Transcriptomic and Metabolomic Analyses Reveal the Attenuating Role of Cordycepin and Cordyceps militaris Extract on Acute Liver Injury Induced by LPS in Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cordyceps Militaris Extract and Cordycepin
2.2. Animals and Experimental Design
2.3. Liver Injury Model
2.4. Sampling
2.5. Serum Biochemical Tests and Liver Indicators
2.6. Liver HE Staining
2.7. Hepatic Transcriptome Analysis
2.8. Untargeted Hepatic Metabolome Analysis
2.9. Statistical Analysis of Data
3. Results
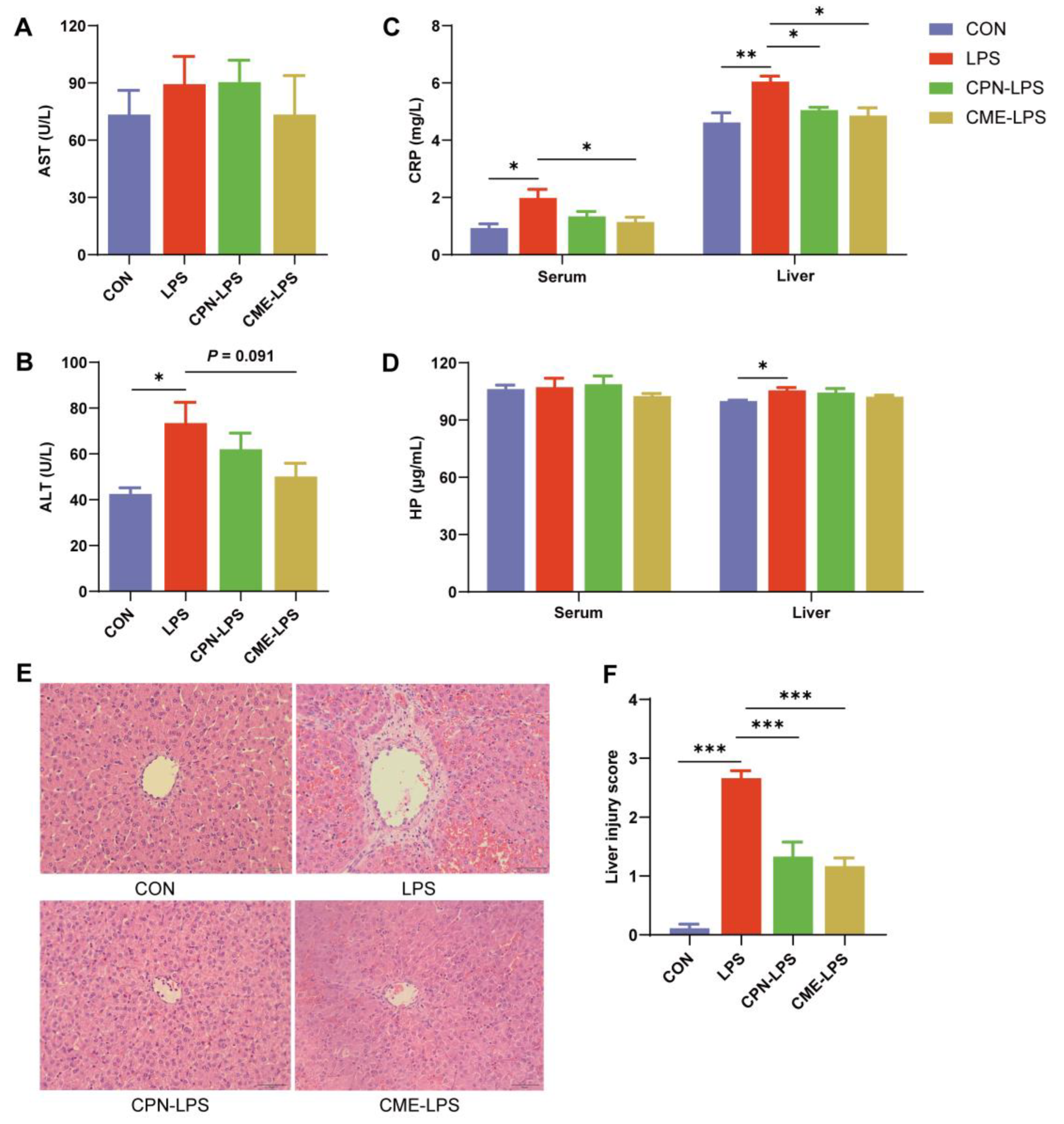
3.1. Ameliorative Effects of Cordyceps Militaris Extract and Cordycepin on LPS-Induced Acute Liver Injury
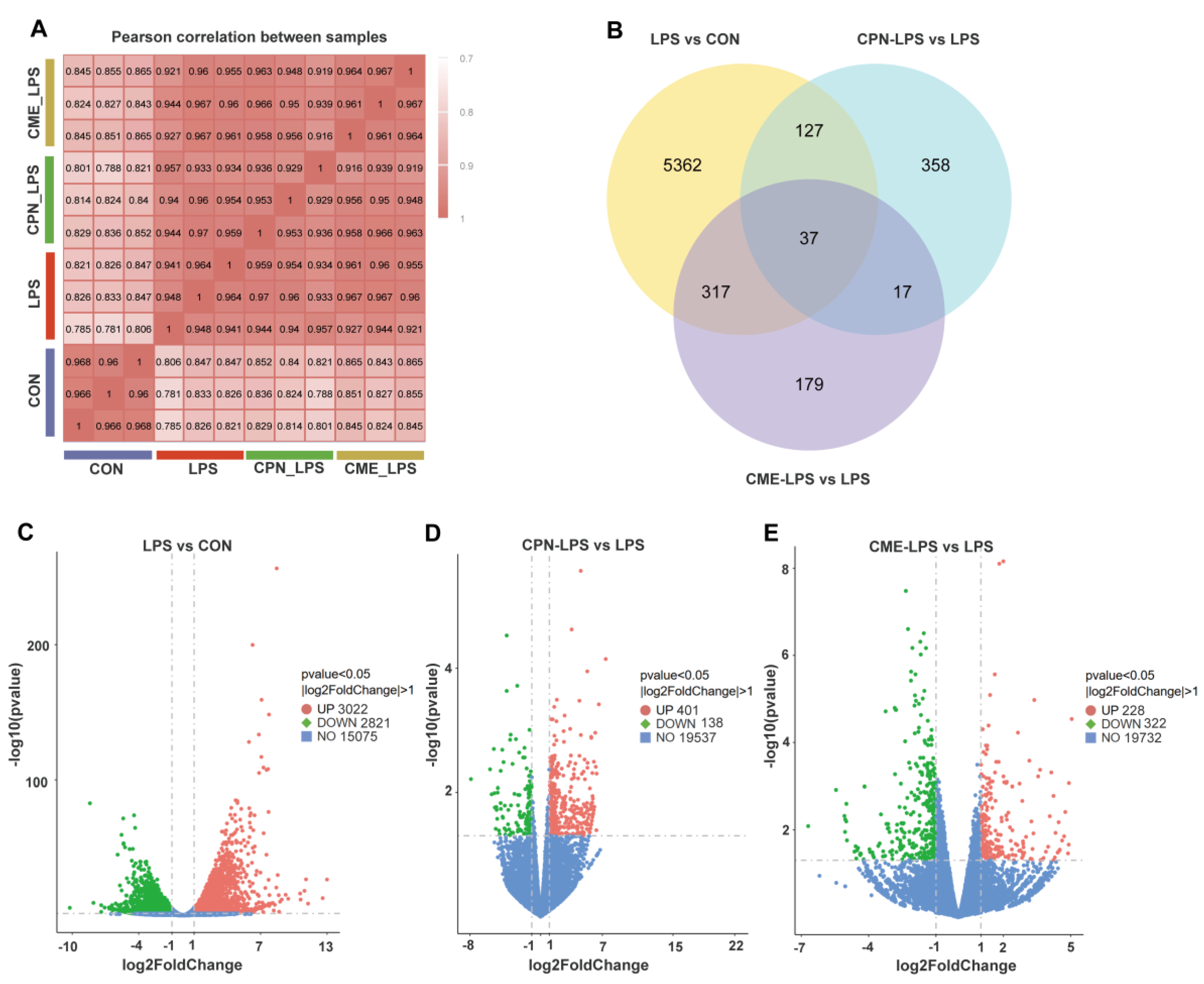
3.2. Transcriptomic Analysis of Piglet Liver
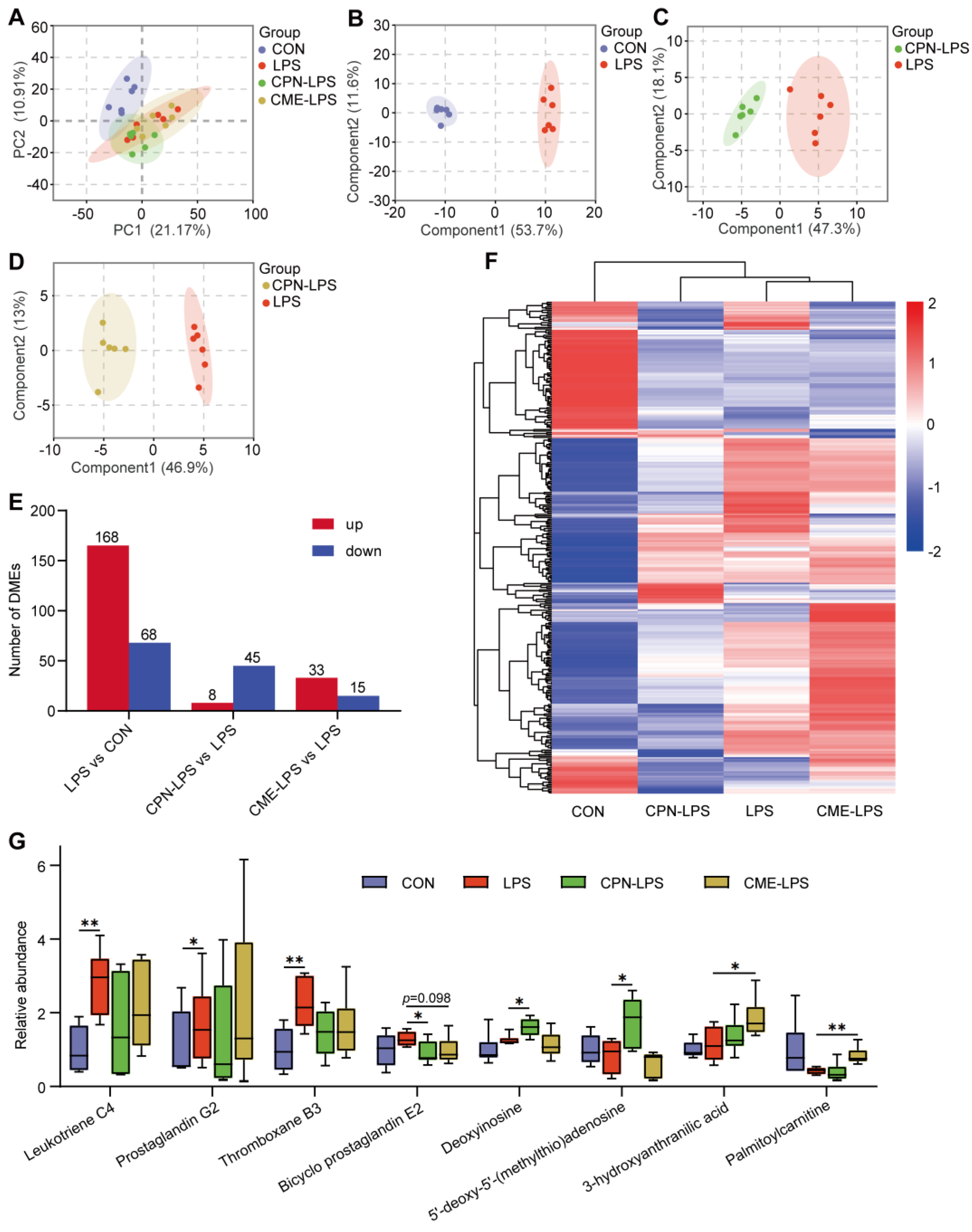
3.3. Metabolomics Analysis of Piglet Liver
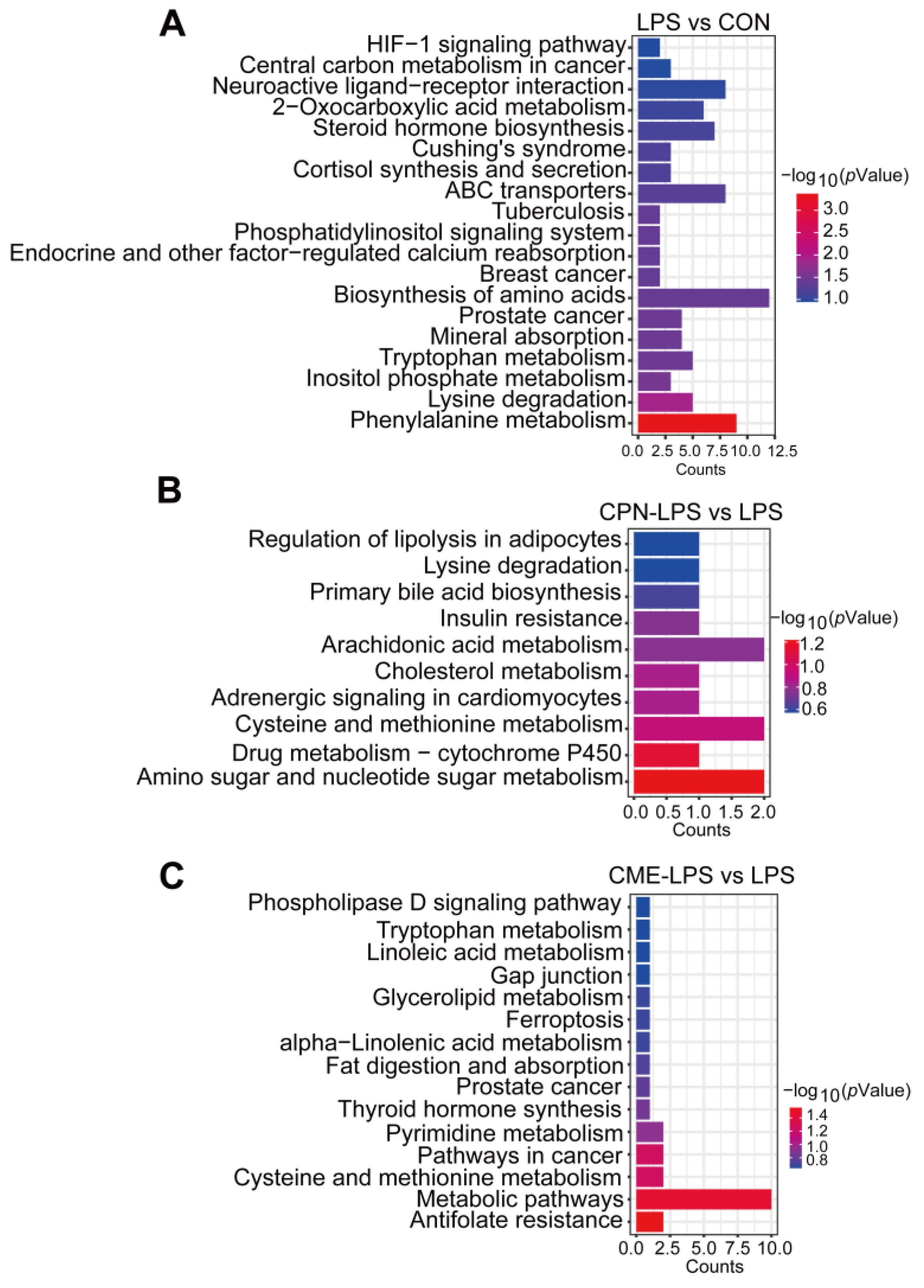
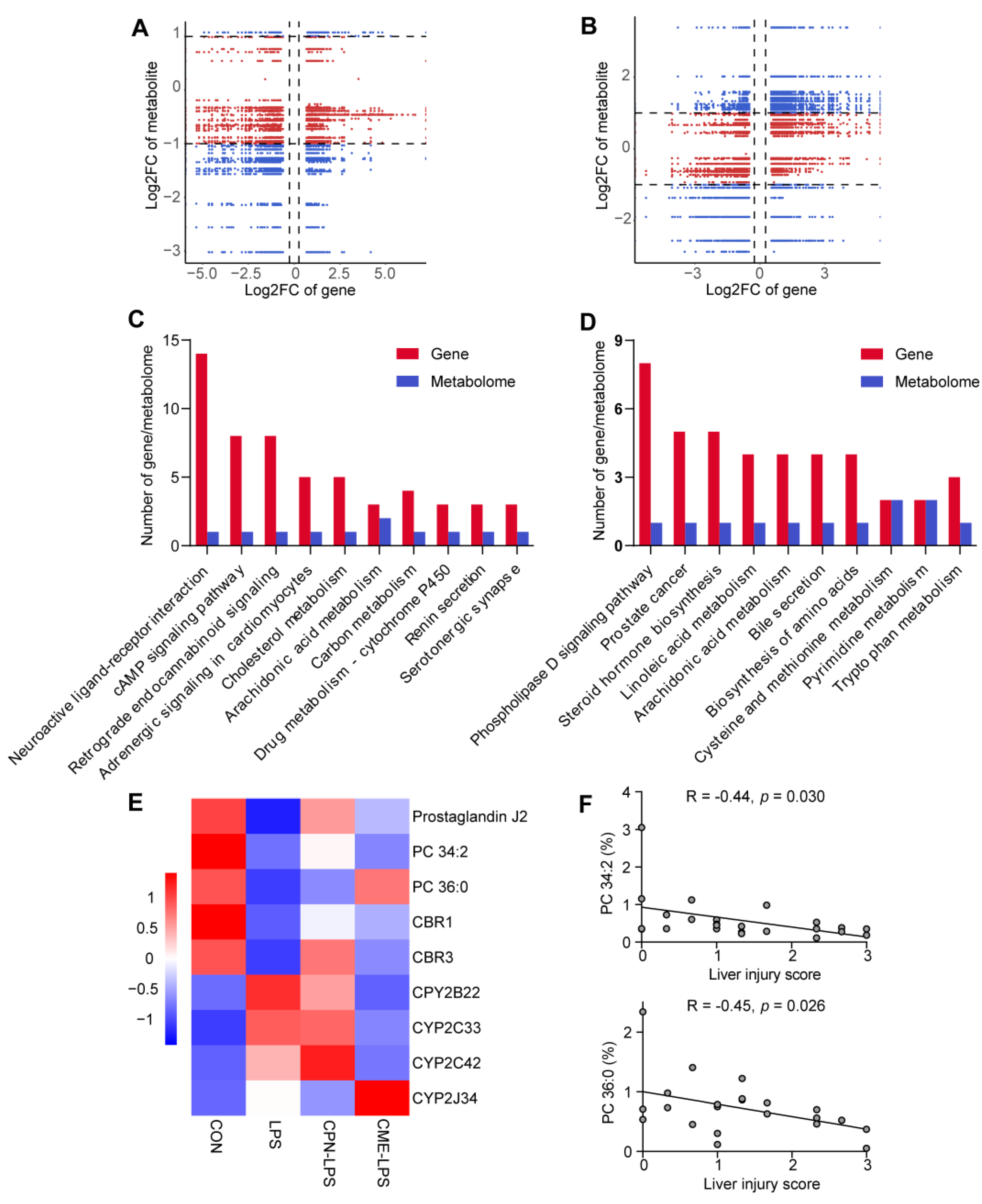
3.4. Combination of Transcriptomic and Metabolomic Analysis on the Protective Effect of Liver Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, J.P. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatology 2010, 52, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Huang, P.; Zheng, C.; Zheng, J.; Yu, J.; Zhang, P.; Wan, M.; Li, F.; Guo, Q.; Yin, Y.; et al. Development and Recovery of Liver Injury in Piglets by Incremental Injection of LPS. Antioxidants 2023, 12, 1143. [Google Scholar] [CrossRef]
- Zhao, D.D.; Wang, J.J.; Fan, J.J.; Fu, Y.; Ma, Y.D.; Li, C.; Wu, G.F.; Liu, M.; Lin, S.M.; Hu, J.M. Taurine Prevents LPS-Induced Liver Injury in Weaned Piglets. Adv. Exp. Med. Biol. 2022, 1370, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef]
- Hong, W.; Fu, W.; Zhao, Q.; Xue, C.; Cai, W.; Dong, N.; Shan, A. Effects of oleanolic acid on acute liver injury triggered by lipopolysaccharide in broiler chickens. Br. Poult. Sci. 2023, 64, 697–709. [Google Scholar] [CrossRef]
- Du, L.; Zheng, Y.; Yang, Y.H.; Huang, Y.J.; Hao, Y.M.; Chen, C.; Wang, B.Z.; Guo, X.; Wu, H.; Su, G.H. Krill oil prevents lipopolysaccharide-evoked acute liver injury in mice through inhibition of oxidative stress and inflammation. Food Funct. 2022, 13, 3853–3864. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, J.; Wan, F.; Tan, D.; Zheng, H.; Xue, H.; Hang, Y.; Lu, Y.; Su, Y. Cordyceps militaris Extract and Cordycepin Alleviate Oxidative Stress, Modulate Gut Microbiota and Ameliorate Intestinal Damage in LPS-Induced Piglets. Antioxidants 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Madaan, K.; Kaur, R. Cordycepin as a Metabolite with Pharmacological Potential: A Review. Int. J. Med. Mushrooms 2022, 24, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Yu, Y.; Zhang, J.; Li, H.; Weng, Q.; Jiang, S.; Tian, S.; Xu, T.; Hu, S.; Yang, G.; et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, L.; Zhu, H.; Wang, F. The Protective Effect of Cordycepin on D-Galactosamine/Lipopolysaccharide-Induced Acute Liver Injury. Mediat. Inflamm. 2017, 2017, 3946706. [Google Scholar] [CrossRef]
- Shao, L.W.; Huang, L.H.; Yan, S.; Jin, J.D.; Ren, S.Y. Cordycepin induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signaling pathways. Oncol. Lett. 2016, 12, 995–1000. [Google Scholar] [CrossRef]
- Wang, X.B.; Liu, P.; Tang, Z.P.; Li, F.H.; Liu, C.H.; Hu, Y.Y.; Xu, L.M. Cordyceps mycelia extract decreases portal hypertension in rats with dimethylnitrosamine-induced liver cirrhosis: A study on its histological basis. Zhong Xi Yi Jie He Xue Bao 2008, 6, 1136–1144. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, L.; Zhu, G.; Zhang, P.; Wu, X.; Yao, X.; Luo, Y.; Yang, Z.; Ren, M.; Wang, X.; et al. The Antiviral Effect of Isatis Root Polysaccharide against NADC30-like PRRSV by Transcriptome and Proteome Analysis. Int. J. Mol. Sci. 2022, 23, 3688. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal Crop Proteomics: Systemic Analysis of Crop Drought Stress Responses Towards Marker-Assisted Selection Breeding. Front. Plant Sci. 2017, 8, 757. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Gao, N.; Gong, Y.; Shi, W.; Wang, X. Transcriptome and Metabolome Analyses Reveal Perfluorooctanoic Acid-Induced Kidney Injury by Interfering with PPAR Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11503. [Google Scholar] [CrossRef]
- Lu, Y.S.; Yao, G.X.; Wang, X.L.; Liu, J.X.; Yu, J.; Qiu, J.; Li, Y.; Qian, Y.Z.; Xu, Y.Y. A comprehensive analysis of metabolomics and transcriptomics reveals new biomarkers and mechanistic insights on DEHP exposures in MCF-7 cells. Chemosphere 2020, 255, 126865. [Google Scholar] [CrossRef]
- Park, S.M.; Kang, S.J.; Choi, M.S.; Kim, S.; Yoon, S.; Oh, J.H. Comparative omics analyses of hepatotoxicity induced by oral azole drugs in mice liver and primary hepatocytes. Toxicol. Mech. Methods 2019, 29, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Zhan, B.; He, W.; Chu, L.; Qiu, D.; Li, N.; Wan, Y.; Zhang, H.; Chen, X.; et al. Therapeutic effect of Schistosoma japonicum cystatin on bacterial sepsis in mice. Parasit. Vectors 2017, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, Y.; Ge, L.; Gong, J.; Weng, C.; Yang, C.; Yang, J.; Fang, Y.; Li, Q.; Zou, T.; et al. Evaluation of the influences of low dose polybrominated diphenyl ethers exposure on human early retinal development. Environ. Int. 2022, 163, 107187. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Sun, Y.; Chu, M.; Xie, Y.; Wang, P.; Li, B.; Li, Z.; Xu, X.; Feng, Y.; Sun, G.; et al. Exploration of Response Mechanisms in the Gills of Pacific Oyster (Crassostrea gigas) to Cadmium Exposure through Integrative Metabolomic and Transcriptomic Analyses. Animals 2024, 14, 2318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, L.; Jiang, S.; Yin, Z.; Tan, G.; Ning, F.; Qin, Z.; Huang, J.; Huang, M.; Jin, J. Salvianolic acid extract prevents Tripterygium wilfordii polyglycosides-induced acute liver injury by modulating bile acid metabolism. J. Ethnopharmacol. 2024, 327, 117939. [Google Scholar] [CrossRef]
- Yi, D.; Hou, Y.; Wang, L.; Ding, B.; Yang, Z.; Li, J.; Long, M.; Liu, Y.; Wu, G. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br. J. Nutr. 2014, 111, 46–54. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Yi, D.; Li, Y.; Ding, B.; Zhu, H.; Liu, J.; Xiao, H.; Wu, G. Dietary supplementation with glutamate precursor α-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs. Amino Acids 2015, 47, 1309–1318. [Google Scholar] [CrossRef]
- Wan, J.Y.; Gong, X.; Zhang, L.; Li, H.Z.; Zhou, Y.F.; Zhou, Q.X. Protective effect of baicalin against lipopolysaccharide/D-galactosamine-induced liver injury in mice by up-regulation of heme oxygenase-1. Eur. J. Pharmacol. 2008, 587, 302–308. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Ringseis, R.; Hillen, S.; Becker, S.; Erhardt, G.; Reiner, G.; Eder, K. The stress signalling pathway nuclear factor E2-related factor 2 is activated in the liver of sows during lactation. Acta Vet. Scand. 2012, 54, 59. [Google Scholar] [CrossRef]
- Giffen, P.S.; Turton, J.; Andrews, C.M.; Barrett, P.; Clarke, C.J.; Fung, K.W.; Munday, M.R.; Roman, I.F.; Smyth, R.; Walshe, K.; et al. Markers of experimental acute inflammation in the Wistar Han rat with particular reference to haptoglobin and C-reactive protein. Arch. Toxicol. 2003, 77, 392–402. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Lu, Y.; Yan, X.; Jin, Z.; Gu, B. Haptoglobin 2-2 Genotype is Related to the Severity of Liver Damage in Hepatitis B Patients with Liver Steatosis. Discov. Med. 2023, 35, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wu, L.; Han, Y.; Chen, J.; Zhu, S.; Yao, Y.; Wang, B.; Li, L. Gut Microbiota-Controlled Tryptophan Metabolism Improves D-Gal/LPS-Induced Acute Liver Failure in C57BL/6 Mice. Engineering 2022, 14, 134–146. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, T.; Chen, J.; Cai, X.; Fu, Y. Uncovering the role of transient receptor potential channels in pterygium: A machine learning approach. Inflamm. Res. 2023, 72, 589–602. [Google Scholar] [CrossRef]
- Su, X.; Li, T.; Zhu, X.; Zheng, P.; Pan, H.; Guo, H. Exploring the impact of nonylphenol exposure on Litopenaeus vannamei at the histological and molecular levels. Ecotoxicol. Environ. Saf. 2024, 279, 116475. [Google Scholar] [CrossRef]
- Declercq, M.; Treps, L.; Geldhof, V.; Conchinha, N.V.; de Rooij, L.; Subramanian, A.; Feyeux, M.; Cotinat, M.; Boeckx, B.; Vinckier, S.; et al. Single-cell RNA sequencing of cystic fibrosis liver disease explants reveals endothelial complement activation. Liver Int. 2024, 44, 2382–2395. [Google Scholar] [CrossRef]
- Qin, Y.; Fan, R.; Liu, Y.; Qiu, S.; Wang, L. Exploring the potential mechanism of Rubus corchorifolius L. fruit polyphenol-rich extract in mitigating non-alcoholic fatty liver disease by integration of metabolomics and transcriptomics profiling. Food Funct. 2023, 14, 9295–9308. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, L.; Duan, Y.; Gao, Y.; Gao, H.; Mao, D.; Luo, Y. Gut microbiota exaggerates triclosan-induced liver injury via gut-liver axis. J. Hazard. Mater. 2022, 421, 126707. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Bai, J.; Zhang, Y.L.; Li, H.H. Integrin CD11b Contributes to Hypertension and Vascular Dysfunction Through Mediating Macrophage Adhesion and Migration. Hypertension 2023, 80, 57–69. [Google Scholar] [CrossRef]
- Jaggi, U.; Matundan, H.H.; Oh, J.J.; Ghiasi, H. Absence of CD80 reduces HSV-1 replication in the eye and delays reactivation but not latency levels. J. Virol. 2024, 98, e0201023. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Yang, Y.; Li, J.; Kang, H.; Tang, W.; Lyon, C.J.; Wan, M. Extracellular Vesicle ITGAM and ITGB2 Mediate Severe Acute Pancreatitis-Related Acute Lung Injury. ACS Nano 2023, 17, 7562–7575. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; He, S.; Liu, Y.; Chen, H.; He, S.; Yin, M.; Zou, D.; Chen, S.; Luo, T.; et al. Resolving the graft ischemia-reperfusion injury during liver transplantation at the single cell resolution. Cell Death Dis. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; You, C.; Zhu, Z.; Wu, W.; Cao, J.; Xie, Q.; Deng, C.; Huang, X.; Hu, S. SLA2 is a prognostic marker in HNSCC and correlates with immune cell infiltration in the tumor microenvironment. Eur. Arch. Otorhinolaryngol. 2024, 281, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, J.; Su, Y.; Li, M.; Wen, J.; Li, S. Cordycepin induces M1/M2 macrophage polarization to attenuate the liver and lung damage and immunodeficiency in immature mice with sepsis via NF-κB/p65 inhibition. J. Pharm. Pharmacol. 2022, 74, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, W.; Jiang, Y.; Ji, J.; Zhang, J.; Wu, L.; Feng, J.; Zheng, Y.; Li, Y.; Cheng, Z.; et al. Cordycepin Protects against Hepatic Ischemia/Reperfusion Injury via Inhibiting MAPK/NF-κB Pathway. Mediat. Inflamm. 2022, 2022, 5676256. [Google Scholar] [CrossRef]
- Feng, X.; Guan, D.; Auen, T.; Choi, J.W.; Salazar Hernández, M.A.; Lee, J.; Chun, H.; Faruk, F.; Kaplun, E.; Herbert, Z.; et al. IL1R1 is required for celastrol’s leptin-sensitization and antiobesity effects. Nat. Med. 2019, 25, 575–582. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Arnts, J.; Vanlerberghe, B.T.K.; Roozen, S.; Crunelle, C.L.; Masclee, A.A.M.; Olde-Damink, S.W.M.; Heeren, R.M.A.; van Nuijs, A.; Neels, H.; Nevens, F.; et al. Diagnostic Accuracy of Biomarkers of Alcohol Use in Patients With Liver Disease: A Systematic Review. Alcohol. Clin. Exp. Res. 2021, 45, 25–37. [Google Scholar] [CrossRef]
- Liang, R.; Yuan, Y.; Bai, Y.; Liu, X.; Chen, J.; Jiang, D.; Meng, D.; Chen, G.; Li, B.; Zhou, L.; et al. Neobavaisoflavone inhibits allergic inflammatory responses by suppressing mast cell activation. Int. Immunopharmacol. 2022, 110, 108953. [Google Scholar] [CrossRef]
- Taylor, E.N.; Beckmann, M.; Villarreal-Ramos, B.; Vordermeier, H.M.; Hewinson, G.; Rooke, D.; Mur, L.A.J.; Koets, A.P. Metabolomic Changes in Naturally MAP-Infected Holstein-Friesian Heifers Indicate Immunologically Related Biochemical Reprogramming. Metabolites 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.F.; Yang, H.Y.; Chen, Z.; Qi, L.Y.; Zhu, D.Y.; Lou, Y.J. Enhanced expressions and activations of leukotriene C4 synthesis enzymes in D-galactosamine/lipopolysaccharide-induced rat fulminant hepatic failure model. World J. Gastroenterol. 2008, 14, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Yang, S. Ischemic preconditioning decreased leukotriene C4 formation by depressing leukotriene C4 synthase expression and activity during hepatic I/R injury in rats. J. Surg. Res. 2012, 178, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Revankar, A.G.; Bagewadi, Z.K.; Shaikh, I.A.; Mannasaheb, B.A.; Ghoneim, M.M.; Khan, A.A.; Asdaq, S.M.B. In-vitro and computational analysis of Urolithin-A for anti-inflammatory activity on Cyclooxygenase 2 (COX-2). Saudi J. Biol. Sci. 2023, 30, 103804. [Google Scholar] [CrossRef]
- Cherian, G. Metabolic and cardiovascular diseases in poultry: Role of dietary lipids. Poult. Sci. 2007, 86, 1012–1016. [Google Scholar] [CrossRef]
- Carnovale, V.; Castaldo, A.; Di Minno, A.; Gelzo, M.; Iacotucci, P.; Illiano, A.; Pinto, G.; Castaldo, G.; Amoresano, A. Oxylipin profile in saliva from patients with cystic fibrosis reveals a balance between pro-resolving and pro-inflammatory molecules. Sci. Rep. 2022, 12, 5838. [Google Scholar] [CrossRef]
- Xu, D.; Tang, L.; Wang, Y.; Pan, J.; Su, C. LC-MS-based rheumatoid arthritis serum metabolomics reveals the role of deoxyinosine in attenuating collagen-induced arthritis in mice. Heliyon 2024, 10, e30903. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, X.; Tan, J.; Zhang, R.; Li, M.; Liu, C.; Wu, C.; Li, X. Lactobacillus reuteri mitigates hepatic ischemia/reperfusion injury by modulating gut microbiota and metabolism through the Nrf2/HO-1 signaling. Biol. Direct 2024, 19, 23. [Google Scholar] [CrossRef]
- Espejo, L.S.; DeNicola, D.; Chang, L.M.; Hofschneider, V.; Haskins, A.E.; Balsa, J.; Freitas, S.S.; Antenor, A.; Hamming, S.; Hull, B.; et al. The Emerging Role of 3-Hydroxyanthranilic Acid on C. elegans Aging Immune Function. bioRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Rao, D.; Zhao, R.; Hu, Y.; Li, H.; Chun, Z.; Zheng, S. Revealing of Intracellular Antioxidants in Dendrobium nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Metabolites 2023, 13, 702. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wang, W.; Jordan, P.M.; Barth, S.A.; Hofstetter, R.K.; Xu, J.; Zhang, X.; Cai, Y.; Menge, C.; Chen, X.; et al. Mycobacterium tuberculosis-Induced Prostaglandin J2 and 15-Deoxy-Prostaglandin J2 Inhibit Inflammatory Signals in Human M1 Macrophages via a Negative Feedback Loop. J. Immunol. 2023, 210, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Jacobs, R.L.; Watts, J.L.; Rottiers, V.; Jiang, K.; Finnegan, D.M.; Shioda, T.; Hansen, M.; Yang, F.; Niebergall, L.J.; et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 2011, 147, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, X.; Liu, C.; Wang, N.; Yin, F.; Wu, H.; Cao, S.; Zhao, W.; Wu, H.; Zhou, A. Metabolomic and biochemical changes in the plasma and liver of toxic milk mice model of Wilson disease. J. Pharm. Biomed. Anal. 2024, 246, 116255. [Google Scholar] [CrossRef]
- Strehse, J.S.; Protopapas, N.; Maser, E. Carbonyl reductase sniffer from the model organism daphnia: Cloning, substrate determination and inhibitory sensitivity. Chem. Biol. Interact. 2019, 307, 29–36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.; Li, E.; Xiong, S.; Sun, Y.; Cheng, W.; Su, Y.; Lu, Y. Transcriptomic and Metabolomic Analyses Reveal the Attenuating Role of Cordycepin and Cordyceps militaris Extract on Acute Liver Injury Induced by LPS in Piglets. Animals 2024, 14, 2873. https://doi.org/10.3390/ani14192873
Tan D, Li E, Xiong S, Sun Y, Cheng W, Su Y, Lu Y. Transcriptomic and Metabolomic Analyses Reveal the Attenuating Role of Cordycepin and Cordyceps militaris Extract on Acute Liver Injury Induced by LPS in Piglets. Animals. 2024; 14(19):2873. https://doi.org/10.3390/ani14192873
Chicago/Turabian StyleTan, Ding, Endian Li, Shijie Xiong, Yue Sun, Wenbo Cheng, Yong Su, and Yang Lu. 2024. "Transcriptomic and Metabolomic Analyses Reveal the Attenuating Role of Cordycepin and Cordyceps militaris Extract on Acute Liver Injury Induced by LPS in Piglets" Animals 14, no. 19: 2873. https://doi.org/10.3390/ani14192873
APA StyleTan, D., Li, E., Xiong, S., Sun, Y., Cheng, W., Su, Y., & Lu, Y. (2024). Transcriptomic and Metabolomic Analyses Reveal the Attenuating Role of Cordycepin and Cordyceps militaris Extract on Acute Liver Injury Induced by LPS in Piglets. Animals, 14(19), 2873. https://doi.org/10.3390/ani14192873






