Pathohistological Findings after Bilateral Ovariectomy in Mares with Behavioral Problems
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Group Determination, Clinical History, Premedication, and Preoperative Examination
2.2. Hormone Concentrations
2.3. Surgical Procedure and Owner Consult
2.4. Macro- and Microscopical Evaluation of the Ovaries
2.5. Immunohistochemical Staining and Evaluation
2.6. Statistical Analysis
3. Results
3.1. Animals, Clinical History, and Pre-OP Examinations
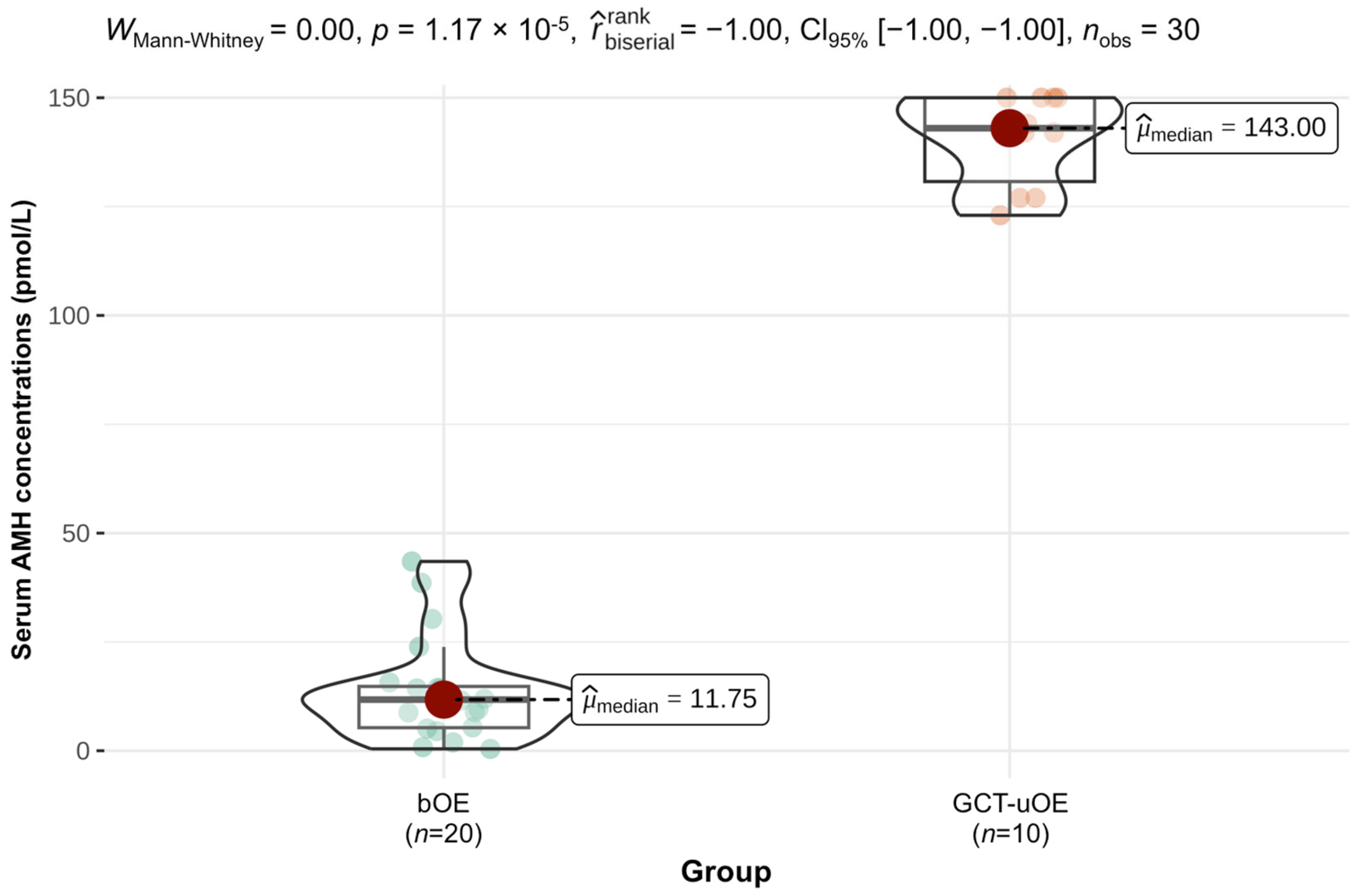
3.2. Results of Hormone Measurements
3.3. Outcome of Surgery and Improvement of Behavior
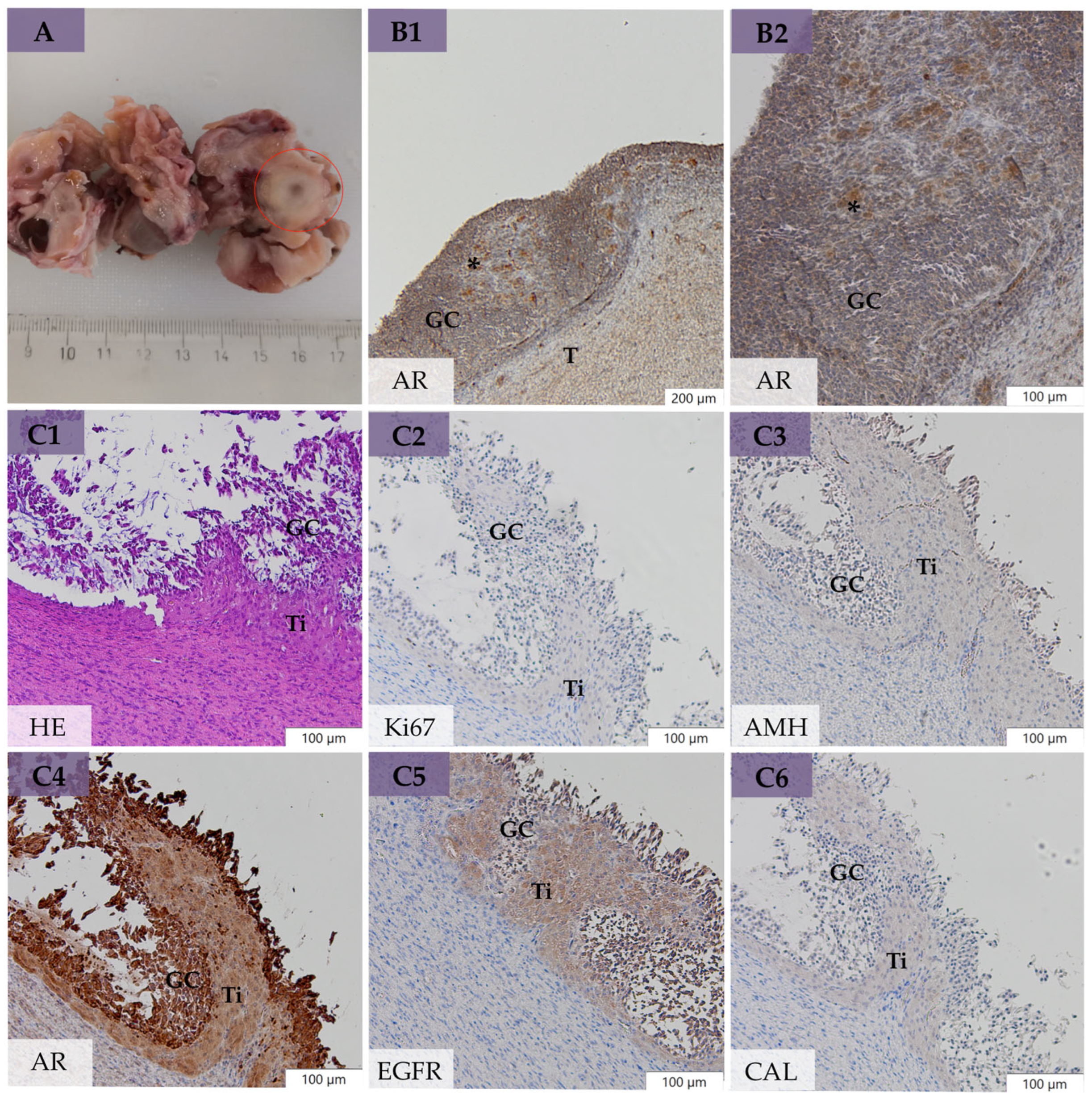
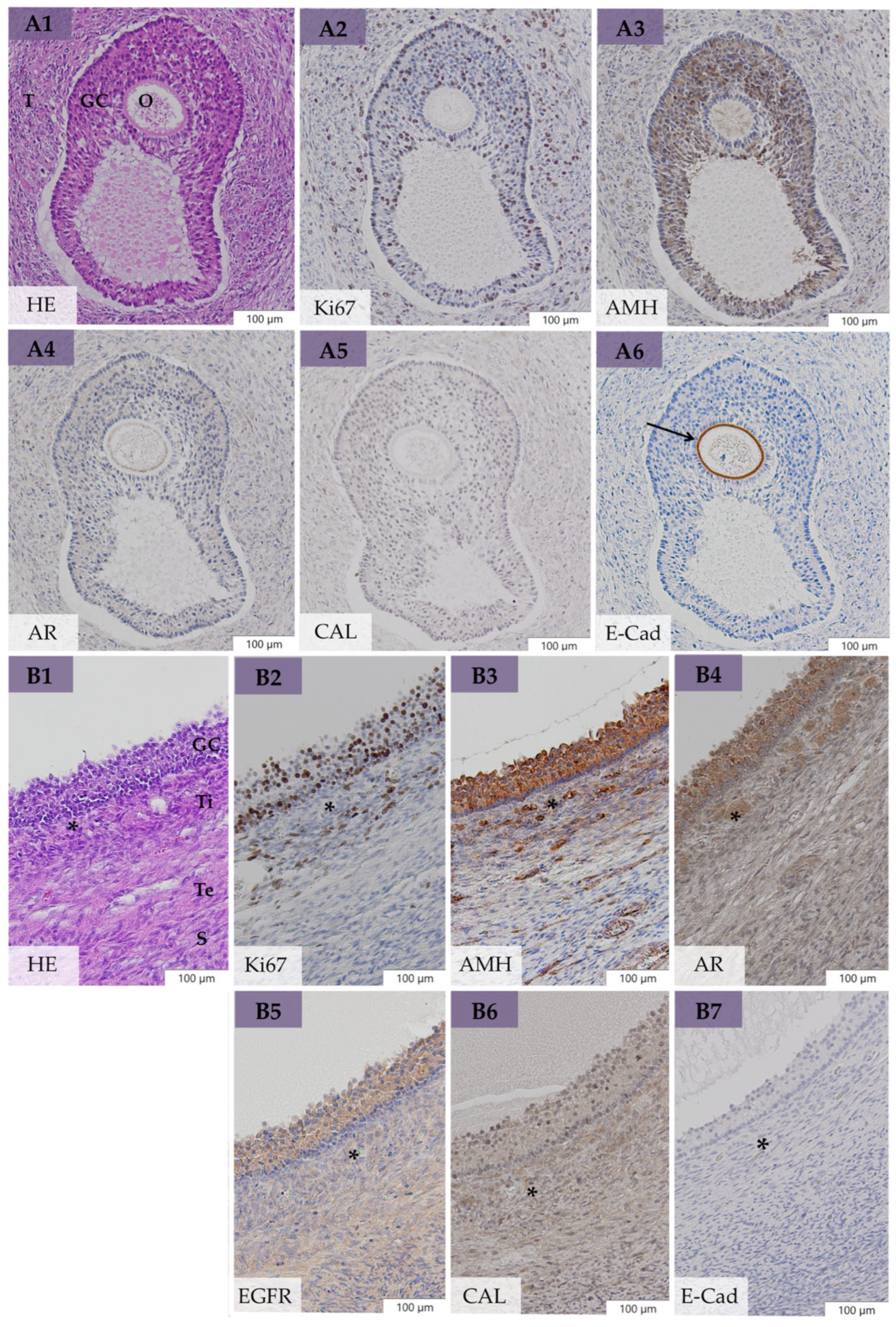
3.4. Macro- and Microscopical Findings on the Ovaries
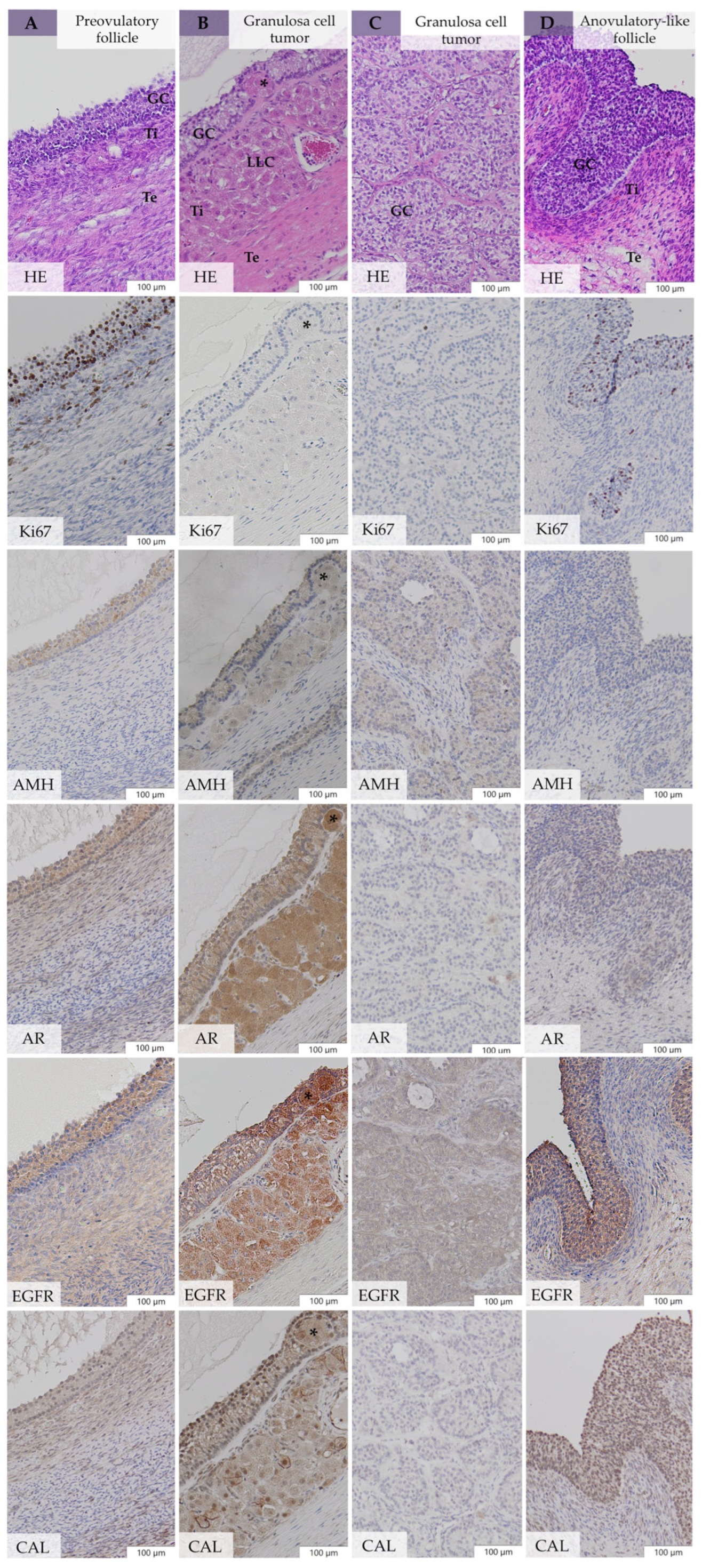
3.5. Immunohistochemical Evaluation
3.5.1. Expression of Ki67, AMH, AR, EGFR, and CAL in Large Follicular Structures of bOE
3.5.2. Expression of Ki67, AMH, AR, EGFR, and CAL in Neoplastic Cells of GCT-uOE
3.5.3. Comparative Immunohistochemistry between bOE and GCT-uOE
3.5.4. Correlation of Serum AMH and Immunohistochemical AMH Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jorgensen, J.S.; Vivrette, S.; Correa, M.; Mansmann, R.A. Significance of the Estrous Cycle on Athletic Performance in Mares. In Proceedings of the 42nd Annual Convention Am. Equine Pract, Denver, CO, USA, 8–9 December 1996; Volume 42, pp. 98–100. [Google Scholar]
- Stout, T.A.E.; Colenbrander, B. Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Anim. Reprod. Sci. 2004, 82–83, 633–643. [Google Scholar] [CrossRef]
- Pryor, P.; Tibary, A. Management of estrus in the performance mare. Clin. Tech. Equine Pract. 2005, 4, 197–209. [Google Scholar] [CrossRef]
- McDonnell, S.M. Behavior problem: Ovaries or not? In Proceedings of the 63rd Annual Convention of the American Association of Equine Practitioners, San Antonio, TX, USA, 17–21 November 2017. [Google Scholar]
- Melgaard, D.T.; Korsgaard, T.S.; Thoefner, M.S.; Petersen, M.R.; Pedersen, H.G. Moody Mares—Is Ovariectomy a Solution? Animals 2020, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Nout-Lomas, Y.S.; Beacom, C.L. Granulosa cell tumours: Examining the “moody” mare. Equine Vet. Educ. 2015, 27, 515–518. [Google Scholar] [CrossRef]
- Kennedy, P.; Miller, R. The female genital system. In Pathology in Domestic Animals, 4th ed.; Jubb, K.V.F., Kennedy, P.C., Almer, N., Eds.; Academic Press: New York, NY, USA, 1993; pp. 366–367. [Google Scholar]
- McCue, P.M.; Roser, J.F.; Munro, C.J.; Liu, I.K.M.; Lasley, B.L. Granulosa Cell Tumors of the Equine Ovary. Vet. Clin. N. Am. Equine Pract. 2006, 22, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Aurich, C.; Kaps, M. Suppression of reproductive behaviour and gonadal function in female horses—An update. Reprod. Domest. Anim. 2022, 57, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.R. A review of oestrus suppression techniques in mares. Equine Vet. Educ. 2022, 34, 141–151. [Google Scholar] [CrossRef]
- Gynäkologische Praktiken bei Sportstuten. Available online: https://www.tierschutz-tvt.de/alle-merkblaetter-und-stellungnahmen/?no_cache=1&download=TVT-Stellungn._Gyn%C3%A4kologische_Praktiken_bei_Sportstuten_Sept._2017_01.pdf&did=238 (accessed on 3 January 2024).
- Röcken, M.; Mosel, M.; Seyrek-Intas, K.; Seyrek-Intas, D.; Litzke, F.; Verver, J.; Rijkenhuizen, A.B. Unilateral and bilateral laparoscopic ovariectomy in 157 mares: A retrospective multicenter study. Vet. Surg. 2011, 40, 1009–1014. [Google Scholar] [CrossRef]
- Hooper, R.N.; Taylor, T.S.; Varner, D.D.; Blanchard, T.L. Effects of bilateral ovariectomy via colpotomy in mares: 23 cases (1984-1990). J. Am. Vet. Med. Assoc. 1993, 203, 1043–1046. [Google Scholar] [CrossRef]
- Collar, E.M.; Duesterdieck-Zellmer, K.F.; Huber, M.J.; Semevolos, S.A.; Parker, J.E.; Husby, K.A. Outcome of Bilateral Equid Laparoscopic Ovariectomies. Vet. Surg. 2021, 50, 975–983. [Google Scholar] [CrossRef]
- Roessner, H.A.; Kurtz, K.A.; Caron, J.P. Laparoscopic Ovariectomy Diminishes Estrus-Associated Behavioral Problems in Mares. J. Equine Vet. Sci. 2015, 35, 250–253. [Google Scholar] [CrossRef]
- Kamm, J.L.; Hendrickson, D.A. Clients’ Perspectives on the Effects of Laparoscopic Ovariectomy on Equine Behavior and Medical Problems. J. Equine Vet. Sci. 2007, 27, 435–438. [Google Scholar] [CrossRef]
- Devick, I.F.; Leise, B.S.; McCue, P.M.; Rao, S.; Hendrickson, D.A. Ovarian histopathology, pre- and post-operative endocrinological analysis and behavior alterations in 27 mares undergoing bilateral standing laparoscopic ovariectomy. Can. Vet. J. 2020, 61, 181. [Google Scholar] [PubMed]
- Straticò, P.; Hattab, J.; Guerri, G.; Carluccio, A.; Bandera, L.; Celani, G.; Marruchella, G.; Varasano, V.; Petrizzi, L. Behavioral Disorders in Mares with Ovarian Disorders, Outcome after Laparoscopic Ovariectomy: A Case Series. Vet. Sci. 2023, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.R. Can ovariectomy be justified on grounds of behaviour? Equine Vet. Educ. 2016, 28, 58–59. [Google Scholar] [CrossRef]
- Mccue, P.M. Review of Ovarian Abnormalities in the Mare. Proc. Am. Assoc. Equine Pract. 1998, 44, 125–133. [Google Scholar]
- Dolin, A.; Schweiger, P.; Waselau, M.; Egerbacher, M.; Walter, I. Immunohistochemical markers for equine granulosa cell tumors: A pilot study. J. Equine Sci. 2023, 34, 37–46. [Google Scholar] [CrossRef]
- Ellenberger, C.; Bartmann, C.P.; Hoppen, H.O.; Kratzsch, J.; Aupperle, H.; Klug, E.; Schoon, D.; Schoon, H.A. Histomorphological and Immunohistochemical Characterization of Equine Granulosa Cell Tumours. J. Comp. Pathol. 2007, 136, 167–176. [Google Scholar] [CrossRef]
- Hoque, S.; Derar, R.I.; Senba, H.; Osawa, T.; Kano, K.; Taya, K.; Miyake, Y. Localization of inhibin α-, βA- and βB-subunits and aromatase in ovarian follicles with granulosa theca cell tumor (GTCT) in 6 mares. J. Vet. Med. Sci. 2003, 65, 713–717. [Google Scholar] [CrossRef]
- Neto, A.C.A.; Ball, B.A.; Browne, P.; Conley, A.J. Cellular localization of androgen synthesis in equine granulosa-theca cell tumors: Immunohistochemical expression of 17α-hydroxylase/17,20-lyase cytochrome P450. Theriogenology 2010, 74, 393–401. [Google Scholar] [CrossRef]
- Müller, K.; Ellenberger, C.; Hoppen, H.O.; Schoon, H.A. Immunohistochemical study of angiogenesis and angiogenic factors in equine granulosa cell tumours. Res. Vet. Sci. 2012, 92, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.L.A. Studies to Characterize Ovarian Tumours in the Mare. Ph.D. Thesis, University of Glasgow, Scottland, UK, 13 August 2021. [Google Scholar]
- King, L.A.; Okagaki, T.; Gallup, D.G.; Twiggs, L.B.; Messing, M.J.; Carson, L.F. Mitotic count, nuclear atypia, and immunohistochemical determination of Ki-67, c-myc, p21-ras, c-erbB2, and p53 expression in granulosa cell tumors of the ovary: Mitotic count and Ki-67 are indicators of poor prognosis. Gynecol. Oncol. 1996, 61, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Balan, R.A.; Căruntu, I.D.; Giuşcă, S.E.; Lozneanu, L.; Păvăleanu, I.; Socolov, R.V. Immunohistochemical significance of ER alpha, inhibin a, calretinin, and Ki67 expression in granulosa cell ovarian tumors. Rom. J. Morphol. Embryol. 2017, 58, 753–760. [Google Scholar] [PubMed]
- Akhter, S.; Islam, N.; Kabir, E.; Begum, S.; Gaffar, T.; Khan, A. Expression of Ki-67 in Ovarian Tumors and its Correlation with Type, Grade and Stage. J. Histopathol. Cytopathol. 2019, 3, 15–26. [Google Scholar]
- Visser, J.A.; de Jong, F.H.; Laven, J.S.E.; Themmen, A.P.N. Anti-Müllerian hormone: A new marker for ovarian function. Reproduction 2006, 131, 1–9. [Google Scholar] [CrossRef]
- Ball, B.A.; Conley, A.J.; MacLaughlin, D.T.; Grundy, S.A.; Sabeur, K.; Liu, I.K.M. Expression of anti-Müllerian hormone (AMH) in equine granulosa-cell tumors and in normal equine ovaries. Theriogenology 2008, 70, 968–977. [Google Scholar] [CrossRef]
- Tsogtgerel, M.; Tagami, M.; Watanabe, K.; Murase, H.; Hirosawa, Y.; Kobayashi, Y.; Nambo, Y. Case report: The case of a 17 kg ovarian granulosa cell tumor in a breton draft mare. J. Equine Sci. 2021, 32, 67–72. [Google Scholar] [CrossRef]
- Nelissen, S.; Miller, A.D. Comparison of anti-Müllerian hormone and inhibin immunolabeling in canine and equine granulosa cell tumors. J. Vet. Diagn. Investig. 2022, 34, 1027–1031. [Google Scholar] [CrossRef]
- Almeida, J.; Ball, B.A.; Conley, A.J.; Place, N.J.; Liu, I.K.M.; Scholtz, E.L.; Mathewson, L.; Stanley, S.D.; Moeller, B.C. Biological and clinical significance of anti-Müllerian hormone determination in blood serum of the mare. Theriogenology 2011, 76, 1393–1403. [Google Scholar] [CrossRef]
- Ball, B.A.; Almeida, J.; Conley, A.J. Determination of serum anti-Müllerian hormone concentrations for the diagnosis of granulosa-cell tumours in mares. Equine Vet. J. 2013, 45, 199–203. [Google Scholar] [CrossRef]
- Watson, E.D.; Thomson, S.R. Immunolocalization of aromatase P-450 in ovarian tissue from pregnant and nonpregnant mares and in ovarian tumours. J. Reprod. Fertil. 1996, 108, 239–244. [Google Scholar] [CrossRef]
- Mlodawska, W.; Slomczynska, M. Immunohistochemical localization of aromatase during the development and atresia of ovarian follicles in prepubertal horses. Theriogenology 2010, 74, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Toka, F.N.; Jurka, P. Role of Cadherins in Cancer—A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Almstedt, K.; Battista, M.J.; Seeger, A.; Jäkel, J.; Brenner, W.; Hasenburg, A. Immunohistochemical markers of prognosis in adult granulosa cell tumors of the ovary—A review. J. Ovarian Res. 2023, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Huggins, L.; Norris, J.; Conley, A.; Dini, P. Abnormal mare behaviour is rarely associated with changes in hormonal markers of granulosa cell tumours: A retrospective study. Equine Vet. J. 2023, 1, 1–9. [Google Scholar] [CrossRef]
- Overbeck, W.; Jäger, K.; Schoon, H.A.; Witte, T.S. Comparison of cytological and histological examinations in different locations of the equine uterus-an in vitro study. Theriogenology 2013, 79, 1262–1268. [Google Scholar] [CrossRef]
- Weir, B.; Rowlands, I. Functional anatomy of the hystricomorph ovary. Symp. Zool. Soc. Lond. 1974, 34, 303–330. [Google Scholar]
- Gomes, R.G.; Lisboa, L.A.; Silva, C.B.; Max, M.C.; Marino, P.C.; Oliveira, R.L.; González, S.M.; Barreiros, T.R.R.; Marinho, L.S.R.; Seneda, M.M. Improvement of development of equine preantral follicles after 6 days of in vitro culture with ascorbic acid supplementation. Theriogenology 2015, 84, 750–755. [Google Scholar] [CrossRef]
- Müller, K.; Ellenberger, C.; Schoon, H.A. Histomorphological and immunohistochemical study of angiogenesis and angiogenic factors in the ovary of the mare. Res. Vet. Sci. 2009, 87, 421–431. [Google Scholar] [CrossRef]
- Van Niekerk, C.H.; Gerneke, W.H.; Van Heerden, J.S. Anatomical and histological observations on the reproductive tract of mares with abnormal oestrous cycles. J. South Afr. Vet. Assoc. 1973, 44, 141–152. [Google Scholar]
- Kennedy, P.C.; Cullen, J.M.; Edwards, J.F.; Goldschmidt, M.H.; Larsen, S.; Munson, L.; Nielsen, S. Tumors of the ovary. In World Health Organization International Histological Classification of Tumors of Domestic Animals. Histological Classification of Tumors of the Genital System of Domestic Animals, 2nd ed.; Kennedy, P.C., Cullen, J.M., Edwards, J.F., Goldschmidt, M.H., Larsen, L., Munson, L., Nielsen, L., Eds.; Armed Forces Institute of Pathology, American Registry of Pathology: Washington, DC, USA, 1998; Volume 4, pp. 24–31. [Google Scholar]
- Matos, A.C.H.; Consalter, A.; dos Santos Batista, B.P.; Fonseca, A.B.M.; Ferreira, A.M.; Leite, J. Immunohistochemical expression of HER-2 and Ki-67 in granulosa cell tumor in bitches. Reprod. Domest. Anim. 2021, 56, 667–672. [Google Scholar] [CrossRef]
- Witte, T.S.; Wolf, N.; Walter, I.; Hahn, J.A.; Zerbe, H. Pathohistological findings in bilateral removed ovaries of mares with behavioral problems. J. Equine Vet. Sci. 2023, 125, 104754. [Google Scholar] [CrossRef]
- Watson, E.D.; Al-zi’abi, M.O. Characterization of morphology and angiogenesis in follicles of mares during spring transition and the breeding season. Reproduction 2002, 124, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Imboden, I.; Janett, F.; Burger, D.; Crowe, M.A.; Hässig, M.; Thun, R. Influence of immunization against GnRH on reproductive cyclicity and estrous behavior in the mare. Theriogenology 2006, 66, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Elhay, M.; Newbold, A.; Britton, A.; Turley, P.; Dowsett, K.; Walker, J. Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH. Aust. Vet. J. 2007, 85, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, D.; Burger, D.; Gremmes, S.; Szunyog, K.; Röthemeier, S.; Sieme, H. Active immunisation against GnRH as treatment for unilateral granulosa theca cell tumour in mares. Equine Vet. J. 2020, 53, 740–745. [Google Scholar] [CrossRef]
- Sherlock, C.E.; Lott-Ellis, K.; Bergren, A.; Withers, J.M.; Fews, D.; Mair, T.S. Granulosa cell tumours in the mare: A review of 52 cases. Equine Vet. Educ. 2016, 28, 75–82. [Google Scholar] [CrossRef]
- Ball, B.A.; Conley, A.J.; Almeida, J.; Esteller-Vico, A.; Crabtree, J.; Munro, C.; Liu, I.K.M. A retrospective analysis of 2,253 cases submitted for endocrine diagnosis of possible granulosa cell tumors in mares. J. Equine Vet. Sci. 2014, 34, 307–313. [Google Scholar] [CrossRef]
- Crabtree, J. Review of seven cases of granulosa cell tumour of the equine ovary. Vet. Rec. 2011, 169, 251. [Google Scholar] [CrossRef]
- Conley, A.J.; Ball, B.A. Endocrine Testing for Reproductive Conditions in Horses. In Interpretation of Equine Laboratory Diagnostics; Pusterla, N., Higgins, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1, pp. 409–418. [Google Scholar]
- Renaudin, C.D.; Kelleman, A.A.; Keel, K.; McCracken, J.L.; Ball, B.A.; Ferris, R.A.; McCue, P.M.; Dujovne, G.; Conley, A.J. Equine granulosa cell tumours among other ovarian conditions: Diagnostic challenges. Equine Vet. J. 2021, 53, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Claes, A.; Ball, B.A.; Corbin, C.J.; Conley, A.J. Anti-Müllerian hormone as a diagnostic marker for equine cryptorchidism in three cases with equivocal testosterone concentrations. J. Equine Vet. Sci. 2014, 34, 442–445. [Google Scholar] [CrossRef]
- Stabenfeldt, G.H.; Hughes, J.P.; Kennedy, P.C.; Meagher, D.M.; Neely, D.P. Clinical findings, pathological changes and endocrinological secretory patterns in mares with ovarian tumours. J. Reprod. Fertil. Suppl. 1979, 27, 277–285. [Google Scholar]
- Bailey, M.T.; Troedsson, M.H.T.; Wheaton, J.E. Inhibin concentrations in mares with granulosa cell tumors. Theriogenology 2002, 57, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Haag, K.T.; Magalhães-Padilha, D.M.; Fonseca, G.R.; Wischral, A.; Gastal, M.O.; King, S.S.; Jones, K.L.; Figueiredo, J.R.; Gastal, E.L. Equine preantral follicles obtained via the Biopsy Pick-Up method: Histological evaluation and validation of a mechanical isolation technique. Theriogenology 2013, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van der Zaag, E.J.; Rijkenhuizen, A.B.M.; Kalsbeek, H.C.; Peperkamp, N.H.M.T. A mare with colic caused by an ovarian tumour. Vet. Q. 1996, 18, 60–62. [Google Scholar] [CrossRef]
- Chopin, J.; Chopin, L.; Knott, L.; Kretser, D.; Dowsett, K. Unusual ovarian activity in a mare preceding the development of an ovarian granulosa cell tumour. Aust. Vet. J. 2002, 80, 32–36. [Google Scholar] [CrossRef]
- Crabtree, J.; Brennan, M.; Foote, A.; Pycock, J. Granulosa cell tumour: An interesting case in a pregnant mare. Equine Vet. Educ. 2013, 25, 4–10. [Google Scholar] [CrossRef]
- Castillo, J.M.; Tse, M.P.Y.; Dockweiler, J.C.; Cheong, S.H.; De Amorim, M.D. Bilateral granulosa cell tumor in a cycling mare. Can. Vet. J. 2019, 60, 480–484. [Google Scholar]
- McCue, P.M.; LeBlanc, M.M.; Akita, G.Y.; Pascoe, J.R.; Witherspoon, D.M.; Stabenfeldt, G.H. Granulosa Cell Tumors in two cycling mares. J. Equine Vet. Sci. 1991, 11, 281–282. [Google Scholar] [CrossRef]
- McCue, P.M.; Squires, E.L. Persistent anovulatory follicles in the mare. Theriogenology 2002, 58, 541–543. [Google Scholar]
- Crabtree, J. Update on the management of the anovulatory follicle in horses. In Practice 2020, 42, 171–176. [Google Scholar] [CrossRef]
- Ellenberger, C.; Müller, K.; Schoon, H.A.; Wilsher, S.; Allen, W. Histological and immunohistochemical characterization of equine anovulatory haemorrhagic follicles (AHFs). Reprod. Domest. Anim. 2009, 44, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kölling, M.; Allen, W. Anovulatory haemorrhagic follicles (AHFs) and ovulation failure in the mare. Reprod. Domest. Anim. 2006, 41, 308. [Google Scholar]

| Antibody | Function—Diagnostic Relevance | Clone/Host | Source | Dilution | Pretreatment | Positive Control |
|---|---|---|---|---|---|---|
| Ki67 | Cell proliferation—Tumor marker | MIB-1 Mouse | Agilent Technologies, Santa Clara, CA, USA | 1:1000 in PBS | 0.01 M Citrate buffer pH 6.0 | Lymph node |
| AMH | Hormone—Produced in granulosa cells | Polyclonal Rabbit | Genetex, Irvine, CA, USA | 1:5000 in PBS | Tris EDTA pH 9.0 | Fetal gonads |
| AR | Enzyme complex— Conversion of androgens into estrogens | Polyclonal Rabbit | BioVision, Waltham, MA, USA | 1:1000 in PBS | Tris EDTA pH 9.0 | Testes |
| EGFR | Transmembrane protein with function in ovulation—Tumor marker | 4575 Mouse | NeoBiotechnologies, Aachen, Germany | 1:200 in PBS | Tris EDTA pH 9.0 | Skin |
| CAL | Calcium-binding protein—Tumor marker | Polyclonal Rabbit | Chemicon, Temecula, CA, USA | 1:5000 in PBS | 0.01 M Citrate buffer pH 6.0 | Cerebrum |
| E-Cad | Adhering cell junction—Tumor suppression activity, Tumor marker | Polyclonal Rabbit | Sigma Prestige, St. Louis, MI, USA | 1:500 in PBS | 0.01 M Citrate buffer pH 6.0 | Kidney |
| Behavioral Problem | Frequency of Occurrence | |
|---|---|---|
| bOE | GCT-uOE | |
| Moodiness | 50% (10/20) | 40% (4/10) |
| Stressed manner | 30% (6/20) | 20% (2/10) |
| Unwillingness to be ridden | 45% (9/20) | 30% (3/10) |
| Aggressiveness towards people | 15% (3/20) | 0% (0/10) |
| Aggressiveness towards other horses | 30% (6/20) | 0% (0/10) |
| Stallion-like behavior | 5% (1/20) | 60% (6/20) |
| Increased flank sensitivity | 25% (5/20) | 0% (0/20) |
| Colic symptoms | 40% (8/20) | 10% (1/10) |
| Prolonged or constant estrous signs | 20% (4/20) | 10% (1/10) |
| No estrous signs | 5% (1/20) | 40% (4/10) |
| No abnormal behavior | 5% (1/20) | 10% (1/10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, N.; Hahn, J.A.; Walter, I.; Zablotski, Y.; Zerbe, H.; Witte, T.S. Pathohistological Findings after Bilateral Ovariectomy in Mares with Behavioral Problems. Animals 2024, 14, 2899. https://doi.org/10.3390/ani14192899
Wolf N, Hahn JA, Walter I, Zablotski Y, Zerbe H, Witte TS. Pathohistological Findings after Bilateral Ovariectomy in Mares with Behavioral Problems. Animals. 2024; 14(19):2899. https://doi.org/10.3390/ani14192899
Chicago/Turabian StyleWolf, Nadine, Joachim A. Hahn, Ingrid Walter, Yury Zablotski, Holm Zerbe, and Tanja S. Witte. 2024. "Pathohistological Findings after Bilateral Ovariectomy in Mares with Behavioral Problems" Animals 14, no. 19: 2899. https://doi.org/10.3390/ani14192899





