Daily Activity Rhythms of Animals in the Southwest Mountains, China: Influences of Interspecific Relationships and Seasons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
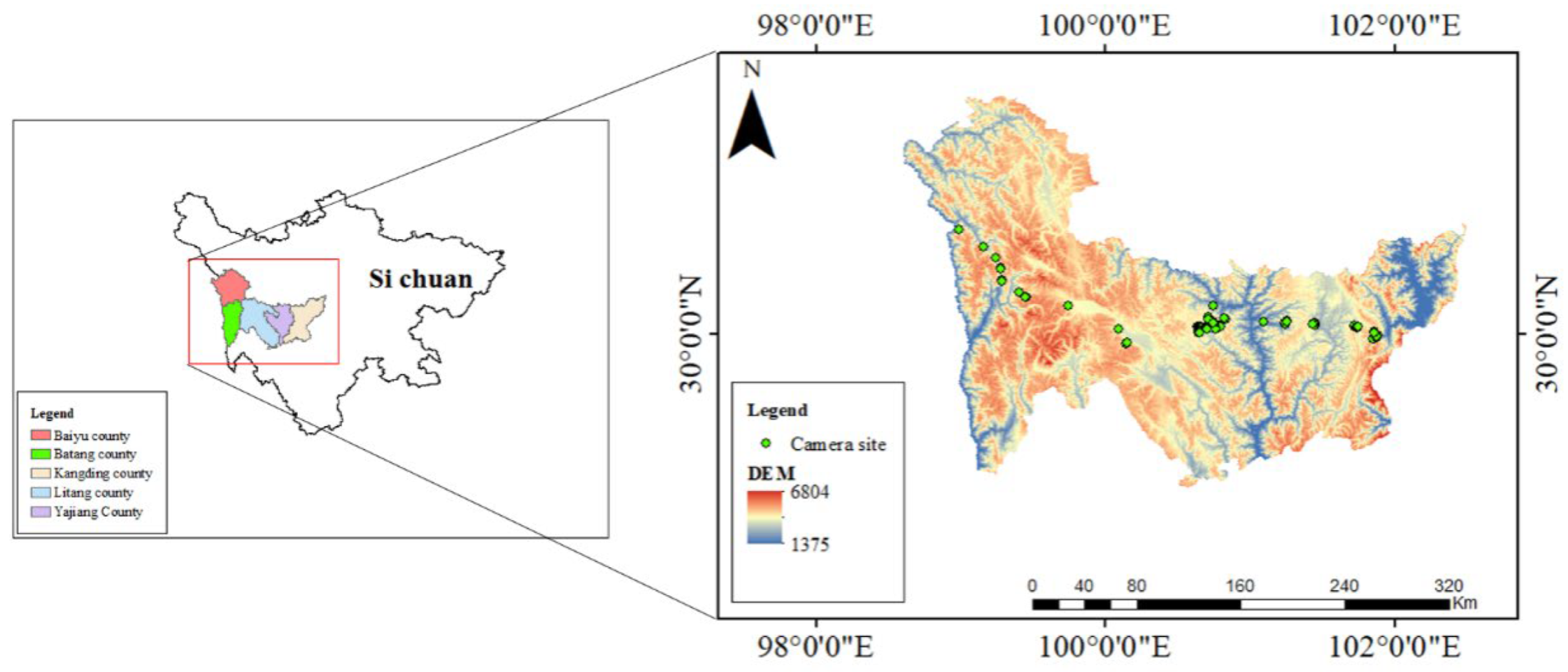
2.1. Study Area
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
3.1. Species Overview
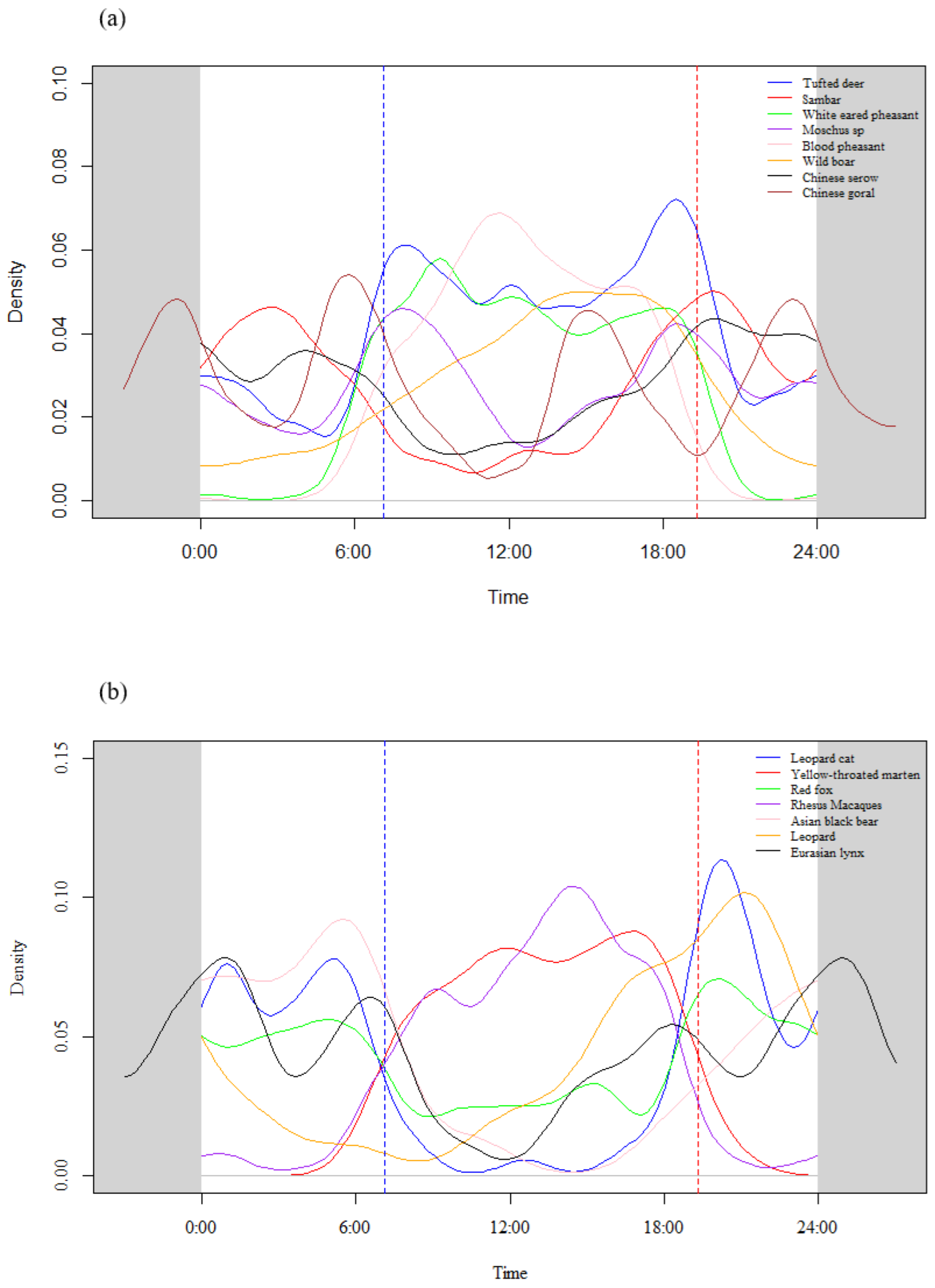
3.2. Daily Activity Rhythm Types
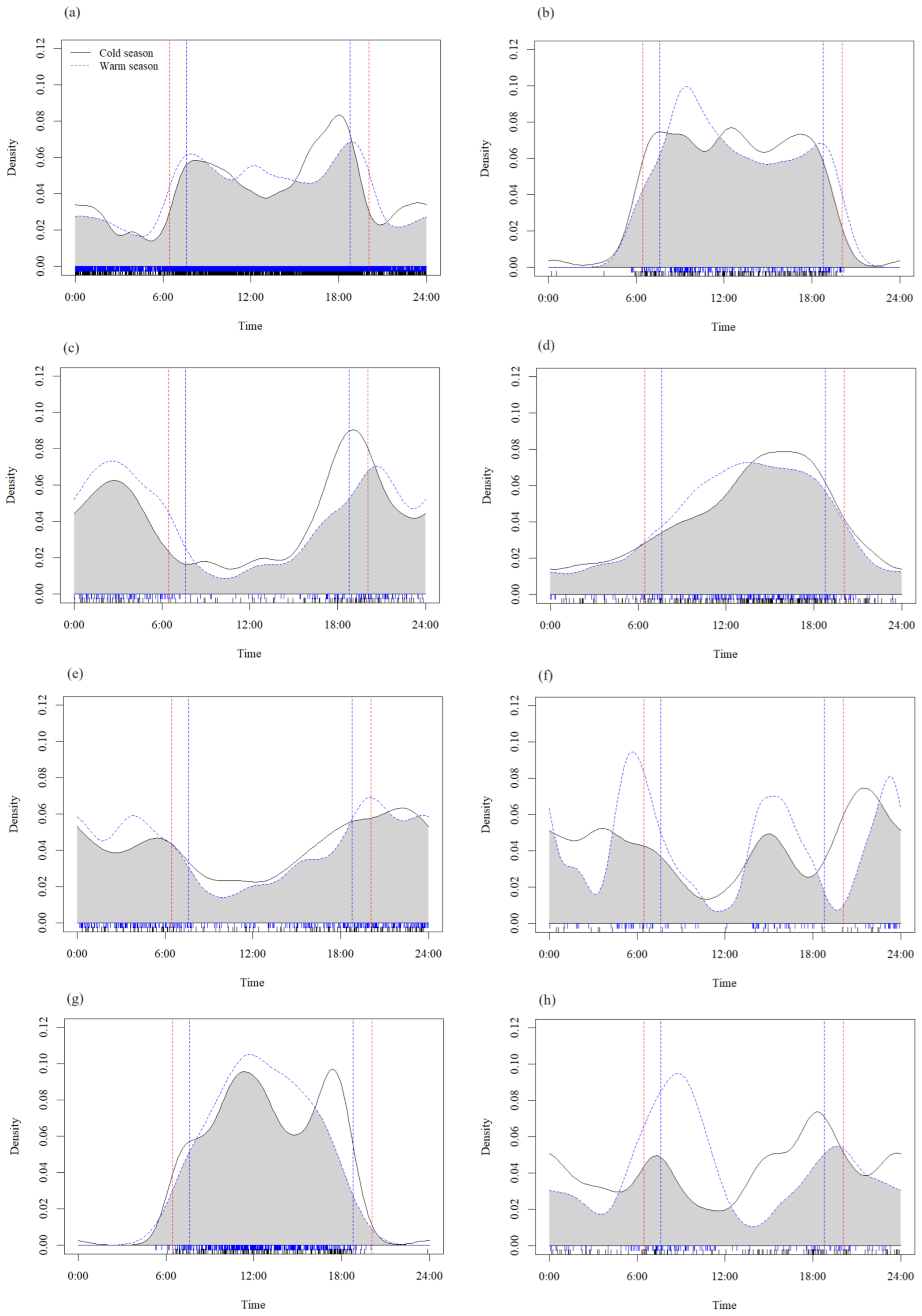
3.3. Seasonal Variation in Daily Activity Patterns
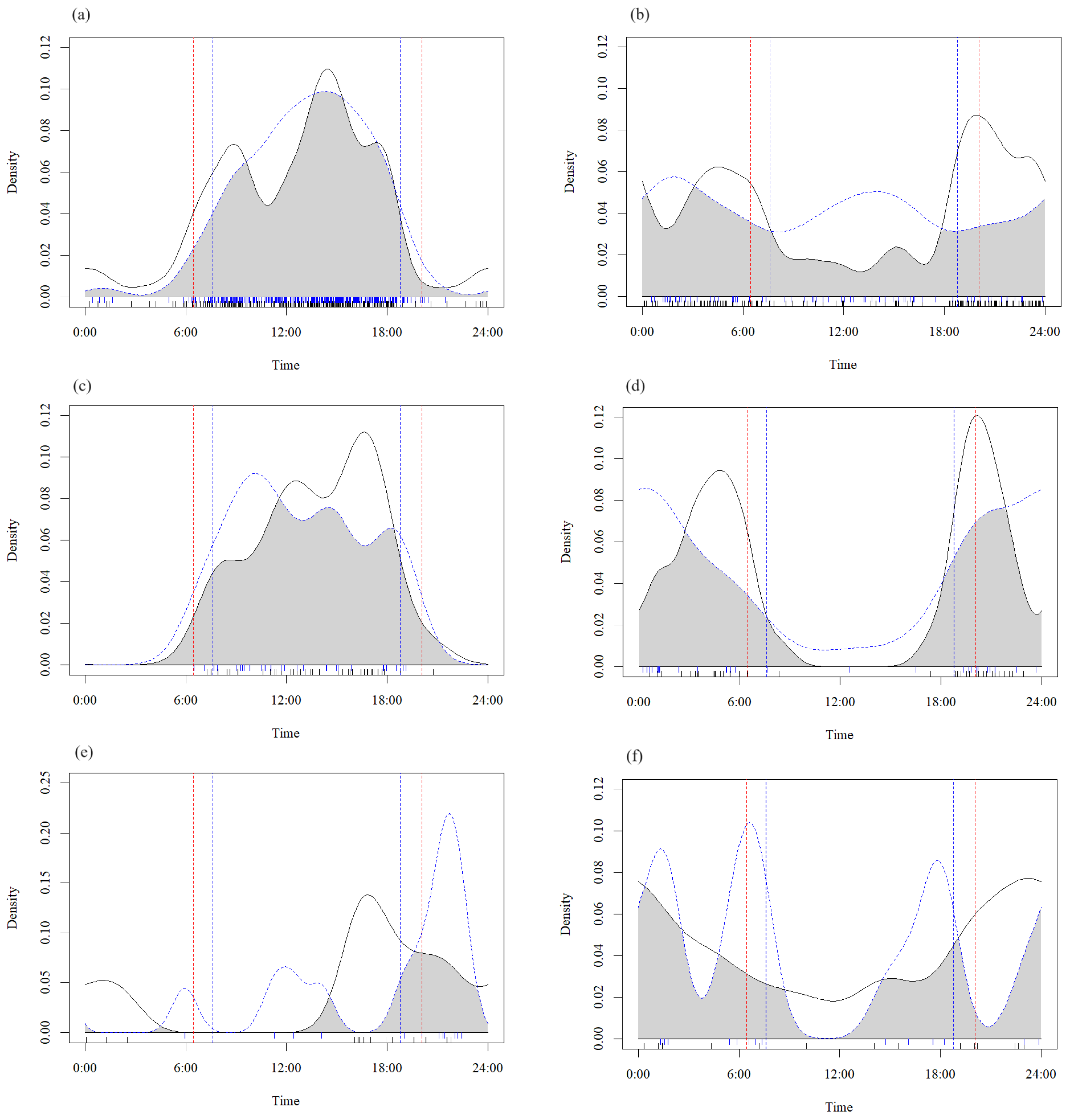
3.4. Seasonal Variation in Interspecific Relationships
3.5. Influences of the Seasons on Interspecific Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; McShea, W.J.; Wang, D.; Gu, X.; Zhang, X.; Zhang, L.; Shen, X. Retreat of large carnivores across the giant panda distribution range. Nat. Ecol. Evol. 2020, 4, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Li, Z.; Duo, L.; Li, S.; Wang, T. Competition and coexistence among terrestrial mammalian carnivores. Biodivers. Sci. 2021, 29, 81–97. [Google Scholar]
- Finn, K.T.; Janse van Vuuren, A.K.; Hart, D.W.; Süess, T.; Zöttl, M.; Bennett, N.C. Seasonal Changes in Locomotor Activity Patterns of Wild Social Natal Mole-Rats (Cryptomys hottentotus natalensis). Front. Ecol. Evol. 2022, 10, 819393. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, Z.; Zhang, L.; Wang, X.; Li, M.; Xiang, Z. Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps. Animals 2019, 9, 1071. [Google Scholar] [CrossRef]
- Karanth, K.U.; Srivathsa, A.; Vasudev, D.; Puri, M.; Parameshwaran, R.; Kumar, N.S. Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161860. [Google Scholar] [CrossRef]
- Andersen, G.E.; Johnson, C.N.; Jones, M.E. Space use and temporal partitioning of sympatric Tasmanian devils and spotted-tailed quolls. Austral Ecol. 2020, 45, 355–365. [Google Scholar] [CrossRef]
- Gómez-Ortiz, Y.; Monroy-Vilchis, O.; Castro-Arellano, I. Temporal coexistence in a carnivore assemblage from central Mexico: Temporal-domain dependence. Mammal Res. 2019, 64, 333–342. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Kanamori, T.; Matsukawa, A.; Tangah, J.; Tuuga, A.; Malim, P.T.; Bernard, H.; Ahmad, A.H.; Matsuda, I.; Hanya, G. Temporal activity patterns suggesting niche partitioning of sympatric carnivores in Borneo, Malaysia. Sci. Rep. 2021, 11, 19819. [Google Scholar] [CrossRef]
- Viana, D.S.; Granados, J.E.; Fandos, P.; Pérez, J.M.; Cano-Manuel, F.J.; Burón, D.; Fandos, G.; Aguado, M.Á.P.; Figuerola, J.; Soriguer, R.C. Linking seasonal home range size with habitat selection and movement in a mountain ungulate. Mov. Ecol. 2018, 6, 1. [Google Scholar] [CrossRef]
- Semenzato, P.; Cagnacci, F.; Ossi, F.; Eccel, E.; Morellet, N.; Hewison, A.J.M.; Sturaro, E.; Ramanzin, M. Behavioural heat-stress compensation in a cold-adapted ungulate: Forage-mediated responses to warming Alpine summers. Ecol. Lett. 2021, 24, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, Y.N.; White, K.S.; Waite, J.N. Staying close to home: Ecological constraints on space use and range fidelity in a mountain ungulate. Ecol. Evol. 2021, 11, 11051–11064. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Liao, M.; Dong, W.; Wu, B.; Li, D. Temporal and Spatial Activity Patterns of Sympatric Wild Ungulates in Qinling Mountains, China. Animals 2022, 12, 1666. [Google Scholar] [CrossRef]
- Karanth, K.K. Wildlife in the Matrix: Spatio-Temporal Patterns of Herbivore Occurrence in Karnataka, India. Environ. Manag. 2016, 57, 189–206. [Google Scholar] [CrossRef]
- Mueller, M.A.; Drake, D.; Allen, M.L. Coexistence of coyotes (Canis latrans) and red foxes (Vulpes vulpes) in an urban landscape. PLoS ONE 2018, 13, e0190971. [Google Scholar] [CrossRef]
- Vance, R.R. Competition and Mechanism of Coexistence in Three Sympatric of Intertidal Hermit Crabs. Ecology 1972, 53, 1062–1074. [Google Scholar] [CrossRef]
- Barraclough, T.G.; Vogler, A.P.; Harvey, P.H. Revealing the Factors That Promote Speciation. Philos. Trans. R. Soc. B: Biol. Sci. 1998, 353, 241–249. [Google Scholar] [CrossRef]
- Prigioni, C.; Balestrieri, A.; Remonti, L.; Cavada, L. Differential use of food and habitat by sympatric carnivores in the eastern Italian Alps. Ital. J. Zool. 2008, 75, 173–184. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Y.-Y.; Liu, Z.-X.; Dayananda, B.; Fu, X.-B.; Yuan, D.; Tu, Z.-B.; Luo, C.-P.; Li, J.-Q. Temporal niche patterns of large mammals in Wanglang National Nature Reserve, China. Glob. Ecol. Conserv. 2020, 22, e01015. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Tracy, C.R.; Christian, K.A. Ecological Relations Among Space, Time, and Thermal Niche Axes. Ecology 1986, 67, 609–615. [Google Scholar] [CrossRef]
- Frey, S.; Fisher, J.T.; Burton, A.C.; Volpe, J.P. Investigating animal activity patterns and temporal niche partitioning using camera-trap data: Challenges and opportunities. Remote Sens. Ecol. Conserv. 2017, 3, 123–132. [Google Scholar] [CrossRef]
- Ahumada, J.A.; Hurtado, J.; Lizcano, D.J. Monitoring the Status and Trends of Tropical Forest Terrestrial Vertebrate Communities from Camera Trap Data: A Tool for Conservation. PLoS ONE 2013, 8, e73707. [Google Scholar] [CrossRef]
- Ferreguetti, Á.C.; Tomas, W.M.; Bergallo, H.G. Density, occupancy, and activity pattern of two sympatric deer (Mazama) in the Atlantic Forest, Brazil. J. Mammal. 2015, 96, 1245–1254. [Google Scholar] [CrossRef]
- Fiore, A.D.; Rodman, P.S. Time Allocation Patterns of Lowland Woolly Monkeys (Lagothrix lagotricha poeppigii) in a Neotropical Terra Firma Forest. Int. J. Primatol. 2001, 22, 449–480. [Google Scholar] [CrossRef]
- Dussault, C.; Poulin, M.; Courtois, R.; Ouellet, J.-p. Temporal and spatial distribution of moose-vehicle accidents in the Laurentides Wildlife Reserve, Quebec, Canada. Wildl. Biol. 2006, 12, 415–425. [Google Scholar] [CrossRef]
- Ebensperger, L.A.; Sobrero, R.; Campos, V.; Giannoni, S.M. Activity, range areas, and nesting patterns in the viscacha rat, Octomys mimax. J. Arid Environ. 2008, 72, 1174–1183. [Google Scholar] [CrossRef]
- Fortin, D.; Beyer, H.L.; Boyce, M.S.; Smith, D.W.; Duchesne, T.; Mao, J.S. Wolves influence elk movements: Behavior shapes a trophic cascade in yellowstone national park. Ecology 2005, 86, 1320–1330. [Google Scholar] [CrossRef]
- Ross, J.; Hearn, A.J.; Johnson, P.J.; Macdonald, D.W. Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. J. Zool. 2013, 290, 96–106. [Google Scholar] [CrossRef]
- Linkie, M.; Ridout, M.S. Assessing tiger–prey interactions in Sumatran rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Jȩdrzejewski, W.; Schmidt, K.; Okarma, H.; Ruczyński, I.; Śniezko, S.; Gula, R. Daily Patterns and Duration of Wolf Activity in the Białowieza Forest, Poland. J. Mammal. 2003, 84, 243–253. [Google Scholar] [CrossRef]
- Foster, V.C.; Sarmento, P.; Sollmann, R.; Tôrres, N.; Jácomo, A.T.A.; Negrões, N.; Fonseca, C.; Silveira, L. Jaguar and Puma Activity Patterns and Predator-Prey Interactions in Four Brazilian Biomes. Biotropica 2013, 45, 373–379. [Google Scholar] [CrossRef]
- Rossa, M.; Lovari, S.; Ferretti, F. Spatiotemporal patterns of wolf, mesocarnivores and prey in a Mediterranean area. Behav. Ecol. Sociobiol. 2021, 75, 32. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Z.; Zheng, C.; Fang, J. Biodiversity in China’s mountains. Front. Ecol. Environ. 2006, 4, 347–352. [Google Scholar] [CrossRef]
- Li, X.; Hu, W.; Pu, C.; Li, Q.; Yu, Q.; Hu, Z.; Bleisch, W.V.; Jiang, X.-L. Camera-trapping monitoring platform for mammals and pheasants in the Longitudinal Range and Gorge Region of Southwest China: Protocol, progress and future outlook. Biodivers. Sci. 2020, 28, 1090–1096. [Google Scholar] [CrossRef]
- Li, X.; Bleisch, W.V.; Liu, X.; Hu, W.; Jiang, X. Human disturbance and prey occupancy as predictors of carnivore richness and biomass in a Himalayan hotspot: Drivers affecting carnivores richness and biomass. Anim. Conserv. 2021, 24, 64–72. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; He, X.; Li, D.; Hu, Q.; Zhang, Y.; Ran, J. Free-ranging livestock affected the spatiotemporal behavior of the endangered snow leopard (Panthera uncia). Ecol. Evol. 2023, 13, e9992. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Li, H.; Xiao, Y.; Zhang, D.; Smith, J.L.D.; Ge, J.; Wang, T. Assessing mammal species richness and occupancy in a Northeast Asian temperate forest shared by cattle. Divers. Distrib. 2021, 27, 857–872. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, P.; Wu, Y.; Dai, Q.; Luo, G.; Zhou, H.; Zhao, D.; Ran, J. Livestock limits snow leopard’s space use by suppressing its prey, blue sheep, at Gongga Mountain, China. Glob. Ecol. Conserv. 2021, 29, e01728. [Google Scholar] [CrossRef]
- Petridou, M.; Benson, J.; Gimenez, O.; Kati, V. Spatiotemporal Patterns of Wolves, and Sympatric Predators and Prey Relative to Human Disturbance in Northwestern Greece. Diversity 2023, 15, 184. [Google Scholar] [CrossRef]
- Jia, J.; Fang, Y.; Li, X.; Song, K.; Xie, W.; Bu, C.; Sun, Y. Temporal Activity Patterns of Sympatric Species in the Temperate Coniferous Forests of the Eastern Qinghai-Tibet Plateau. Animals 2023, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Nouvellet, P.; Rasmussen, G.; Macdonald, D.; Courchamp, F. Noisy clocks and silent sunrises: Measurement methods of daily activity pattern. J. Zool. 2011, 286, 179–184. [Google Scholar] [CrossRef]
- Gomez, H.; Wallace, R.; Ayala, G.; Tejada, R. Dry season activity periods of some Amazonian mammals. Stud. Neotrop. Fauna Environ. 2005, 40, 91–95. [Google Scholar] [CrossRef]
- Ridout, M.; Linkie, M. Estimating Overlap of Daily Activity Patterns From Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Romero-Muñoz, A.; Maffei, L.; Cuéllar, E.; Noss, A.J. Temporal separation between jaguar and puma in the dry forests of southern Bolivia. J. Trop. Ecol. 2010, 26, 303–311. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Xiang, Y.; Zhou, L.; Wang, H.; Bai, M.; Deng, S. Diversity and spatial and temporal distribution pattern of large and medium-sized mammals in the eastern Qinghai-Tibet Plateau. J. Sichuan For. Sci. Technol. 2024, 45, 48–62. [Google Scholar] [CrossRef]
- Ikeda, T.; Uchida, K.; Matsuura, Y.; Takahashi, H.; Yoshida, T.; Kaji, K.; Koizumi, I. Seasonal and Diel Activity Patterns of Eight Sympatric Mammals in Northern Japan Revealed by an Intensive Camera-Trap Survey. PLoS ONE 2016, 11, e0163602. [Google Scholar] [CrossRef]
- Bogdan, V.; Jůnek, T.; Jůnková Vymyslická, P. Temporal overlaps of feral cats with prey and competitors in primary and human-altered habitats on Bohol Island, Philippines. PeerJ 2016, 4, e2288. [Google Scholar] [CrossRef]
- Ramesh, T.; Kalle, R.; Sankar, K.; Qureshi, Q. Spatio-temporal partitioning among large carnivores in relation to major prey species in Western Ghats. J. Zool. 2012, 287, 269–275. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, F.; Li, D.; Chen, Z.; Chen, P. Spatial–Temporal Patterns of Sympatric Asiatic Black Bears (Ursus thibetanus) and Brown Bears (Ursus arctos) in Northeastern China. Animals 2022, 12, 1262. [Google Scholar] [CrossRef]
- Zhang, D.; An, B.; Chen, L.; Sun, Z.; Mao, R.; Zhao, C.; Zhang, L. Camera Trapping Reveals Spatiotemporal Partitioning Patterns and Conservation Implications for Two Sympatric Pheasant Species in the Qilian Mountains, Northwestern China. Animals 2022, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Ordiz, A.; Sæbø, S.; Kindberg, J.; Swenson, J.E.; Støen, O.-G. Seasonality and human disturbance alter brown bear activity patterns: Implications for circumpolar carnivore conservation? Anim. Conserv. 2017, 20, 51–60. [Google Scholar] [CrossRef]
- Lizcano, D.J.; Cavelier, J. Daily and seasonal activity of the mountain tapir (Tapirus pinchaque) in the Central Andes of Colombia. J. Zool. 2000, 252, 429–435. [Google Scholar] [CrossRef]
- Monterroso, P.; Alves, P.C.; Ferreras, P. Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: Implications for species coexistence. Behav. Ecol. Sociobiol. 2014, 68, 1403–1417. [Google Scholar] [CrossRef]
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked Seasonal Variation in Structure and Function of Gut Microbiota in Forest and Alpine Musk Deer. Front. Microbiol. 2021, 12, 699797. [Google Scholar] [CrossRef]
- Brown, J.s.; Laundré, J.W.; Gurung, M. The Ecology of Fear: Optimal Foraging, Game Theory, and Trophic Interactions. J. Mammal. 1999, 80, 385–399. [Google Scholar] [CrossRef]
- Liu, H.; Li, L.; Ma, Q.; Wan, D.; Zhang, S.; Zhu, Y. Review on Wild Boar Research. Sichuan J. Zool. 2011, 30. [Google Scholar]
- Ferretti, F.; Pacini, G.; Belardi, I.; ten Cate, B.; Sensi, M.; Oliveira, R.; Rossa, M.; Burrini, L.; Lovari, S. Recolonizing wolves and opportunistic foxes: Interference or facilitation? Biol. J. Linn. Soc. 2020, 132, 196–210. [Google Scholar] [CrossRef]
- Marinho, P.H.; Fonseca, C.R.; Sarmento, P.; Fonseca, C.; Venticinque, E.M. Temporal niche overlap among mesocarnivores in a Caatinga dry forest. Eur. J. Wildl. Res. 2020, 66, 34. [Google Scholar] [CrossRef]
- Liu, X.; Wu, P.; Shao, X.; Songer, M.; Cai, Q.; He, X.; Zhu, Y. Diversity and activity patterns of sympatric animals among four types of forest habitat in Guanyinshan Nature Reserve in the Qinling Mountains, China. Environ. Sci. Pollut. Res. 2017, 24, 16465–16477. [Google Scholar] [CrossRef]
- Viviano, A.; Mori, E.; Fattorini, N.; Mazza, G.; Lazzeri, L.; Panichi, A.; Strianese, L.; Mohamed, W.F. Spatiotemporal Overlap between the European Brown Hare and Its Potential Predators and Competitors. Animals 2021, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Massé, A.; Côté, S.D. Spatiotemporal variations in resources affect activity and movement patterns of white-tailed deer (Odocoileus virginianus) at high density. Can. J. Zool. 2013, 91, 252–263. [Google Scholar] [CrossRef]
- Pauli, J.N.; Zuckerberg, B.; Whiteman, J.P.; Porter, W. The subnivium: A deteriorating seasonal refugium. Front. Ecol. Environ. 2013, 11, 260–267. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, B.; Wang, Y.; Zeren, Z.; Fang, C.; Jin, G.; Leng, Z.; Guan, T.; Sun, Z. Activity Rhythm and Seasonal Changes of Elaphodus cephalophus in Baihe National Nature Reserve, Sichuan Province. J. Sichuan For. Sci. Technol. 2021, 42, 27–32. [Google Scholar] [CrossRef]
- Peng, K.; Chen, X.; Wen, P.; Wei, Y.; Yang, Z.; Dai, Q. The Activity Rhythm Survey of Ungulates in Baishuihe National Nature Reserve Based on Infrared Camera Trapping. J. Sichuan For. Sci. Technol. 2021, 42, 76–82. [Google Scholar] [CrossRef]
- Sun, J.X.; Li, J.Q.; Wan, J.Q.; Li, S.; Guan, T.P.; Wang, J.; Xia, W.; Xu, H.G. Study on the activity rhythms of nine ungulates in summer and autumn in sichuan. J. Ecol. Rural Environ. 2018, 34, 1003–1009. [Google Scholar] [CrossRef]
- Gordon, I.J.; Prins, H.H.T. The Ecology of Browsing and Grazing II; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Cui, X.; Tang, J.; Zhang, Q.; Zhou, H.; Hong, M.; Wei, W.; Zhang, Z. Spatio-temporal variations in Takin (Budorcas tibetanus) habitats in the five mountains of Sichuan, China. Glob. Ecol. Conserv. 2023, 42, e02390. [Google Scholar] [CrossRef]
| Orders | Species | Diets | Total | Cold Season | Warm Season |
|---|---|---|---|---|---|
| Carnivora | Vulpes vulpes(red fox) | Carnivore | 215 | 146 | 69 |
| Carnivora | Prionailurus bengalensis (leopard cat) | Carnivore | 73 | 44 | 29 |
| Carnivora | Martes flavigula (yellow-throated marten) | Carnivore | 67 | 36 | 31 |
| Carnivora | Lynx lynx (Eurasian lynx) | Carnivore | 33 | 15 | 18 |
| Carnivora | Ursus thibetanus (Asiatic black bear) | Omnivore | 28 | 23 | 5 |
| Carnivora | Panthera pardus (leopard) | Carnivore | 26 | 14 | 12 |
| Artiodactyla | Elaphodus cephalophus (tufted deer) | Herbivore | 4874 | 1615 | 3259 |
| Artiodactyla | Sus scrofa (wild boar) | Omnivore | 467 | 247 | 220 |
| Artiodactyla | Capricornis milneedwardsii (Chinese serow) | Herbivore | 457 | 143 | 314 |
| Artiodactyla | Rusa unicolor (Sambar) | Herbivore | 272 | 123 | 149 |
| Artiodactyla | Moschus spp. | Herbivore | 246 | 125 | 121 |
| Artiodactyla | Naemorhedus caudatus (Chinese goral) | Herbivore | 115 | 27 | 88 |
| Primates | Macaca mulatta (macaque) | Omnivore | 576 | 234 | 342 |
| Galliformes | Ithaginis cruentus (blood pheasant) | Herbivore | 532 | 187 | 345 |
| Galliformes | Crossoptilon crossoptilon (white-eared pheasant) | Herbivore | 393 | 212 | 181 |
| Total | 8369 | 3173 | 5196 | ||
| Species | RAI | Dawn (06:00–08:00) | Day (8:00–18:00) | Dusk (18:00–20:00) | Night (20:00–06:00) | Rhythm Types |
|---|---|---|---|---|---|---|
| Yellow-throated marten | 0.36 | 10.45 | 80.60 | 7.46 | 1.49 | Daytime |
| Macaque | 3.06 | 7.99 | 80.38 | 6.94 | 4.69 | Daytime |
| White-eared pheasant | 2.02 | 13.74 | 70.48 | 12.72 | 3.05 | Daytime |
| Blood pheasant | 2.48 | 9.59 | 84.21 | 5.26 | 0.94 | Daytime |
| Tufted deer | 13.75 | 10.50 | 52.05 | 13.91 | 23.53 | Mostly daytime |
| Moschus spp. | 1.37 | 13.01 | 39.02 | 13.41 | 34.55 | Mostly daytime |
| Wild boar | 1.78 | 7.07 | 63.60 | 11.56 | 17.77 | Mostly daytime |
| Leopard cat | 0.50 | 5.48 | 5.48 | 13.70 | 75.34 | Mostly nighttime |
| Asiatic black bear | 0.24 | 17.39 | 4.35 | 8.70 | 69.57 | Mostly nighttime |
| Red fox | 1.05 | 8.37 | 23.72 | 13.02 | 54.88 | Cathemeral |
| Sambar | 1.58 | 5.51 | 22.43 | 14.71 | 57.35 | Cathemeral |
| Chinese serow | 1.40 | 8.97 | 25.16 | 12.25 | 53.61 | Cathemeral |
| Chinese goral | 1.11 | 12.17 | 34.78 | 2.61 | 50.43 | Cathemeral |
| Leopard | 0.27 | 0.00 | 34.62 | 11.54 | 53.85 | Cathemeral |
| Eurasian lynx | 0.66 | 15.15 | 21.21 | 9.09 | 54.55 | Cathemeral |
| Species 1 | Species 2 | Warm Season | Cold Season | ||||
|---|---|---|---|---|---|---|---|
| Δ | CI | p | Δ | CI | p | ||
| Yellow-throated marten | White-eared pheasant | 0.83 | 0.68–0.92 | 0.14 | 0.93 | 0.74–0.94 | 0.72 |
| Yellow-throated marten | Blood pheasant | 0.87 | 0.70–0.93 | 0.26 | 0.88 | 0.71–0.93 | 0.24 |
| Yellow-throated marten | Moschus spp. | 0.64 | 0.50–0.76 | 0 | 0.57 | 0.46–0.71 | 0 |
| Yellow-throated marten | Tufted deer | 0.76 | 0.63–0.85 | 0.01 | 0.71 | 0.60–0.80 | 0 |
| Red fox | White-eared pheasant | 0.6 | 0.50–0.72 | 0 | 0.42 | 0.37–0.53 | 0 |
| Red fox | Blood pheasant | 0.54 | 0.45–0.67 | 0 | 0.35 | 0.31–0.47 | 0 |
| Red fox | Moschus spp. | 0.68 | 0.59–0.81 | 0.14 | 0.76 | 0.67–0.85 | 0 |
| Red fox | Tufted deer | 0.78 | 0.68–0.86 | 0.10 | 0.6 | 0.55–0.69 | 0 |
| Leopard cat | White-eared pheasant | 0.35 | 0.24–0.51 | 0 | 0.27 | 0.22–0.41 | 0 |
| Leopard cat | Blood pheasant | 0.22 | 0.16–0.41 | 0 | 0.2 | 0.16–0.35 | 0 |
| Species 1 | Species 2 | Warm Season | Cold Season | ||||
|---|---|---|---|---|---|---|---|
| Δ | CI | p | Δ | CI | p | ||
| Yellow-throated marten | White-eared pheasant | 0.83 | 0.68–0.92 | 0.14 | 0.93 | 0.74–0.94 | 0.72 |
| Yellow-throated marten | Blood pheasant | 0.87 | 0.70–0.93 | 0.26 | 0.88 | 0.71–0.93 | 0.24 |
| Yellow-throated marten | Moschus spp. | 0.64 | 0.50–0.76 | 0 | 0.57 | 0.46–0.71 | 0 |
| Yellow-throated marten | Tufted deer | 0.76 | 0.63–0.85 | 0.01 | 0.71 | 0.60–0.80 | 0 |
| Red fox | White-eared pheasant | 0.6 | 0.50–0.72 | 0 | 0.42 | 0.37–0.53 | 0 |
| Red fox | Blood pheasant | 0.54 | 0.45–0.67 | 0 | 0.35 | 0.31–0.47 | 0 |
| Red fox | Moschus spp. | 0.68 | 0.59–0.81 | 0.14 | 0.76 | 0.67–0.85 | 0 |
| Red fox | Tufted deer | 0.78 | 0.68–0.86 | 0.10 | 0.6 | 0.55–0.69 | 0 |
| Leopard cat | White-eared pheasant | 0.35 | 0.24–0.51 | 0 | 0.27 | 0.22–0.41 | 0 |
| Leopard cat | Blood pheasant | 0.22 | 0.16–0.41 | 0 | 0.2 | 0.16–0.35 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, Q.; Jiang, Q.; Zhou, H.; Zhang, Z.; Zhou, H.; Wei, W.; Hong, M. Daily Activity Rhythms of Animals in the Southwest Mountains, China: Influences of Interspecific Relationships and Seasons. Animals 2024, 14, 2842. https://doi.org/10.3390/ani14192842
Li Q, Zhang Q, Jiang Q, Zhou H, Zhang Z, Zhou H, Wei W, Hong M. Daily Activity Rhythms of Animals in the Southwest Mountains, China: Influences of Interspecific Relationships and Seasons. Animals. 2024; 14(19):2842. https://doi.org/10.3390/ani14192842
Chicago/Turabian StyleLi, Qiuxian, Qian Zhang, Qingsong Jiang, Huaqiang Zhou, Zejun Zhang, Hong Zhou, Wei Wei, and Mingsheng Hong. 2024. "Daily Activity Rhythms of Animals in the Southwest Mountains, China: Influences of Interspecific Relationships and Seasons" Animals 14, no. 19: 2842. https://doi.org/10.3390/ani14192842






