Evaluation of Female Recipient Infertility and Donor Spermatogonial Purification for Germ Cell Transplantation in Paralichthys olivaceus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Heat and Busulfan Treatment for Recipient Fish
2.3. Preparation SSCs from Donors
2.4. Transplantation and Post-Transplantation Evaluation
2.5. Histology and Immunofluorescence Analysis
2.6. Quantitative Real-Time PCR
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
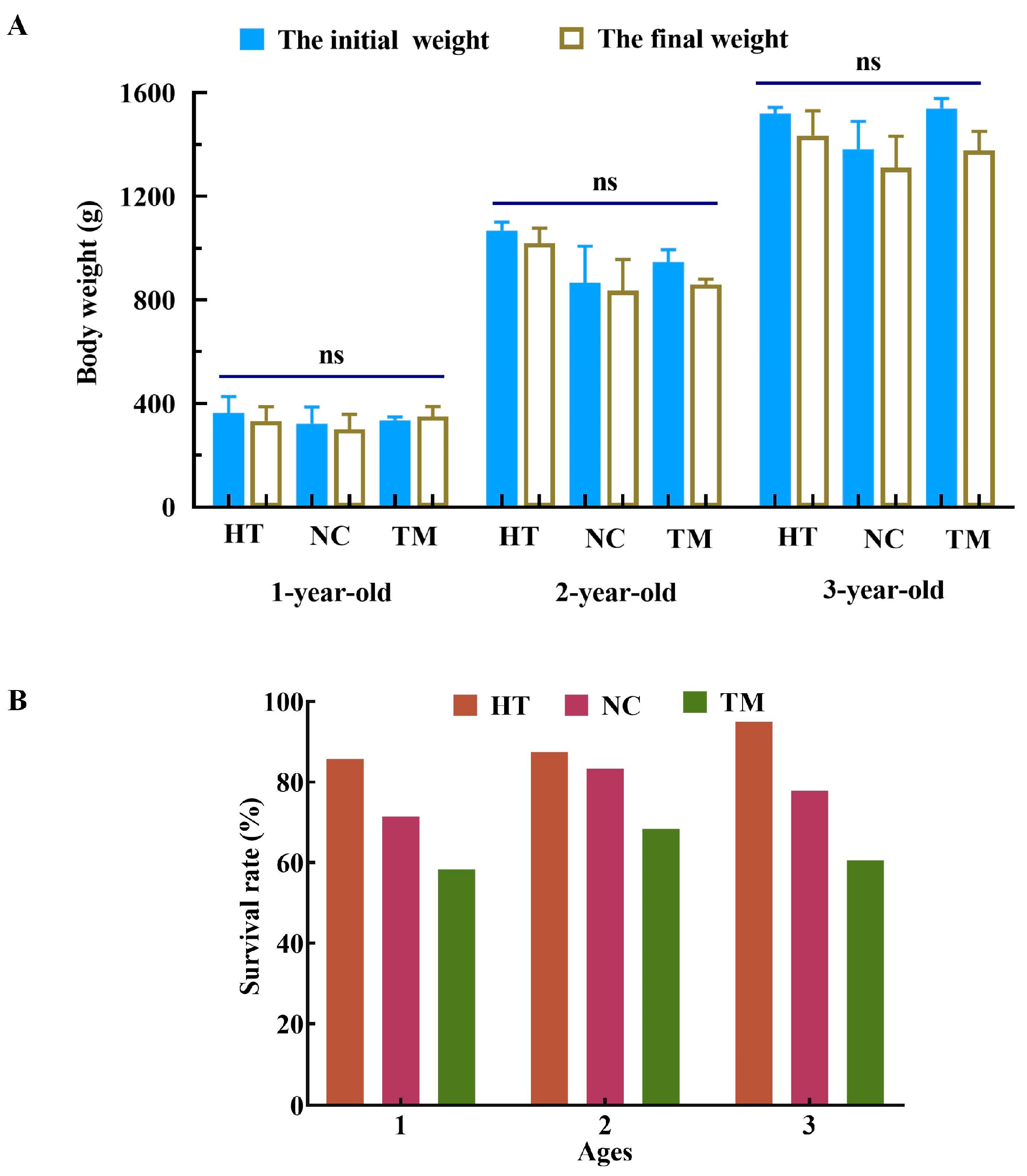
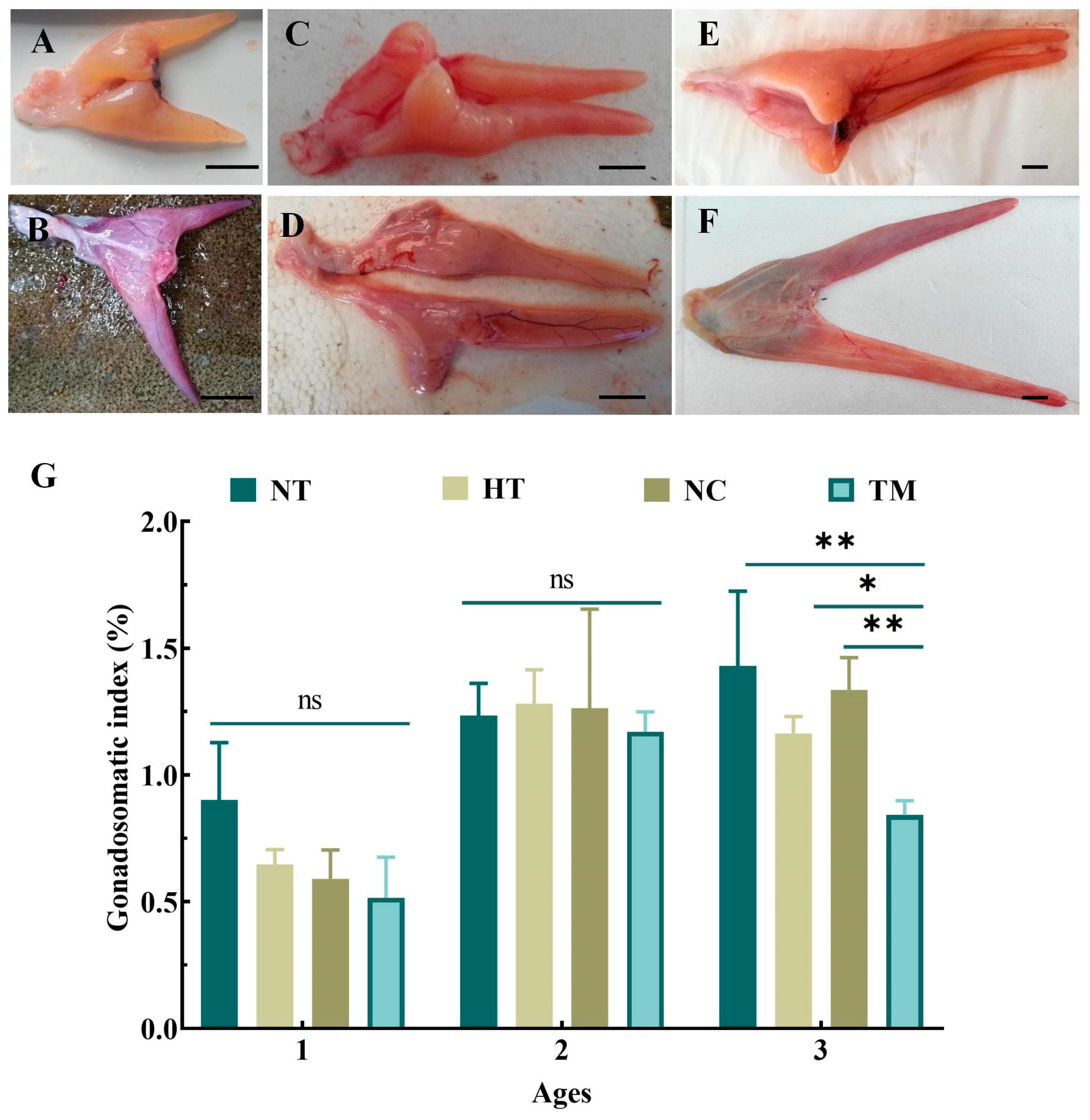
3.1. Survival and Growth after Busulfan Treatment
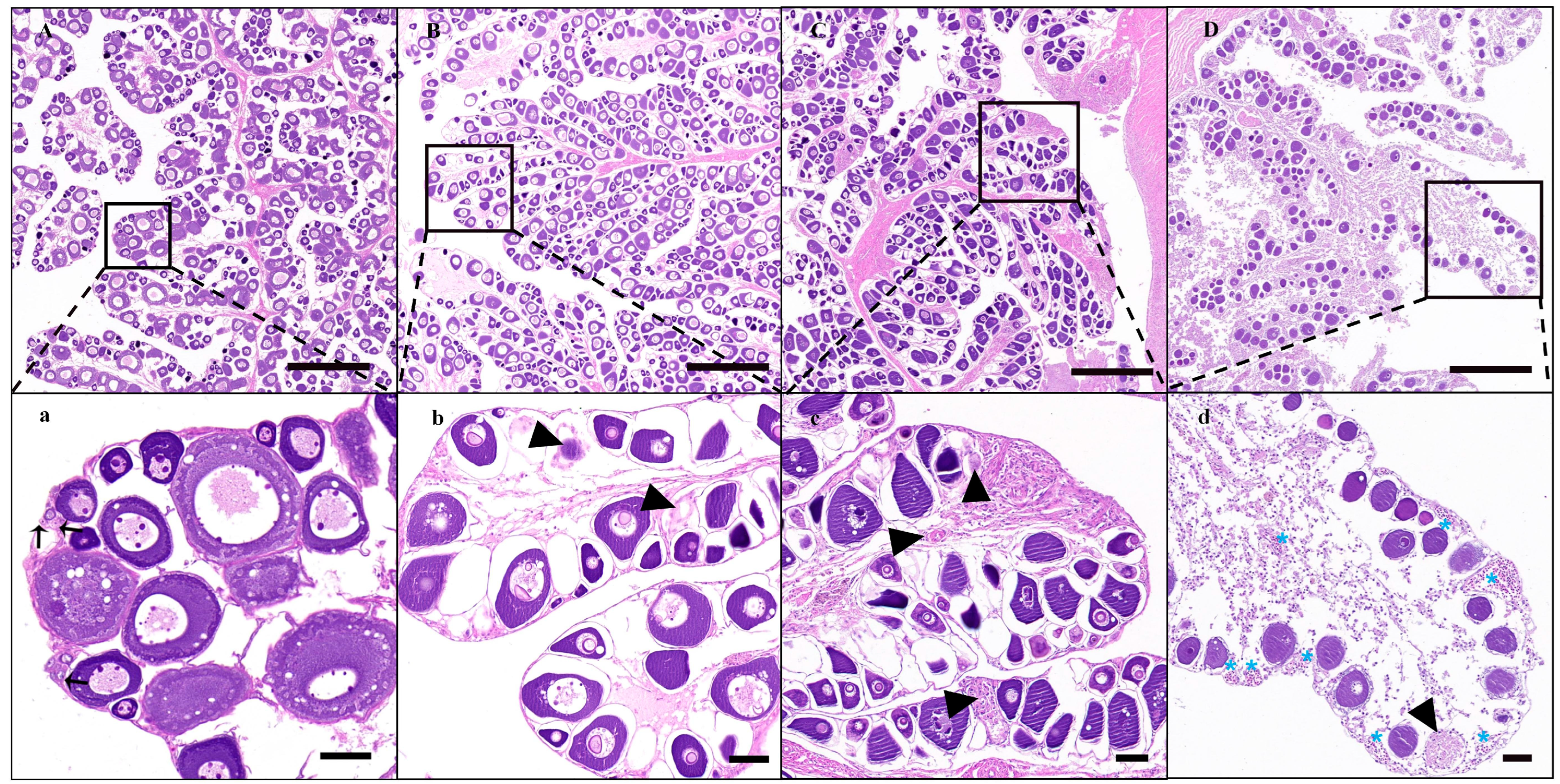
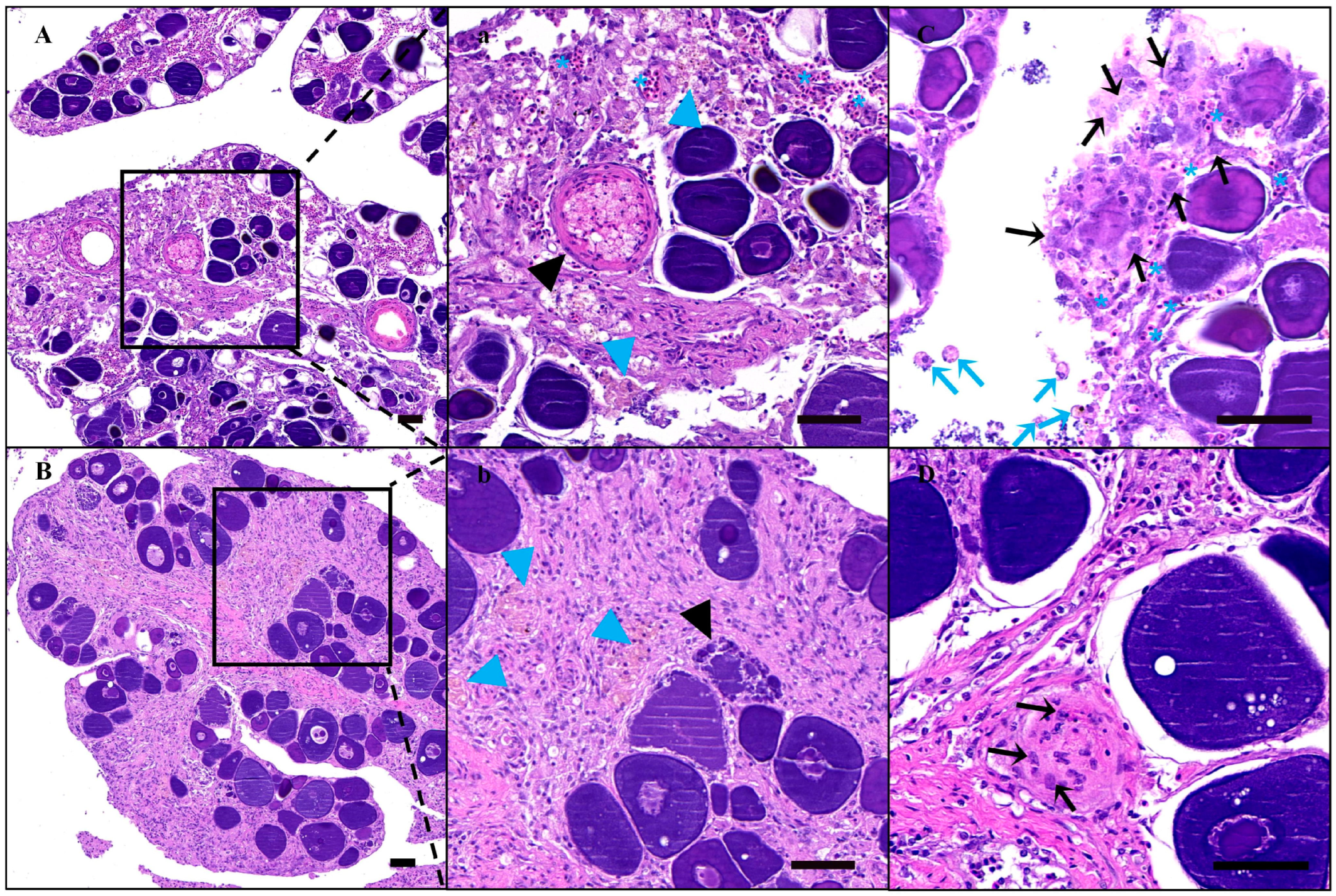
3.2. Apoptosis of Endogenous Germ Cells in the Recipient after Busulfan Treatment
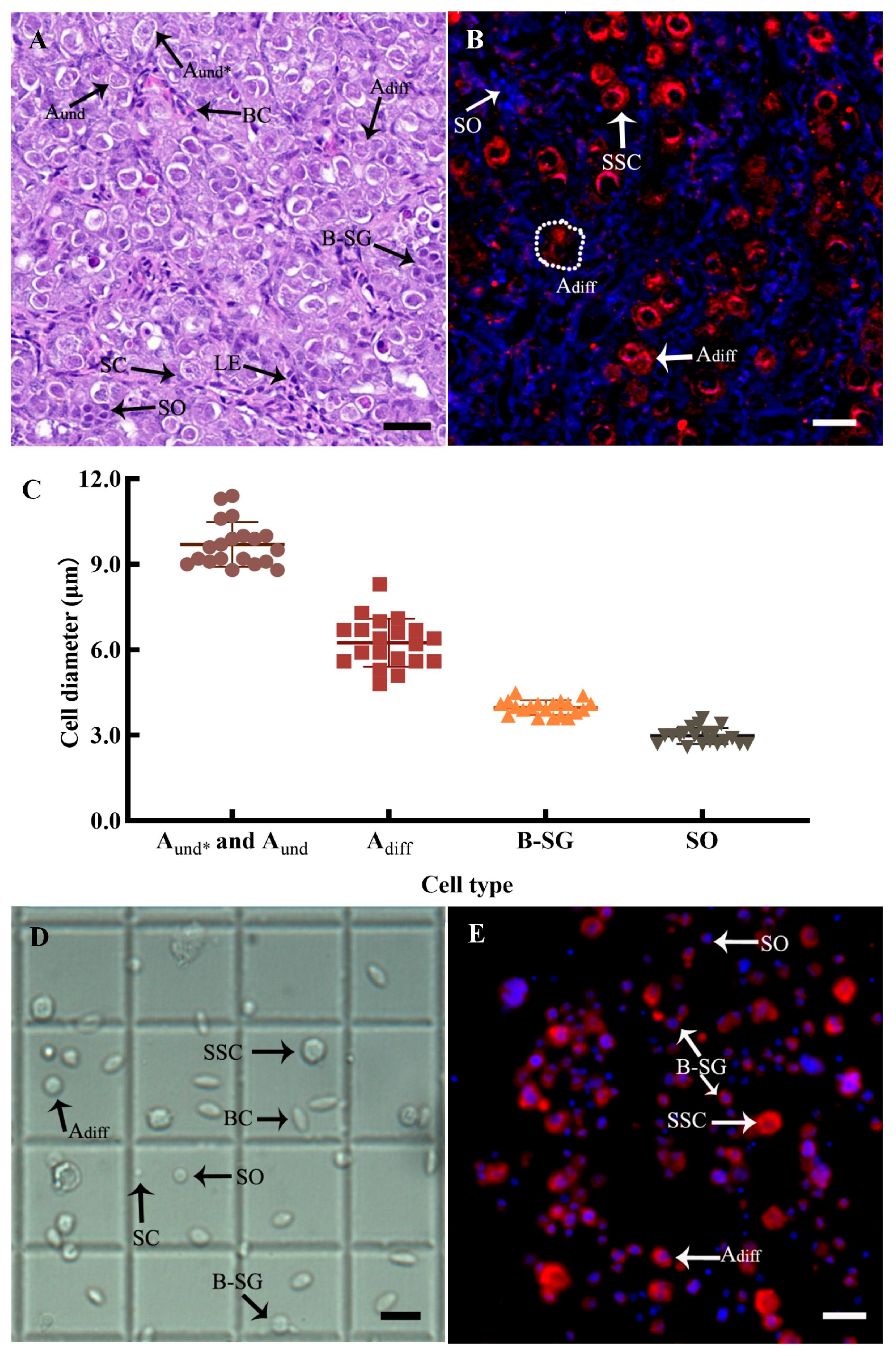
3.3. SSC Identification
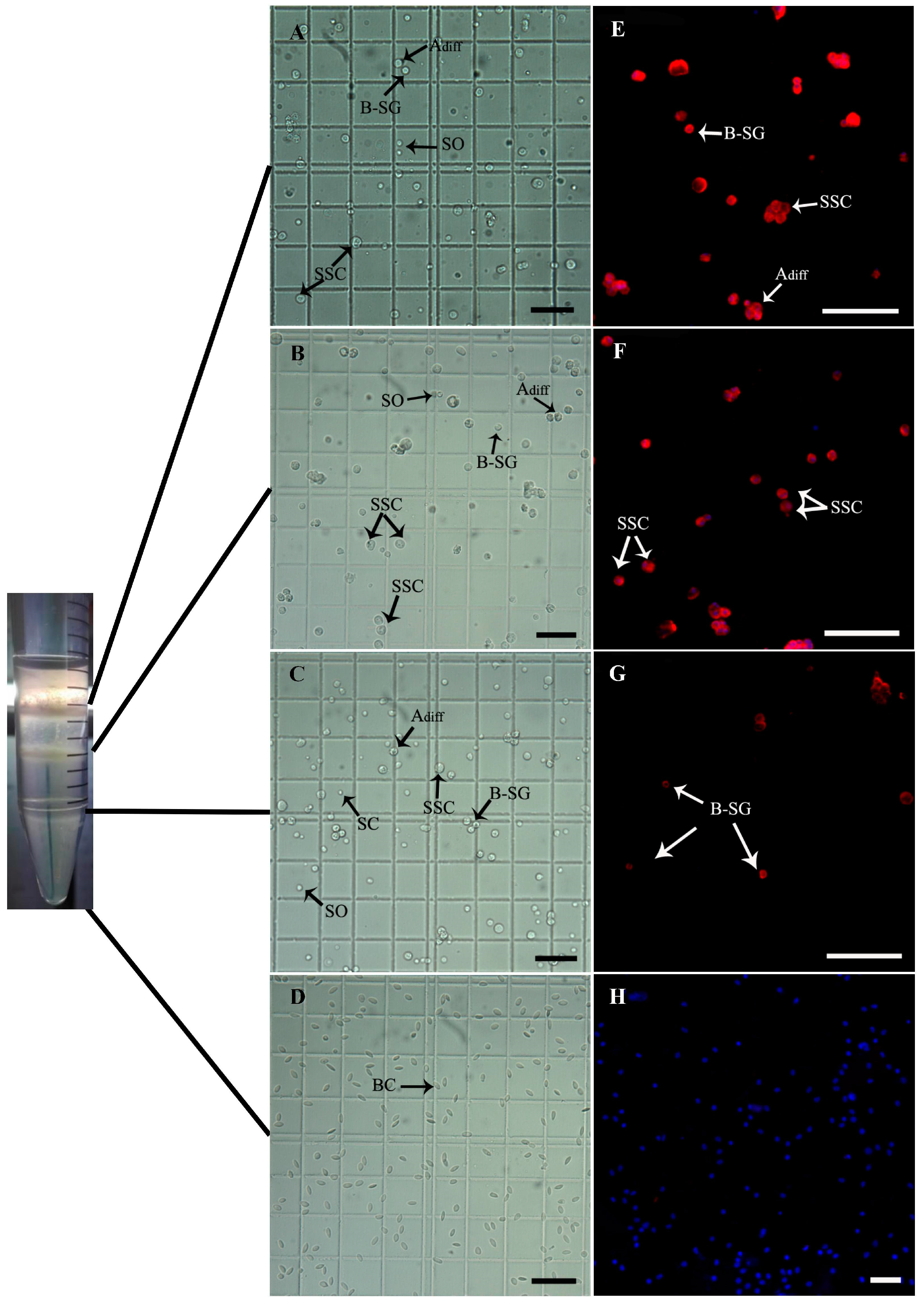
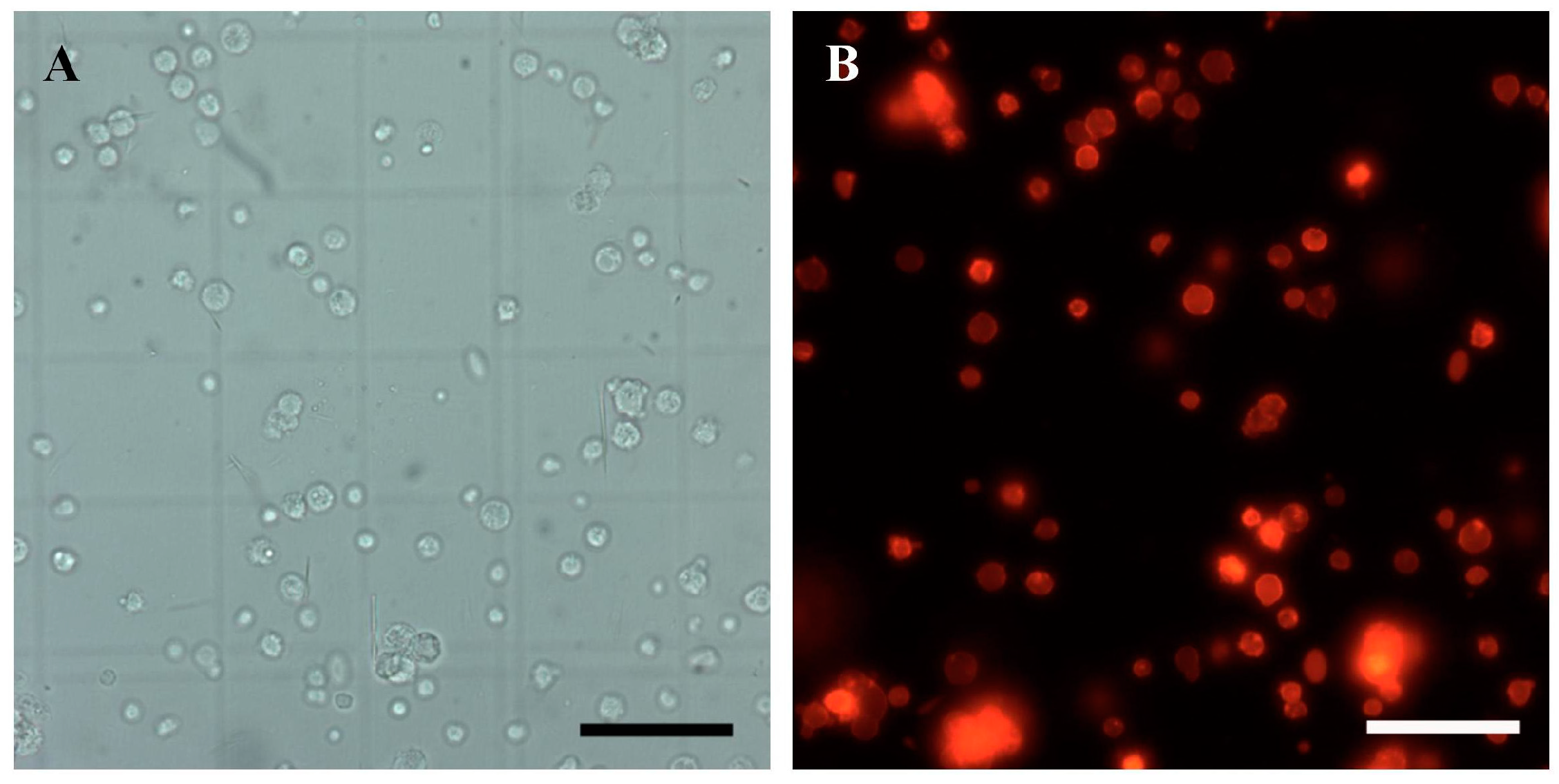
3.4. SSC Isolation and Purification from Donor Testes
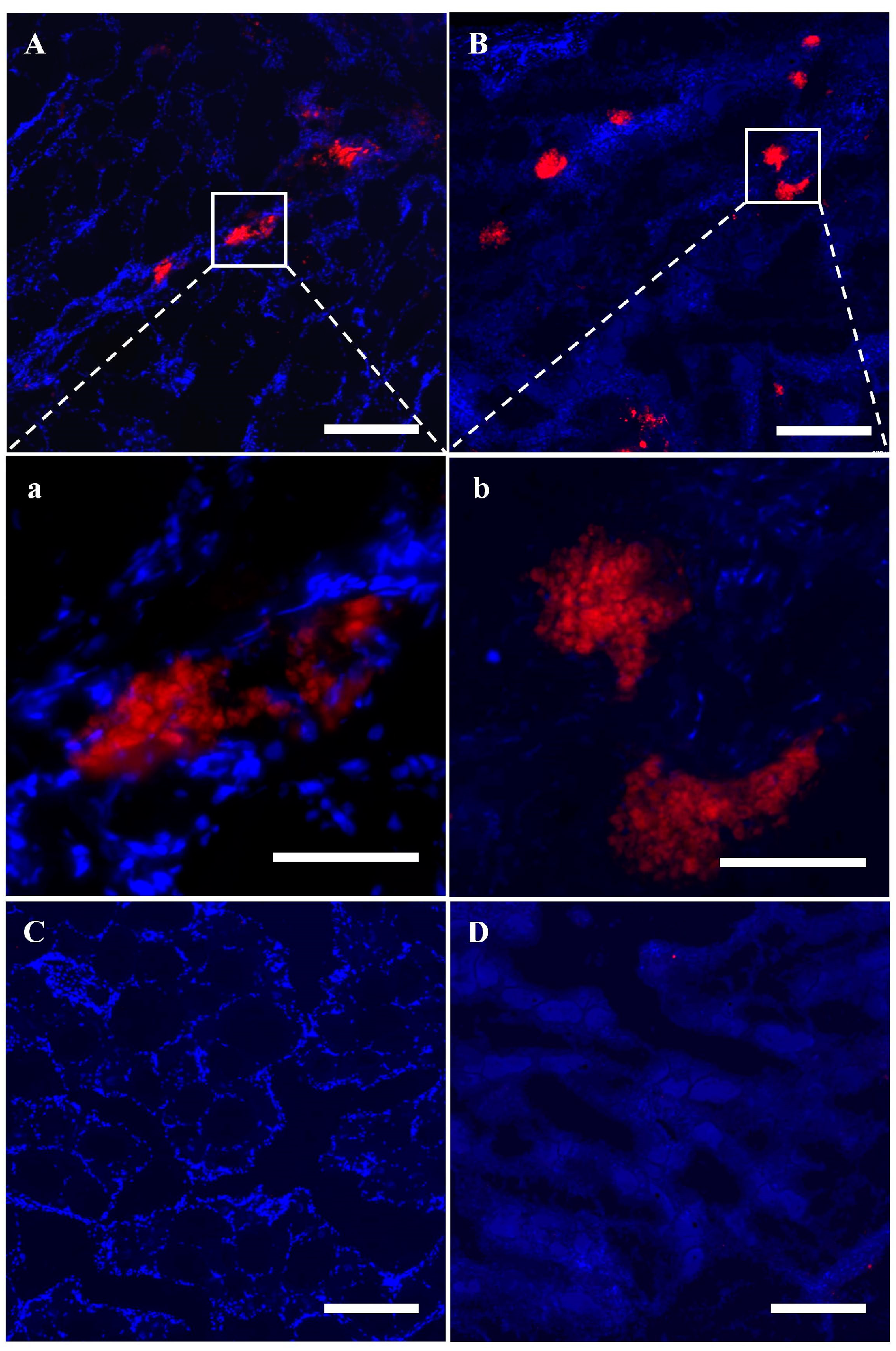
3.5. SSC Transplantation and Fate in the Ovaries of Sterile Recipients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralph, L.; Brinster, J.W.Z. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef]
- Honaramooz, A. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol. Reprod. 2003, 69, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Giassetti, M.; Ciccarelli, M.; Oatley, J. Spermatogonial stem cell transplantation: Insights and outlook for domestic animals. Annu. Rev. Anim. Biosci. 2019, 7, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Yoshizaki, G.; Takeuchi, T. Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol. Reprod. 2003, 69, 1142–1149. [Google Scholar] [CrossRef]
- Kawakami, Y.; Goto-Kazeto, R.; Saito, T.; Fujimoto, T.; Higaki, S.; Takahashi, Y.; Arai, K.; Yamaha, E. Generation of germ-line chimera zebrafish using primordial germ cells isolated from cultured blastomeres and cryopreserved embryoids. Int. J. Dev. Biol. 2010, 54, 1493–1501. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Arai, K.; Yamaha, E. Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol. Reprod. 2008, 78, 159–166. [Google Scholar] [CrossRef]
- Okutsu, T.; Suzuki, K.; Takeuchi, Y.; Takeuchi, T.; Yoshizaki, G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc. Natl. Acad. Sci. USA 2006, 103, 2725–2729. [Google Scholar] [CrossRef]
- Morita, T.; Kumakura, N.; Morishima, K.; Mitsuboshi, T.; Ishida, M.; Hara, T.; Kudo, S.; Miwa, M.; Ihara, S.; Higuchi, K.; et al. Production of donor-derived offspring by allogeneic transplantation of spermatogonia in the yellowtail (Seriola quinqueradiata). Biol. Reprod. 2012, 86, 176. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Ichikawa, M.; Hayashi, M.; Iwasaki, Y.; Miwa, M.; Shikina, S.; Okutsu, T. Sexual plasticity of ovarian germ cells in rainbow trout. Development 2010, 137, 1227–1230. [Google Scholar] [CrossRef]
- Nayak, R.; Franek, R.; Sindelka, R.; Psenicka, M. Enhancement of zebrafish sperm production via a large body-sized surrogate with germ cell transplantation. Commun. Biol. 2023, 6, 412. [Google Scholar] [CrossRef]
- Majhi, S.K.; Hattori, R.S.; Rahman, S.M.; Suzuki, T.; Strussmann, C.A. Experimentally induced depletion of germ cells in sub-adult Patagonian pejerrey (Odontesthes hatcheri). Theriogenology 2009, 71, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Okutsu, T.; Shikina, S.; Sakamoto, T.; Mochizuki, M.; Yoshizaki, G. Successful production of functional Y eggs derived from spermatogonia transplanted into female recipients and subsequent production of YY supermales in rainbow trout, Oncorhynchus mykiss. Aquaculture 2015, 446, 298–302. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ino, Y.; Shigenaga, K.; Katayama, T.; Kuroyanagi, M.; Yoshiura, Y. Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 2018, 493, 302–313. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Takeuchi, Y.; Ino, Y.; Wang, J.; Iwata, G.; Kabeya, N.; Yazawa, R.; Yoshizaki, G. Efficient production of donor-derived gametes from triploid recipients following intra-peritoneal germ cell transplantation into a marine teleost, Nibe croaker (Nibea mitsukurii). Aquaculture 2017, 478, 35–47. [Google Scholar] [CrossRef]
- Wong, T.T.; Saito, T.; Crodian, J.; Collodi, P. Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol. Reprod. 2011, 84, 1190–1197. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, Z.; Wang, Y.; Yu, Q.; Wang, G.; He, Z.; Liu, Y.; Jiang, X.; Kang, X.; Hou, J. Production of donor-derived offsprings by allogeneic transplantation of oogonia in the adult Japanese flounder (Paralichthys olivaceus). Aquaculture 2021, 543, 736977. [Google Scholar] [CrossRef]
- Sato, M.; Morita, T.; Katayama, N.; Yoshizaki, G. Production of genetically diversified fish seeds using spermatogonial transplantation. Aquaculture 2014, 422–423, 218–224. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Fujinuma, K.; Iwasaki, Y.; Okutsu, T.; Shikina, S.; Yazawa, R.; Takeuchi, Y. Spermatogonial transplantation in fish: A novel method for the preservation of genetic resources. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 55–61. [Google Scholar] [CrossRef]
- Ye, H.; Li, C.J.; Yue, H.M.; Du, H.; Yang, X.G.; Yoshino, T.; Hayashida, T.; Takeuchi, Y.; Wei, Q.W. Establishment of intraperitoneal germ cell transplantation for critically endangered Chinese sturgeon Acipenser sinensis. Theriogenology 2017, 94, 37–47. [Google Scholar] [CrossRef]
- Wang, W.; Liang, S.; Zou, Y.; Wang, L.; You, F. Effects of busulfan on somatic cells after inhibiting germ cells in the gonads of the young olive flounder Paralichthys olivaceus. Anim. Reprod. Sci. 2021, 228, 106746. [Google Scholar] [CrossRef]
- Milstone, D.S.; Nóbrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de França, L.R.; Schulz, R.W. Spermatogonial Stem Cell Niche and Spermatogonial Stem Cell Transplantation in Zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar] [CrossRef]
- Silva, M.A.; Costa, G.M.; Lacerda, S.M.; Brandao-Dias, P.F.; Kalapothakis, E.; Silva Junior, A.F.; Alvarenga, E.R.; Franca, L.R. Successful xenogeneic germ cell transplantation from Jundia catfish (Rhamdia quelen) into adult Nile tilapia (Oreochromis niloticus) testes. Gen. Comp. Endocrinol. 2016, 230–231, 48–56. [Google Scholar] [CrossRef]
- de Siqueira-Silva, D.H.; Silva, A.P.; Ninhaus-Silveira, A.; Verissimo-Silveira, R. The effects of temperature and busulfan (Myleran) on the yellowtail tetra Astyanax altiparanae (Pisces, Characiformes) spermatogenesis. Theriogenology 2015, 84, 1033–1042. [Google Scholar] [CrossRef]
- Tani, R.; Yazawa, R.; Kamio, S.; Kawamura, W.; Morita, T.; Takeuchi, Y.; Yoshizaki, G. Establishment of surrogate broodstock technology in Scombridae species by germ cell transplantation. Aquac. Res. 2022, 53, 2760–2771. [Google Scholar] [CrossRef]
- Li, Q.; Fujii, W.; Naito, K.; Yoshizaki, G. Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol. Reprod. Dev. 2017, 84, 1100–1111. [Google Scholar] [CrossRef]
- Dobrinski, I. Germ cell transplantation in pigs--advances and applications. Soc. Reprod. Fertil. Suppl. 2006, 62, 331–339. [Google Scholar] [CrossRef]
- Majhi, S.K.; Rasal, A.R.; Kushwaha, B.; Raizada, S. Heat and chemical treatments in adult Cyprinus carpio (Pisces cypriniformes) rapidly produce sterile gonads. Anim. Reprod. Sci. 2017, 183, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, G.; Zhang, X.; Wang, Y.; Sun, Z.; Si, F.; Jiang, X.; Liu, H. Production and verification of a 2nd generation clonal group of Japanese flounder, Paralichthys olivaceus. Sci. Rep. 2016, 6, 35776. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, Q.; Song, L.; Jiang, X.; Wang, Q.; Ren, J.; Wang, Y.; Ren, Y. Identification, separation and enrichment of oogonia from Japanese flounder (Paralichthys olivaceus). Chin. J. Cell Biol. 2018, 40, 1510–1517. [Google Scholar] [CrossRef]
- Shao, C.; Bao, B.; Xie, Z.; Chen, X.; Li, B.; Jia, X.; Yao, Q.; Ortí, G.; Li, W.; Li, X.; et al. The genome and transcriptome of Japanese flounder provide insights into flatfish asymmetry. Nat. Genet. 2016, 49, 119–124. [Google Scholar] [CrossRef]
- Hou, J.; Wang, G.; Zhang, X.; Sun, Z.; Liu, H.; Wang, Y. Cold-shock induced androgenesis without egg irradiation and subsequent production of doubled haploids and a clonal line in Japanese flounder, Paralichthys olivaceus. Aquaculture 2016, 464, 642–646. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lacerda, S.M.; Batlouni, S.R.; Costa, G.M.; Segatelli, T.M.; Quirino, B.R.; Queiroz, B.M.; Kalapothakis, E.; Franca, L.R. A new and fast technique to generate offspring after germ cells transplantation in adult fish: The Nile tilapia (Oreochromis niloticus) model. PLoS ONE 2010, 5, e10740. [Google Scholar] [CrossRef]
- Watanabe, S.; Strussmann, C.A. Germ cell transplantation using sexually competent fish: An approach for rapid propagation of endangered and valuable germlines. PLoS ONE 2009, 4, e6132. [Google Scholar] [CrossRef]
- Majhi, S.K.; Hattori, R.S.; Rahman, S.M.; Strussmann, C.A. Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS ONE 2014, 9, e95294. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; de Franca, L.R.; Lareyre, J.J.; Le Gac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Gui, J.; Hong, Y. Fish germ cells. Sci. China Life Sci. 2010, 53, 435–446. [Google Scholar] [CrossRef]
- Leal, M.C.; Cardoso, E.R.; Nobrega, R.H.; Batlouni, S.R.; Bogerd, J.; Franca, L.R.; Schulz, R.W. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod. 2009, 81, 177–187. [Google Scholar] [CrossRef]
- Loir, M. Spermatogonia of rainbow trout: II. in vitro study of the influence of pituitary hormones, growth factors and steroids on mitotic activity. Mol. Reprod. Dev. 1999, 53, 434–442. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Higuchi, K.; Yatabe, T.; Miwa, M.; Yoshizaki, G. Development of spermatogonial cell transplantation in Nibe croaker, Nibea mitsukurii (Perciformes, Sciaenidae). Biol. Reprod. 2009, 81, 1055–1063. [Google Scholar] [CrossRef]
- Wu, J.; Liao, M.; Zhu, H.; Kang, K.; Mu, H.; Song, W.; Niu, Z.; He, X.; Bai, C.; Li, G.; et al. CD49f-positive testicular cells in Saanen dairy goat were identified as spermatogonia-like cells by miRNA profiling analysis. J. Cell. Biochem. 2014, 115, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Kise, K.; Morita, T.; Yazawa, R.; Takeuchi, Y.; Yoshizaki, G. Flow-cytometric enrichment of Pacific bluefin tuna type A spermatogonia based on light-scattering properties. Theriogenology 2017, 101, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Psenicka, M.; Saito, T.; Linhartova, Z.; Gazo, I. Isolation and transplantation of sturgeon early-stage germ cells. Theriogenology 2015, 83, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Piferrer, F.; Chen, S.L.; Shen, Z.G. Sex Control in Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, Q.; Liu, Y.; Wang, Y.; Wang, Q.; Jiang, X.; Yu, Q.; Zhang, H. Effects of estradiol-17β on survival, growth performance, gonadal structure and sex ratio of the tiger puffer, Takifugu rubripes (Temminck & Schlegel, 1850), fingerlings. Aqua. Res. 2018, 49, 1638–1646. [Google Scholar] [CrossRef]
- Morishima, K.; Fujimoto, T.; Sato, M.; Kawae, A.; Zhao, Y.; Yamaha, E.; Arai, K. Cold-shock eliminates female nucleus in fertilized eggs to induce androgenesis in the loach (Misgurnus anguillicaudatus), a teleost fish. BMC Biotechnol. 2011, 11, 116. [Google Scholar] [CrossRef]

| Gender | Recipients (♀) | Donors (♂) | ||
|---|---|---|---|---|
| Age | 1 | 2 | 3 | 1 |
| Body weight (g) | 340.00 ± 26.94 | 960.00 ± 52.55 | 1480.00 ± 41.75 | 262.36 ± 24.05 |
| Gonadosomatic index (GSI) | 0.59 ± 0.02 | 1.03 ± 0.10 | 1.21 ± 0.10 | 0.08 ± 0.00 |
| Gonadal development period | Phase II | End of phase II | Phase III | Phase II |
| Primer Name | Primer Sequences (5′–3′) | Amplicon (bp) |
|---|---|---|
| vasa-F | TAGTTCCCTCGTGGTTAGAAGAGT | 133 |
| vasa-R | GCTGTGCTGTCCTGAGAGAATC | |
| UbcE-F | TTACTGTCCATTTCCCCACTGAC | 127 |
| UbcE-R | GACCACTGTGACCTCAAGATG |
| Cell Suspension | Cell Survival Rate (%) | SSC Proportion (%) | SSC Counts (×104/mg Testis Tissue) |
|---|---|---|---|
| Before enrichment | 91.67 ± 0.88 a | 18.21 ± 2.77 a | 3.38 ± 0.51 a |
| 1 | 90.57 ± 2.03 a | 64.35 ± 3.50 b | 1.52 ± 1.11 a |
| 2 | 86.68 ± 1.01 a | 61.83 ± 7.16 b | |
| 3 | 88.82 ± 2.20 a | 14.60 ± 1.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Tao, Y.; Sun, Z.; Wang, Y.; Li, W.; He, Z.; Wang, G.; Yang, Y.; Hou, J. Evaluation of Female Recipient Infertility and Donor Spermatogonial Purification for Germ Cell Transplantation in Paralichthys olivaceus. Animals 2024, 14, 2887. https://doi.org/10.3390/ani14192887
Ren Y, Tao Y, Sun Z, Wang Y, Li W, He Z, Wang G, Yang Y, Hou J. Evaluation of Female Recipient Infertility and Donor Spermatogonial Purification for Germ Cell Transplantation in Paralichthys olivaceus. Animals. 2024; 14(19):2887. https://doi.org/10.3390/ani14192887
Chicago/Turabian StyleRen, Yuqin, Yuehong Tao, Zhaohui Sun, Yufen Wang, Weidong Li, Zhongwei He, Guixing Wang, Yucong Yang, and Jilun Hou. 2024. "Evaluation of Female Recipient Infertility and Donor Spermatogonial Purification for Germ Cell Transplantation in Paralichthys olivaceus" Animals 14, no. 19: 2887. https://doi.org/10.3390/ani14192887
APA StyleRen, Y., Tao, Y., Sun, Z., Wang, Y., Li, W., He, Z., Wang, G., Yang, Y., & Hou, J. (2024). Evaluation of Female Recipient Infertility and Donor Spermatogonial Purification for Germ Cell Transplantation in Paralichthys olivaceus. Animals, 14(19), 2887. https://doi.org/10.3390/ani14192887





