390/ani14192827
Chicago/Turabian StyleKo, Seongju, Sangjin Ahn, Ho-Hyun Kwak, Heung-Myong Woo, and Junhyung Kim. 2024. "Establishing Joint Orientation Angles of the Limbs in Korean Raccoon Dogs (Nyctereutes procyonoides koreensis) Using Computed Tomographic Imaging" Animals 14, no. 19: 2827. https://doi.org/10.3390/ani14192827
APA StyleKo, S., Ahn, S., Kwak, H.-H., Woo, H.-M., & Kim, J. (2024). Establishing Joint Orientation Angles of the Limbs in Korean Raccoon Dogs (Nyctereutes procyonoides koreensis) Using Computed Tomographic Imaging. Animals, 14(19), 2827. https://doi.org/10.3390/ani14192827






