Changes in Selected Biochemical Markers of Honey Bees Exposed to Fermented Common Tansy Solution (Tanacetum vulgare L.)
Abstract
:Simple Summary
Abstract
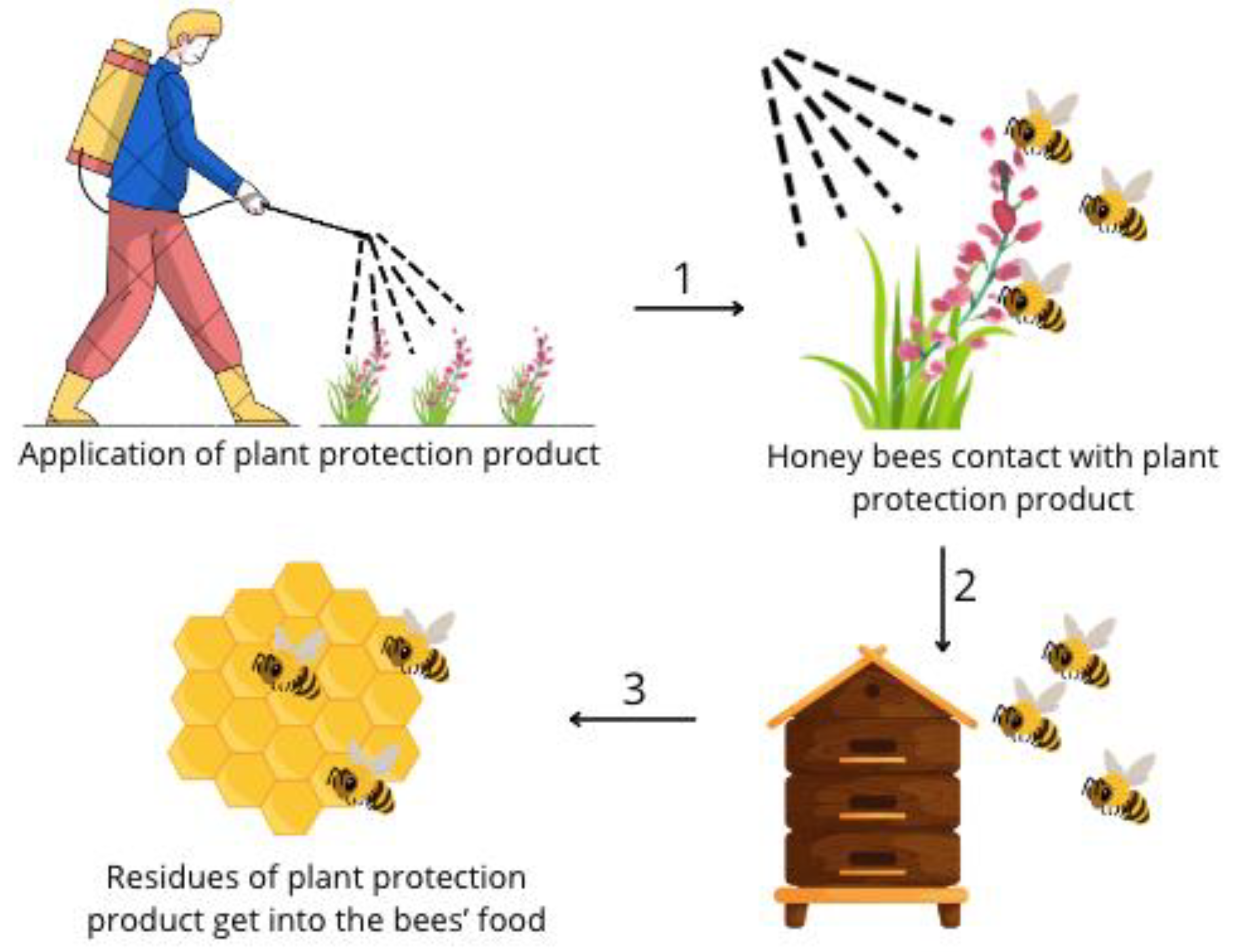
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Extract Exposure
2.3. Hemolymph Collection and Analysis
2.4. Analysis of Selected Biochemical Markers
2.5. Statistical Analysis
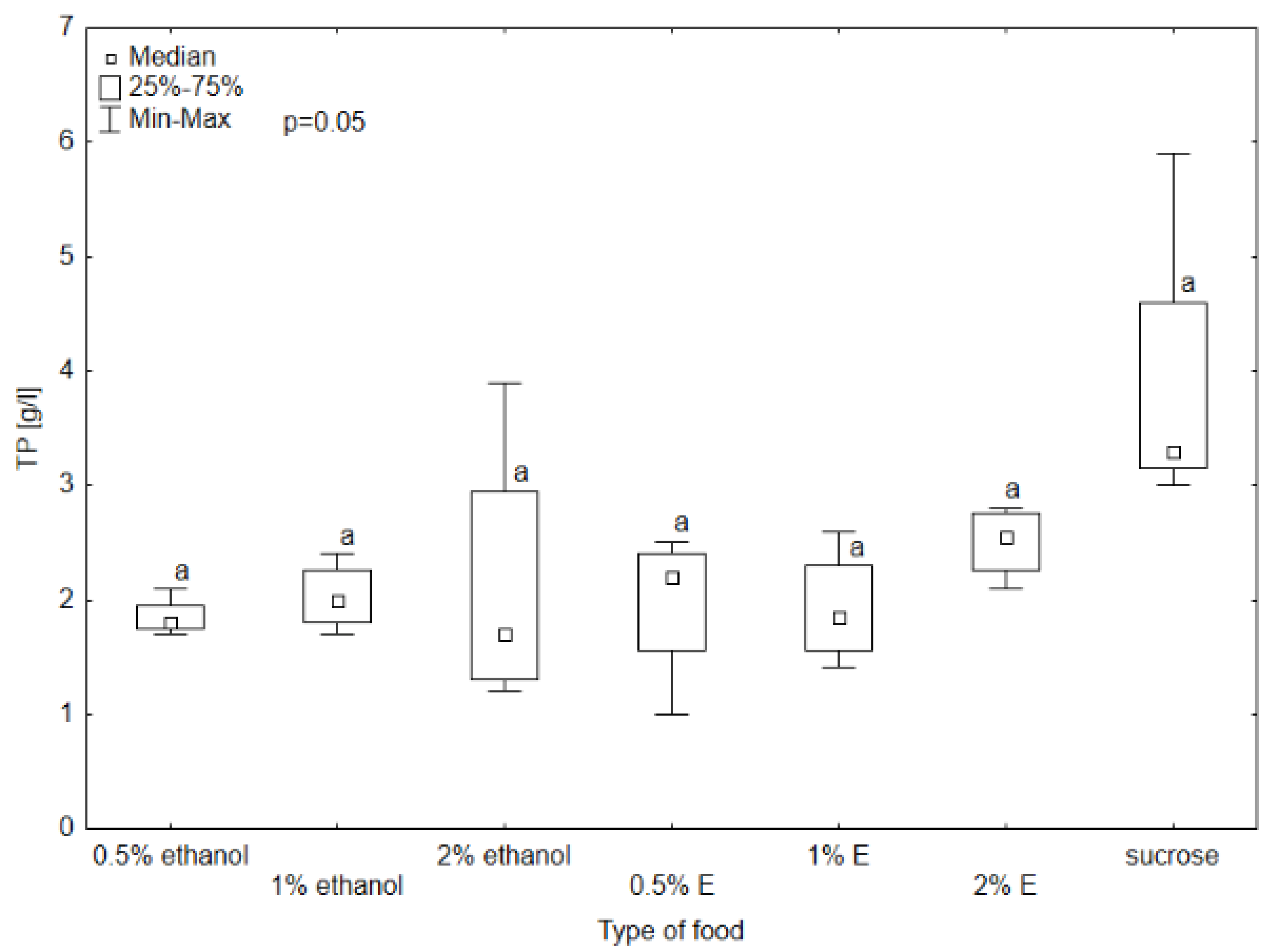
3. Results
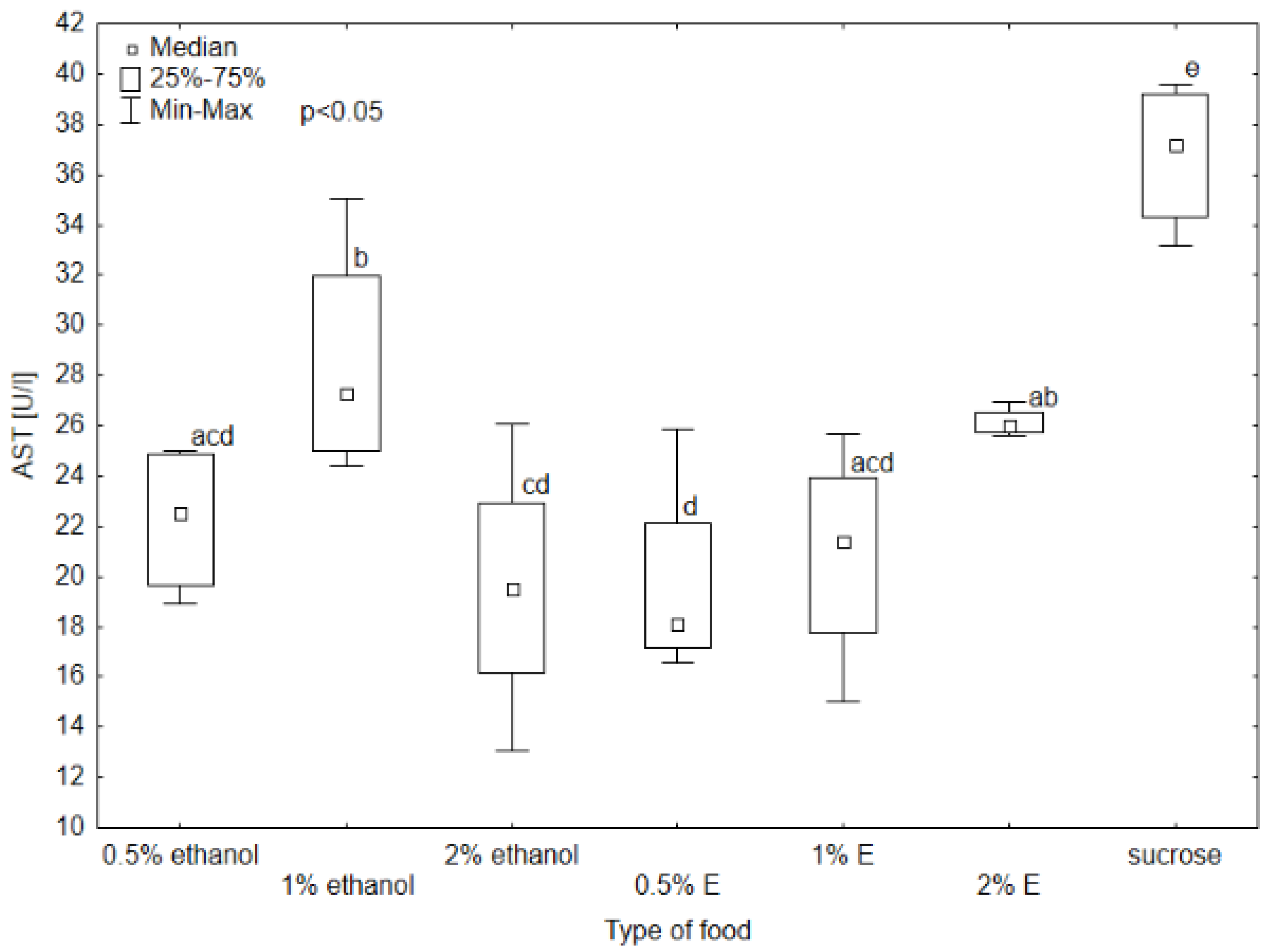
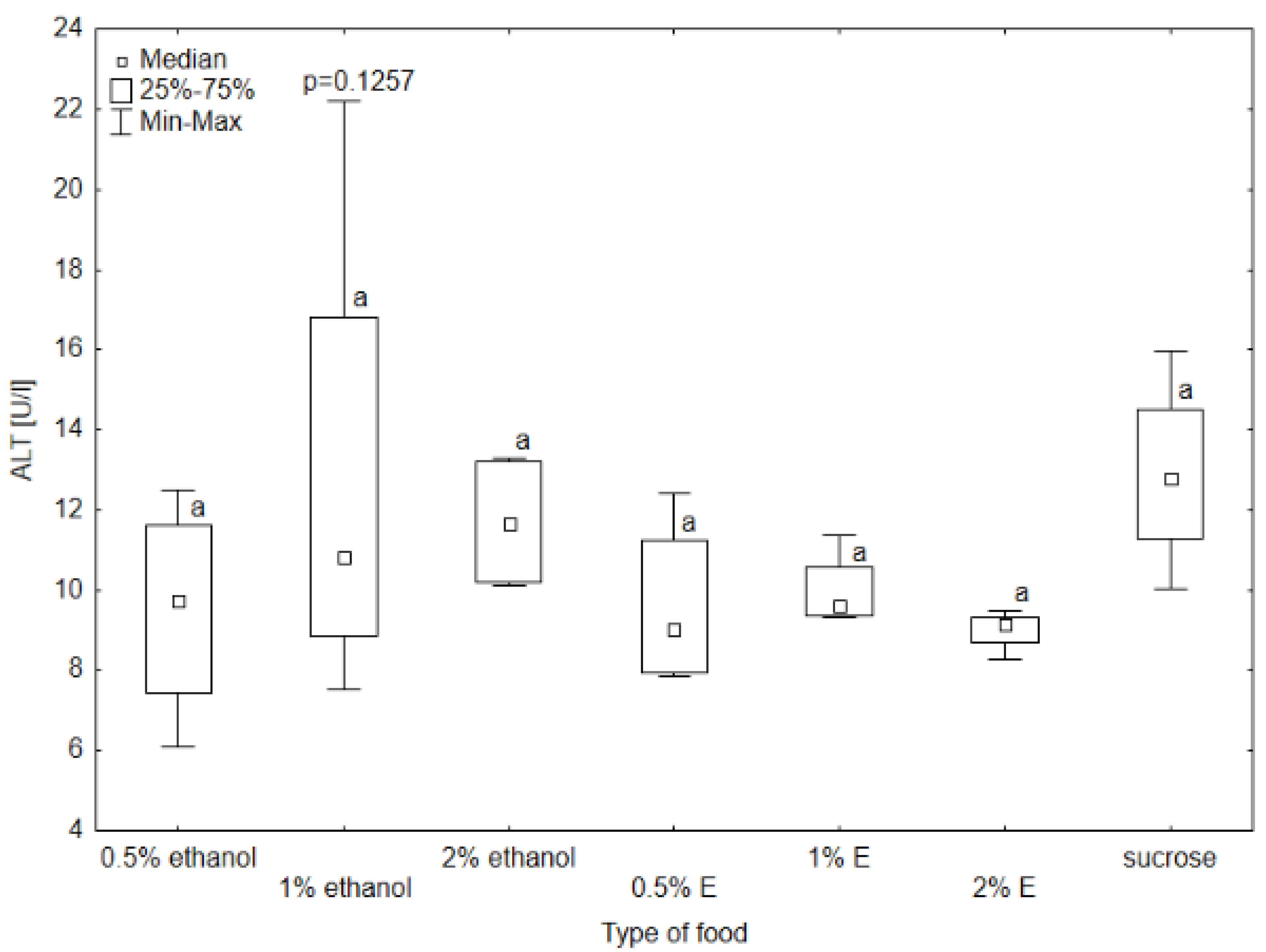
3.1. Results of Selected Biochemical Markers
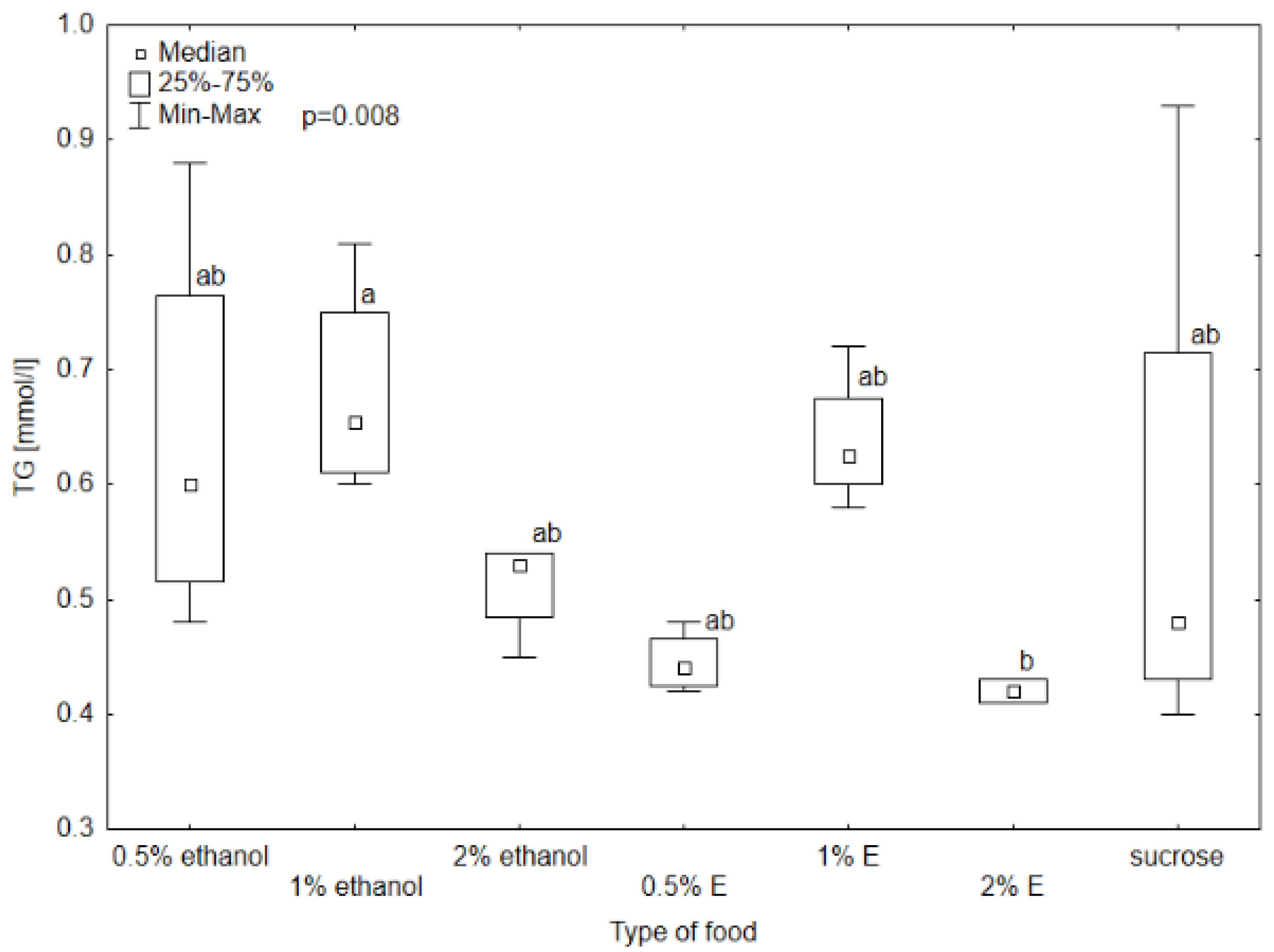
3.2. Selected Non-Enzymatic Biochemical Markers
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalamas, C.; Eleftherohorinos, I.G. Pesticide exposure, safety issues and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. A review on efficacy of biopesticides to control the agricultural insect’s pest. Int. J. Agric. Sci. Res. 2015, 4, 168–179. [Google Scholar]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Impacts of pesticides on honey bees. Beekeep. Bee Conserv.-Adv. Res. 2016, 4, 77–97. [Google Scholar]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides classification and its impact on environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Aioub, A.A.; Ghosh, S.; Al-Farga, A.; Khan, A.N.; Bibi, R.; Elwakeel, A.M.; Nawaz, A.; Sherif, N.T.; Elmasry, S.A.; Ammar, E.E. Back to the origins: Biopesticides as promising alternatives to conventional agrochemicals. Eur. J. Plant Pathol. 2024, 169, 697–713. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C. Ecotoxicity of plant extracts and essential oils: A review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef]
- Alkassab, E.T.; Erler, S.; Steinert, M.; Pistorius, J. Exposure of honey bees to mixtures of microbial biopesticides and their effects on bee survival under laboratory conditions. Environ. Sci. Pollut. Res. 2024, 31, 26618–26627. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, R.; Zawiślak, G.; Rybiński, M.; Zalewska, E.; Walasek-Janusz, M. Usychalność surowców zielarskich (Drying ratio of herbal raw materials). Ann. Hortic. 2020, 30, 21. [Google Scholar] [CrossRef]
- Górska-Drabik, E.; Golan, K.; Kot, I.; Kmieć, K.; Poniewozik, M.; Dzida, K.; Bochniak, A. The effect of pre-harvest treatments with Tanacetum vulgare L. and Satureja montana L. essentian oils (Eos) on the yield and chemical composition of Aronia melanocarpa (Michx.) lliot fruit. Agriculture 2024, 14, 12. [Google Scholar] [CrossRef]
- Acimovic, M.; Puvaca, N. Tanacetum vulgare L.—A systematic review. J. Agron. Technol. Eng. Manag. 2020, 3, 416–422. [Google Scholar]
- LeCain, R. Common tansy (Tanacetum vulgare), Montana State University Extension, MT199911AG Revised 1/14. Agric. Nat. Resour. (Weeds) 2014, 114SA. [Google Scholar]
- Raal, A.; Orav, A.; Greczusznikova, T. Essential oil content and composition in Tanacetum vulgare L. herbs growing wild in Estonia. J. Essent. Oil Bear. Plants 2014, 17, 670–675. [Google Scholar] [CrossRef]
- Karcheva-Bahchevanska, D.; Benbassat, N.; Georgieva, Y.; Lechkova, B.; Ivanova, S.; Ivanov, K.; Todorova, V.; Peychev, L.; Peychev, Z.; Denev, P. A study of the chemical composition, antioxidant potential and acute toxicity of Bulgarian Tanacetum vulgare L. essential oil. Molecules 2023, 28, 6155. [Google Scholar] [CrossRef]
- Derda, M.; Hadaś, E.; Thiem, B.; Wojt, W.J.; Wojtowiak-Giera, A.; Cholewiński, M.; Skrzypczak, Ł. Tanacetum vulgare L. jako roślina o potencjalnych właściwościach leczniczych w Acanthamoeba keratitis. Now. Lek. 2012, 81, 620–625. [Google Scholar]
- Baranauskiene, R.; Kazernaviciute, R.; Pukalskiene, M.; Mazdzieriene, R.; Venskutonis, P.R. Agrorafinery of Tanacetun vulgare L. into valuable products and evaluation of their antioxidant properties and phytochemical composition. Ind. Crops Prod. 2014, 60, 113–122. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Martynov, V.O.; Hladkyi, O.Y.; Kolombar, T.M.; Brygadyrenko, V.V. Impact of essential oil from plants on migratory activity of Sitophilus granarius and Tenebrio molitor. Regul. Mech. Biosyst. 2019, 10, 359–371. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The effect of Tanacatum vulgare essential oil and its main components on some ecological and physiological parameters of Acrobasis advenella (Zinck.) (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2020, 162, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Roman, A. A modified standardized method to extract and store insects hemolymph with use of a glass capillary. J. Apic. Sci. 2020, 64, 165–168. [Google Scholar] [CrossRef]
- Migdał, P.; Bieńkowski, P.; Cebrat, M.; Berbeć, E.; Plotnik, M.; Murawska, A.; Sobkiewicz, P.; Łaszkiewicz, A.; Latarowski, K. Exposure to a 900 MHz electromagnetic field induces a response of the honey bee organism on the level of enzyme activity and the expression of stress-related genes. PLoS ONE 2023, 18, e0285522. [Google Scholar] [CrossRef]
- Schacterle, G.; Pollack, R. Simplified method for quantitative assay of small amounts of protein in biological material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef]
- Łoś, A.; Strachecka, A. Fast and cost-effective biochemical spectrophotometric analysis of solution of insect “blood” and body surface elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Bieńkowski, P.; Strachecka, A.; Roman, A. Effect of the electric field at 50 Hz and variable intensities on biochemical markers in the honey bee’s hemolymph. PLoS ONE 2021, 16, e0252858. [Google Scholar] [CrossRef] [PubMed]
- Migdał, P.; Plotnik, M.; Bieńkowski, P.; Murawska, A.; Berbeć, E.; Sobkiewicz, P.; Zarębski, K.; Latarowski, K. Changes in honey bee nutrition after exposure to radiofrequency electromagnetic field. Eur. Zool. J. 2024, 91, 172–179. [Google Scholar] [CrossRef]
- Catania, R.; Pereira Lima, M.A.; Potrich, M.; Sgolastra, F.; Zappala, L.; Mazzeo, G. Are botanical biopesticides safe for bees (Hymenoptera, Apoidea)? Insects 2023, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, Y.; Chen, Y.; Wang, S.; Chen, Y.; Hu, F.; Zheng, H. Honey bee (Apis mellifera) gut microbiota promotes host endogenous detoxification capability via regulation of P450 gene expression in the digestive tract. Microb. Biotechnol. 2020, 13, 1201–1212. [Google Scholar] [CrossRef]
- Mucci, C.A.; Ramirez, L.; Giffoni, R.S.; Lamattina, L. Cold stress induces specific antioxidant responses in honey bee brood. Apidologie 2021, 52, 596–607. [Google Scholar] [CrossRef]
- Renzi, M.T.; Amichot, M.; Pauron, D.; Tchamitchian, S.; Brunet, J.L.; Kretzschmar, A.; Maini, S.; Belzunces, L.P. Chronic toxicity and physiological changes induced in the honey bee by the exposure to fipronil and Bacillus thuringiensis spores alone or combined. Ecotoxicol. Environ. Saf. 2016, 127, 205–213. [Google Scholar] [CrossRef]
- Cournoyer, A.; Plamondon, L.; Bau-Gaudreault, L.; Deschamps, A.; Dubreuil, P.; Benoit-Biancamano, M.-O. Effects of Varroa destructor on hemolymph sugars and secondary infections in honeybees (Apis mellifera). Appl. Sci. 2022, 12, 11630. [Google Scholar] [CrossRef]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bee. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef]
- Bajda, M.; Łoś, A.; Merska, M. Effect of amphotericin B on the biochemical markers in the haemolymph of honey bees. Med. Weter. 2014, 70, 766–768. [Google Scholar]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid markedly affects hemolymph proteolysis, biomarkers, DNA global methylation and the cuticle proteolytic layer in western honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. CBD supplementation has a positive effect on the activity of the proteolytic system and biochemical markers of honey bees (Apis mellifera) in the apiary. Animals 2022, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Migdał, P.; Murawska, A.; Berbeć, E.; Zarębski, K.; Ratajczak, N.; Roman, A.; Latarowski, K. Biochemical indicators and mortality in honey bee (Apis mellifera) workers after oral exposure to plant protection products and their mixtures. Agriculture 2024, 14, 5. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Bieńkowski, P.; Strachecka, A.; Roman, A. Effect of E-field at frequency 50 Hz on protein, glucose and triglycerides concentration in honeybee hemolymph. Eur. Zool. J. 2021, 88, 1170–1176. [Google Scholar] [CrossRef]
- Even, N.; Devaud, J.-M.; Barron, A.B. General stress responses in honey bee. Insects 2012, 3, 1271–1298. [Google Scholar] [CrossRef]
- Brejcha, M.; Prusakova, D.; Sabova, M.; Peska, V.; Cerny, J.; Kodrik, D.; Konopova, B.; Frydrychova, R.C. Seasonal changes in ultrastructure and gene expression in the fat body of worker honey bees. J. Insect Physiol. 2023, 146, 104504. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insects diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Isani, G.; Bellei, E.; Rudelli, C.; Cabbri, R.; Ferlizza, E.; Andreani, G. SDS-PAGE-based quantitative assay of hemolymph proteins in honeybees: Progress and prospects for field application. Int. J. Mol. Sci. 2023, 24, 10216. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białecka, N.; Garbacz, K.; Berbeć, E.; Murawska, A.; Madras-Majewska, B.; Migdał, P. Changes in Selected Biochemical Markers of Honey Bees Exposed to Fermented Common Tansy Solution (Tanacetum vulgare L.). Animals 2024, 14, 2857. https://doi.org/10.3390/ani14192857
Białecka N, Garbacz K, Berbeć E, Murawska A, Madras-Majewska B, Migdał P. Changes in Selected Biochemical Markers of Honey Bees Exposed to Fermented Common Tansy Solution (Tanacetum vulgare L.). Animals. 2024; 14(19):2857. https://doi.org/10.3390/ani14192857
Chicago/Turabian StyleBiałecka, Natalia, Klaudia Garbacz, Ewelina Berbeć, Agnieszka Murawska, Beata Madras-Majewska, and Paweł Migdał. 2024. "Changes in Selected Biochemical Markers of Honey Bees Exposed to Fermented Common Tansy Solution (Tanacetum vulgare L.)" Animals 14, no. 19: 2857. https://doi.org/10.3390/ani14192857
APA StyleBiałecka, N., Garbacz, K., Berbeć, E., Murawska, A., Madras-Majewska, B., & Migdał, P. (2024). Changes in Selected Biochemical Markers of Honey Bees Exposed to Fermented Common Tansy Solution (Tanacetum vulgare L.). Animals, 14(19), 2857. https://doi.org/10.3390/ani14192857




