The Genetic Characteristics of FT-MIRS-Predicted Milk Fatty Acids in Chinese Holstein Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sample Collection, FT-MIRS, and Fatty Acid Content Reference Value Measurement
2.2. Fatty Acid Content Prediction Model Construction
2.3. Fatty Acid Content Prediction and Correction
2.4. Genetic Parameter Evaluation
2.5. GWAS and Screening of Fatty Acid-Related SNPs
3. Results
3.1. Milk Fatty Acid Content Trait Analysis
3.2. FT-MIRS-Based Fatty Acid Prediction Model
3.3. Genetic Parameter Estimates of Fatty Acid Content
3.3.1. Heritability, Repeatability, and Individual Breeding Value
3.3.2. Genetic and Phenotypic Correlations
3.4. Screening and Identification of Fatty Acid-Related SNPs and Genes
3.4.1. Comparison and Filtering of the Three GWAS Methods
3.4.2. GWAS, Screening, and Identification of Fatty Acid Content Associated SNPs
4. Discussion
4.1. Reference Value of Fatty Acid Measurements and Prediction Model Construction
4.2. Fatty Acid Genetic Parameter Estimates and Comparison Analysis
4.3. Fatty Acid-Related Molecular Markers and Corresponding Genes
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleming, A.; Schenkel, F.S.; Chen, J.; Malchiodi, F.; Bonfatti, V.; Ali, R.A.; Mallard, B.; Corredig, M.; Miglior, F. Prediction of milk fatty acid content with mid-infrared spectroscopy in Canadian dairy cattle using differently distributed model development sets. J. Dairy Sci. 2017, 100, 5073–5081. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Hostmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Soyeurt, H.; Dehareng, F.; Gengler, N.; McParland, S.; Wall, E.; Berry, D.P.; Coffey, M.; Dardenne, P. Mid-infrared prediction of bovine milk fatty acids across multiple breeds, production systems, and countries. J. Dairy Sci. 2011, 94, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Bhavadharini, B.; Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; et al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Res. Care 2020, 8, e000826. [Google Scholar] [CrossRef] [PubMed]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Prediction of fatty acid content in sheep milk by Mid-Infrared spectrometry with a selection of wavelengths by Genetic Algorithms. LWT 2016, 65, 503–510. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, Y.; Zhou, Y.; Li, G.-H.; Zeng, B.-Q.; Zhang, J.-W.; Feng, X.-S. Progress in Pretreatment and Analysis of Fatty Acids in Foods: An Update since 2012. Sep. Purif. Rev. 2019, 50, 203–222. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Chilliard, Y.; Toivonen, V.; Kairenius, P.; Givens, D.I. Trans fatty acids and bioactive lipids in ruminant milk. Adv. Exp. Med. Biol. 2008, 606, 3–65. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2022, 105, 1687–1700. [Google Scholar] [CrossRef]
- Rutten, M.J.; Bovenhuis, H.; Hettinga, K.A.; van Valenberg, H.J.; van Arendonk, J.A. Predicting bovine milk fat composition using infrared spectroscopy based on milk samples collected in winter and summer. J. Dairy Sci. 2009, 92, 6202–6209. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dardenne, P.; Dehareng, F.; Lognay, G.; Veselko, D.; Marlier, M.; Bertozzi, C.; Mayeres, P.; Gengler, N. Estimating fatty acid content in cow milk using mid-infrared spectrometry. J. Dairy Sci. 2006, 89, 3690–3695. [Google Scholar] [CrossRef]
- Tiplady, K.M.; Lopdell, T.J.; Littlejohn, M.D.; Garrick, D.J. The evolving role of Fourier-transform mid-infrared spectroscopy in genetic improvement of dairy cattle. J. Anim. Sci. Biotechnol. 2020, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.; Wells, A.T.; Smith, S.L.; Denholm, S.J.; Wall, E.; Coffey, M.P. Predicting pregnancy status from mid-infrared spectroscopy in dairy cow milk using deep learning. J. Dairy Sci. 2021, 104, 4980–4990. [Google Scholar] [CrossRef] [PubMed]
- Grelet, C.; Dardenne, P.; Soyeurt, H.; Fernandez, J.A.; Vanlierde, A.; Stevens, F.; Gengler, N.; Dehareng, F. Large-scale phenotyping in dairy sector using milk MIR spectra: Key factors affecting the quality of predictions. Methods 2021, 186, 97–111. [Google Scholar] [CrossRef]
- Du, C.; Nan, L.; Yan, L.; Bu, Q.; Ren, X.; Zhang, Z.; Sabek, A.; Zhang, S. Genetic Analysis of Milk Production Traits and Mid-Infrared Spectra in Chinese Holstein Population. Animals 2020, 10, 139. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dardenne, P.; Dehareng, F.; Bastin, C.; Gengler, N. Genetic parameters of saturated and monounsaturated fatty acid content and the ratio of saturated to unsaturated fatty acids in bovine milk. J. Dairy Sci. 2008, 91, 3611–3626. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dehareng, F.; Mayeres, P.; Bertozzi, C.; Gengler, N. Variation of Δ9-desaturase activity in dairy cattle. J. Dairy Sci. 2008, 91, 3211–3224. [Google Scholar] [CrossRef]
- Bresolin, T.; Dórea, J.R.R. Infrared Spectrometry as a High-Throughput Phenotyping Technology to Predict Complex Traits in Livestock Systems. Front. Genet. 2020, 11, 923. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Rinnan, Å.; Nørgaard, L.; Berg, F.v.d.; Thygesen, J.; Bro, R.; Engelsen, S.B. Chapter 2—Data Pre-processing. In Infrared Spectroscopy for Food Quality Analysis and Control; Sun, D.-W., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 29–50. [Google Scholar]
- De Marchi, M.; Penasa, M.; Cecchinato, A.; Mele, M.; Secchiari, P.; Bittante, G. Effectiveness of mid-infrared spectroscopy to predict fatty acid composition of Brown Swiss bovine milk. Animal 2011, 5, 1653–1658. [Google Scholar] [CrossRef]
- Tiplady, K.M.; Lopdell, T.J.; Sherlock, R.G.; Johnson, T.J.J.; Spelman, R.J.; Harris, B.L.; Davis, S.R.; Littlejohn, M.D.; Garrick, D.J. Comparison of the genetic characteristics of directly measured and Fourier-transform mid-infrared-predicted bovine milk fatty acids and proteins. J. Dairy Sci. 2022, 105, 9763–9791. [Google Scholar] [CrossRef] [PubMed]
- Soyeurt, H.; Bastin, C.; Colinet, F.G.; Arnould, V.M.-R.; Berry, D.P.; Wall, E.; Dehareng, F.; Nguyen, H.N.; Dardenne, P.; Schefers, J.; et al. Mid-infrared prediction of lactoferrin content in bovine milk: Potential indicator of mastitis. Animal 2012, 6, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, D.; Zhang, S.; Wang, S.; Wu, X.; Zhang, Q.; Liu, L.; Li, Y.; Qiao, L. Genome wide association study identifies 20 novel promising genes associated with milk fatty acid traits in Chinese Holstein. PLoS ONE 2014, 9, e96186. [Google Scholar] [CrossRef] [PubMed]
- Ferrand-Calmels, M.; Palhiere, I.; Brochard, M.; Leray, O.; Astruc, J.M.; Aurel, M.R.; Barbey, S.; Bouvier, F.; Brunschwig, P.; Caillat, H.; et al. Prediction of fatty acid profiles in cow, ewe, and goat milk by mid-infrared spectrometry. J. Dairy Sci. 2014, 97, 17–35. [Google Scholar] [CrossRef]
- Contla Hernandez, B.; Lopez-Villalobos, N.; Vignes, M. Identifying Health Status in Grazing Dairy Cows from Milk Mid-Infrared Spectroscopy by Using Machine Learning Methods. Animals 2021, 11, 2154. [Google Scholar] [CrossRef]
- Frizzarin, M.; Gormley, I.C.; Berry, D.P.; Murphy, T.B.; Casa, A.; Lynch, A.; McParland, S. Predicting cow milk quality traits from routinely available milk spectra using statistical machine learning methods. J. Dairy Sci. 2021, 104, 7438–7447. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Denise Beaulieu, A.; Barbano, D.M. Feed and Animal Factors Influencing Milk Fat Composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Auldist, M.J.; Johnston, K.A.; White, N.J.; Fitzsimons, W.P.; Boland, M.J. A comparison of the composition, coagulation characteristics and cheesemaking capacity of milk from Friesian and Jersey dairy cows. J. Dairy Res. 2004, 71, 51–57. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed. Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Stoop, W.M.; Bovenhuis, H.; Heck, J.M.; van Arendonk, J.A. Effect of lactation stage and energy status on milk fat composition of Holstein-Friesian cows. J. Dairy Sci. 2009, 92, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, K.; Ward, H.; Berry, S.D.; Ankersmit-Udy, A.; Burrett, A.; Beattie, E.M.; Thomas, N.L.; Harris, B.; Ford, C.A.; Browning, S.R.; et al. Phenotypic population screen identifies a new mutation in bovine DGAT1 responsible for unsaturated milk fat. Sci. Rep. 2015, 5, 8484. [Google Scholar] [CrossRef] [PubMed]
- Niero, G.; Visentin, G.; Ton, S.; De Marchi, M.; Penasa, M.; Cassandro, M. Phenotypic characterisation of milk technological traits, protein fractions, and major mineral and fatty acid composition of Burlina cattle breed. Ital. J. Anim. Sci. 2016, 15, 576–583. [Google Scholar] [CrossRef]
- Gottardo, P.; Penasa, M.; Righi, F.; Lopez-Villalobos, N.; Cassandro, M.; De Marchi, M. Fatty acid composition of milk from Holstein-Friesian, Brown Swiss, Simmental and Alpine Grey cows predicted by mid-infrared spectroscopy. Ital. J. Anim. Sci. 2017, 16, 380–389. [Google Scholar] [CrossRef]
- Soyeurt, H.; Gillon, A.; Vanderick, S.; Mayeres, P.; Bertozzi, C.; Gengler, N. Estimation of heritability and genetic correlations for the major fatty acids in bovine milk. J. Dairy Sci. 2007, 90, 4435–4442. [Google Scholar] [CrossRef]
- Lopez-Villalobos, N.; Spelman, R.J.; Melis, J.; Davis, S.R.; Berry, S.D.; Lehnert, K.; Sneddon, N.W.; Holroyd, S.E.; MacGibbon, A.K.; Snell, R.G. Genetic correlations of milk fatty acid contents predicted from milk mid-infrared spectra in New Zealand dairy cattle. J. Dairy Sci. 2020, 103, 7238–7248. [Google Scholar] [CrossRef]
- Soyeurt, H.; Misztal, I.; Gengler, N. Genetic variability of milk components based on mid-infrared spectral data. J. Dairy Sci. 2010, 93, 1722–1728. [Google Scholar] [CrossRef]
- Bittante, G.; Cecchinato, A. Genetic analysis of the Fourier-transform infrared spectra of bovine milk with emphasis on individual wavelengths related to specific chemical bonds. J. Dairy Sci. 2013, 96, 5991–6006. [Google Scholar] [CrossRef]
- Wang, Q.; Hulzebosch, A.; Bovenhuis, H. Genetic and environmental variation in bovine milk infrared spectra. J. Dairy Sci. 2016, 99, 6793–6803. [Google Scholar] [CrossRef]
- Rovere, G.; de Los Campos, G.; Tempelman, R.J.; Vazquez, A.I.; Miglior, F.; Schenkel, F.; Cecchinato, A.; Bittante, G.; Toledo-Alvarado, H.; Fleming, A. A landscape of the heritability of Fourier-transform infrared spectral wavelengths of milk samples by parity and lactation stage in Holstein cows. J. Dairy Sci. 2019, 102, 1354–1363. [Google Scholar] [CrossRef]
- Zaalberg, R.M.; Shetty, N.; Janss, L.; Buitenhuis, A.J. Genetic analysis of Fourier transform infrared milk spectra in Danish Holstein and Danish Jersey. J. Dairy Sci. 2019, 102, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.C.; Bovenhuis, H.; Visker, M.H.; van Arendonk, J.A. Genome-wide association of milk fatty acids in Dutch dairy cattle. BMC Genet. 2011, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Schopen, G.C.B.; Visker, M.H.P.W.; Koks, P.D.; Mullaart, E.; van Arendonk, J.A.M.; Bovenhuis, H. Whole-genome association study for milk protein composition in dairy cattle. J. Dairy Sci. 2011, 94, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Boichard, D.; Boussaha, M.; Capitan, A.; Rocha, D.; Hozé, C.; Sanchez, M.P.; Tribout, T.; Letaief, R.; Croiseau, P.; Grohs, C.; et al. Experience from large scale use of the EuroGenomics custom SNP chip in cattle. In Proceedings of the 11th World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11–16 February 2018. [Google Scholar]
- Bouwman, A.C.; Visker, M.H.; van Arendonk, J.M.; Bovenhuis, H. Fine mapping of a quantitative trait locus for bovine milk fat composition on Bos taurus autosome 19. J. Dairy Sci. 2014, 97, 1139–1149. [Google Scholar] [CrossRef]
- Buitenhuis, B.; Poulsen, N.A.; Gebreyesus, G.; Larsen, L.B. Estimation of genetic parameters and detection of chromosomal regions affecting the major milk proteins and their post translational modifications in Danish Holstein and Danish Jersey cattle. BMC Genet. 2016, 17, 114. [Google Scholar] [CrossRef]
- Olsen, H.G.; Knutsen, T.M.; Kohler, A.; Svendsen, M.; Gidskehaug, L.; Grove, H.; Nome, T.; Sodeland, M.; Sundsaasen, K.K.; Kent, M.P.; et al. Genome-wide association mapping for milk fat composition and fine mapping of a QTL for de novo synthesis of milk fatty acids on bovine chromosome 13. Genet. Sel. Evol. 2017, 49, 20. [Google Scholar] [CrossRef]
- Palombo, V.; Milanesi, M.; Sgorlon, S.; Capomaccio, S.; Mele, M.; Nicolazzi, E.; Ajmone-Marsan, P.; Pilla, F.; Stefanon, B.; D’Andrea, M. Genome-wide association study of milk fatty acid composition in Italian Simmental and Italian Holstein cows using single nucleotide polymorphism arrays. J. Dairy Sci. 2018, 101, 11004–11019. [Google Scholar] [CrossRef]
- Pegolo, S.; Mach, N.; Ramayo-Caldas, Y.; Schiavon, S.; Bittante, G.; Cecchinato, A. Integration of GWAS, pathway and network analyses reveals novel mechanistic insights into the synthesis of milk proteins in dairy cows. Sci. Rep. 2018, 8, 566. [Google Scholar] [CrossRef]
- Cruz, V.A.R.; Oliveira, H.R.; Brito, L.F.; Fleming, A.; Larmer, S.; Miglior, F.; Schenkel, F.S. Genome-Wide Association Study for Milk Fatty Acids in Holstein Cattle Accounting for the DGAT1 Gene Effect. Animals 2019, 9, 997. [Google Scholar] [CrossRef]
- Iung, L.H.S.; Petrini, J.; Ramírez-Díaz, J.; Salvian, M.; Rovadoscki, G.A.; Pilonetto, F.; Dauria, B.D.; Machado, P.F.; Coutinho, L.L.; Wiggans, G.R.; et al. Genome-wide association study for milk production traits in a Brazilian Holstein population. J. Dairy Sci. 2019, 102, 5305–5314. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Cai, W.; Liu, S.; Yin, H.; Shi, S.; Zhang, Q.; Zhang, S. Genome-Wide Association Study for Milk Protein Composition Traits in a Chinese Holstein Population Using a Single-Step Approach. Front. Genet. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.H.F.; Oliveira, H.R.; Silva, F.F.; Fleming, A.; Miglior, F.; Schenkel, F.S.; Brito, L.F. Genomic analyses for predicted milk fatty acid composition throughout lactation in North American Holstein cattle. J. Dairy Sci. 2020, 103, 6318–6331. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De novo assembly of the cattle reference genome with single-molecule sequencing. GigaScience 2020, 9, giaa021. [Google Scholar] [CrossRef]
- Duggal, P.; Gillanders, E.M.; Holmes, T.N.; Bailey-Wilson, J.E. Establishing an adjusted p-value threshold to control the family-wide type 1 error in genome wide association studies. BMC Genom. 2008, 9, 516. [Google Scholar] [CrossRef]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Harville, D.A. Maximum Likelihood Approaches to Variance Component Estimation and to Related Problems. J. Am. Stat. Assoc. 1977, 72, 320–338. [Google Scholar] [CrossRef]
- Rutten, M.J.; Bovenhuis, H.; van Arendonk, J.A. The effect of the number of observations used for Fourier transform infrared model calibration for bovine milk fat composition on the estimated genetic parameters of the predicted data. J. Dairy Sci. 2010, 93, 4872–4882. [Google Scholar] [CrossRef]
- Narayana, S.G.; Schenkel, F.S.; Fleming, A.; Koeck, A.; Malchiodi, F.; Jamrozik, J.; Johnston, J.; Sargolzaei, M.; Miglior, F. Genetic analysis of groups of mid-infrared predicted fatty acids in milk. J. Dairy Sci. 2017, 100, 4731–4744. [Google Scholar] [CrossRef]
- Fleming, A.; Schenkel, F.S.; Malchiodi, F.; Ali, R.A.; Mallard, B.; Sargolzaei, M.; Jamrozik, J.; Johnston, J.; Miglior, F. Genetic correlations of mid-infrared-predicted milk fatty acid groups with milk production traits. J. Dairy Sci. 2018, 101, 4295–4306. [Google Scholar] [CrossRef]
- Hein, L.; Sorensen, L.P.; Kargo, M.; Buitenhuis, A.J. Genetic analysis of predicted fatty acid profiles of milk from Danish Holstein and Danish Jersey cattle populations. J. Dairy Sci. 2018, 101, 2148–2157. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, T.M.; Olsen, H.G.; Tafintseva, V.; Svendsen, M.; Kohler, A.; Kent, M.P.; Lien, S. Unravelling genetic variation underlying de novo-synthesis of bovine milk fatty acids. Sci. Rep. 2018, 8, 2179. [Google Scholar] [CrossRef] [PubMed]
- Sabek, A.; Li, C.; Du, C.; Nan, L.; Ni, J.; Elgazzar, E.; Ma, Y.; Salem, A.Z.M.; Zhang, S. Effects of parity and days in milk on milk composition in correlation with β-hydroxybutyrate in tropic dairy cows. Trop. Anim. Health Prod. 2021, 53, 270. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Tang, Y.; Zhao, J.; Debrah, A.A.; Shen, Z.; Li, C.; Du, Z. Authentication of organically produced cow milk by fatty acid profile combined with chemometrics: A case study in China. J. Food Compos. Anal. 2023, 120, 105297. [Google Scholar] [CrossRef]
- Libnau, F.; Kvalheim, O.M.; Christy, A.A.; Toft, J. Spectra of water in the near- and mid-infrared region. Vib. Spectrosc. 1994, 7, 243–254. [Google Scholar] [CrossRef]
- Kaylegian, K.E.; Houghton, G.E.; Lynch, J.M.; Fleming, J.R.; Barbano, D.M. Calibration of Infrared Milk Analyzers Modified Milk. J. Dairy Sci. 2006, 89, 2817–2832. [Google Scholar] [CrossRef]
- Wang, Q.; Bovenhuis, H. Validation strategy can result in an overoptimistic view of the ability of milk infrared spectra to predict methane emission of dairy cattle. J. Dairy Sci. 2019, 102, 6288–6295. [Google Scholar] [CrossRef]
- Macedo Mota, L.F.; Bisutti, V.; Vanzin, A.; Pegolo, S.; Toscano, A.; Schiavon, S.; Tagliapietra, F.; Gallo, L.; Ajmone Marsan, P.; Cecchinato, A. Predicting milk protein fractions using infrared spectroscopy and a gradient boosting machine for breeding purposes in Holstein cattle. J. Dairy Sci. 2023, 106, 1853–1873. [Google Scholar] [CrossRef]
- Mishra, P.; Biancolillo, A.; Roger, J.M.; Marini, F.; Rutledge, D.N. New data preprocessing trends based on ensemble of multiple preprocessing techniques. TrAC Trends Anal. Chem. 2020, 132, 116045. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Yin, D.; Fu, Y.; Yuan, X.; Li, X.; Liu, X.; Zhao, S. HIBLUP: An integration of statistical models on the BLUP framework for efficient genetic evaluation using big genomic data. Nucleic Acids Res. 2023, 51, 3501–3512. [Google Scholar] [CrossRef]
- Aguilar, I.; Misztal, I.; Johnson, D.L.; Legarra, A.; Tsuruta, S.; Lawlor, T.J. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Christensen, O.F.; Lund, M.S. Genomic prediction when some animals are not genotyped. Genet. Sel. Evol. 2010, 42, 2. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-efficient, Visualization-enhanced, and Parallel-accelerated Tool for Genome-wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Coppa, M.; Ferlay, A.; Leroux, C.; Jestin, M.; Chilliard, Y.; Martin, B.; Andueza, D. Prediction of milk fatty acid composition by near infrared reflectance spectroscopy. Int. Dairy J. 2010, 20, 182–189. [Google Scholar] [CrossRef]
- Ho, P.N.; Marett, L.C.; Wales, W.J.; Axford, M.; Oakes, E.M.; Pryce, J.E. Predicting milk fatty acids and energy balance of dairy cows in Australia using milk mid-infrared spectroscopy. Anim. Prod. Sci. 2020, 60, 164–168. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, B.; Niu, H.; Zhang, W.; Xu, L.; Xu, L.; Chen, Y.; Zhang, L.; Gao, X.; Gao, H.; et al. Genome wide association study identifies SNPs associated with fatty acid composition in Chinese Wagyu cattle. J. Anim. Sci. Biotechnol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Afseth, N.K.; Martens, H.; Randby, A.; Gidskehaug, L.; Narum, B.; Jørgensen, K.; Lien, S.; Kohler, A. Predicting the fatty acid composition of milk: A comparison of two Fourier transform infrared sampling techniques. Appl. Spectrosc. 2010, 64, 700–707. [Google Scholar] [CrossRef]
- Eskildsen, C.E.; Rasmussen, M.A.; Engelsen, S.B.; Larsen, L.B.; Poulsen, N.A.; Skov, T. Quantification of individual fatty acids in bovine milk by infrared spectroscopy and chemometrics: Understanding predictions of highly collinear reference variables. J. Dairy Sci. 2014, 97, 7940–7951. [Google Scholar] [CrossRef]
- Gupta, M.K. Chapter 2—Basic Oil Chemistry. In Practical Guide to Vegetable Oil Processing, 2nd ed.; Gupta, M.K., Ed.; AOCS Press: Champaign, IL, USA, 2017; pp. 7–25. [Google Scholar]
- Zhao, X.; Song, Y.; Zhang, Y.; Cai, G.; Xue, G.; Liu, Y.; Chen, K.; Zhang, F.; Wang, K.; Zhang, M.; et al. Predictions of Milk Fatty Acid Contents by Mid-Infrared Spectroscopy in Chinese Holstein Cows. Molecules 2023, 28, 666. [Google Scholar] [CrossRef]
- Buitenhuis, B.; Janss, L.L.G.; Poulsen, N.A.; Larsen, L.B.; Larsen, M.K.; Sørensen, P. Genome-wide association and biological pathway analysis for milk-fat composition in Danish Holstein and Danish Jersey cattle. BMC Genom. 2014, 15, 1112. [Google Scholar] [CrossRef]
- Batrakou, D.G.; de Las Heras, J.I.; Czapiewski, R.; Mouras, R.; Schirmer, E.C. TMEM120A and B: Nuclear Envelope Transmembrane Proteins Important for Adipocyte Differentiation. PLoS ONE 2015, 10, e0127712. [Google Scholar] [CrossRef]
- Crouse, W.L.; Das, S.K.; Le, T.; Keele, G.; Holl, K.; Seshie, O.; Craddock, A.L.; Sharma, N.K.; Comeau, M.E.; Langefeld, C.D.; et al. Transcriptome-wide analyses of adipose tissue in outbred rats reveal genetic regulatory mechanisms relevant for human obesity. Physiol. Genom. 2022, 54, 206–219. [Google Scholar] [CrossRef]
- Bangar, Y.C.; Magotra, A.; Chauhan, A.; Yadav, A.S. Genetic polymorphisms of kappa casein gene and its association with milk and composition traits in cows: An updated meta-analysis. Meta Gene 2021, 30, 100948. [Google Scholar] [CrossRef]
- Malik, S.; Sidhu, N.S.; Kumar, S.; Kumar, A.; Rani, R. Kappa-casein alleles in Zebu and cross-bred (1/2 Friesian, 1/4 Jersey, 1/4 Hariana) cattle from India using polymerase chain reaction and sequence-specific oligonucleotide probes (PCR-SSOP). Genet. Anal. Biomol. Eng. 1997, 14, 61–63. [Google Scholar] [CrossRef]
- Hudson, N.J.; Lyons, R.E.; Reverter, A.; Greenwood, P.L.; Dalrymple, B.P. Inferring the in vivo cellular program of developing bovine skeletal muscle from expression data. Gene Expr. Patterns 2013, 13, 109–125. [Google Scholar] [CrossRef]
- Zhang, H.; Takeda, H.; Tsuji, T.; Kamiya, N.; Rajderkar, S.; Louie, K.; Collier, C.; Scott, G.; Ray, M.; Mochida, Y.; et al. Generation of Evc2/Limbin global and conditional KO mice and its roles during mineralized tissue formation. Genesis 2015, 53, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Z.Y.; Xue, G.; Qin, X.J.; Wu, J.F.; Zhang, G. ADORA1 is a diagnostic-related biomarker and correlated with immune infiltrates in papillary thyroid carcinoma. J. Cancer 2021, 12, 3997–4010. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Osorio, J.S.; Loor, J.J. Purinergic signaling gene network expression in bovine polymorphonuclear neutrophils during the peripartal period. J. Dairy Sci. 2013, 96, 7675–7683. [Google Scholar] [CrossRef] [PubMed]
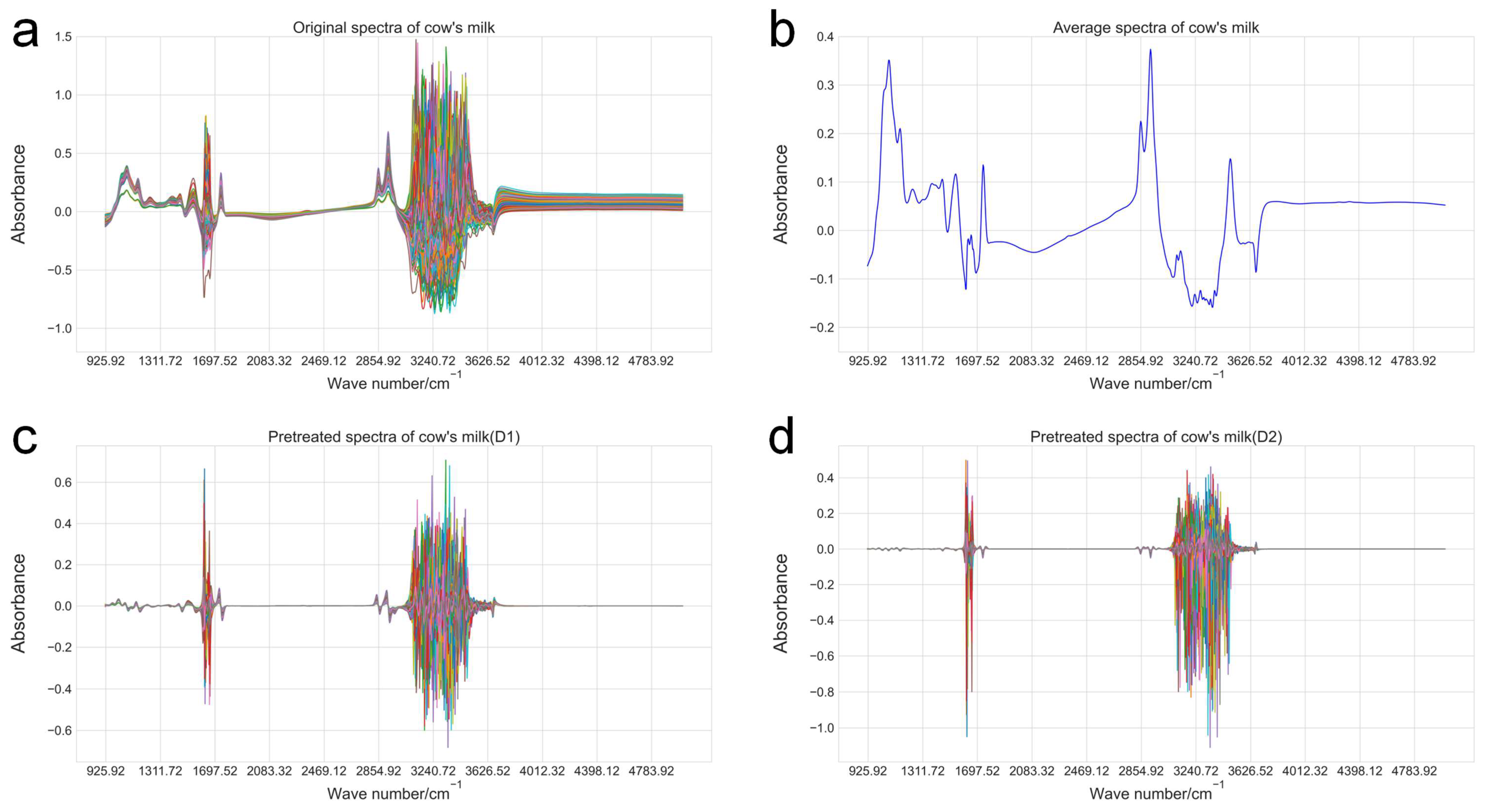
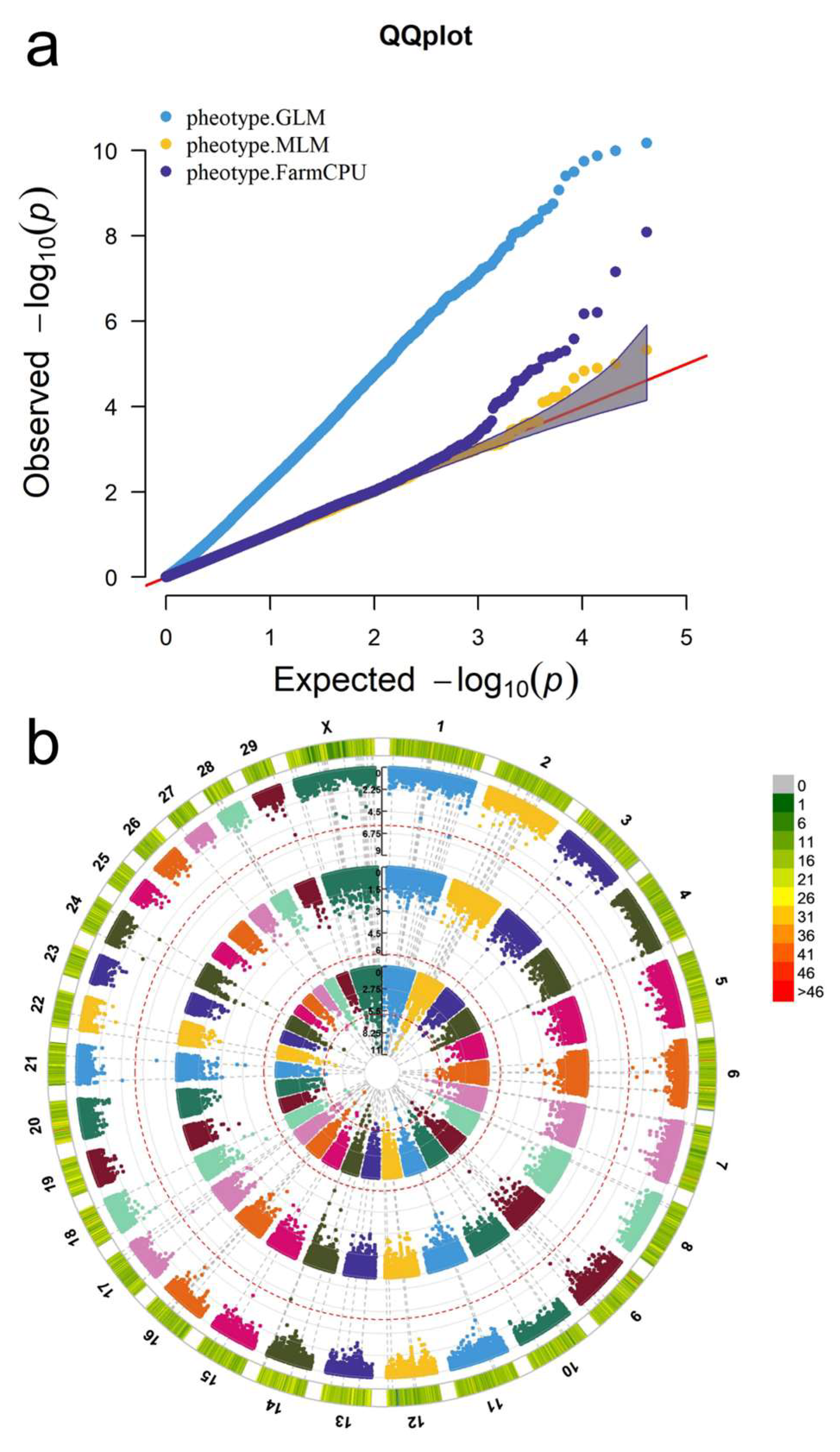
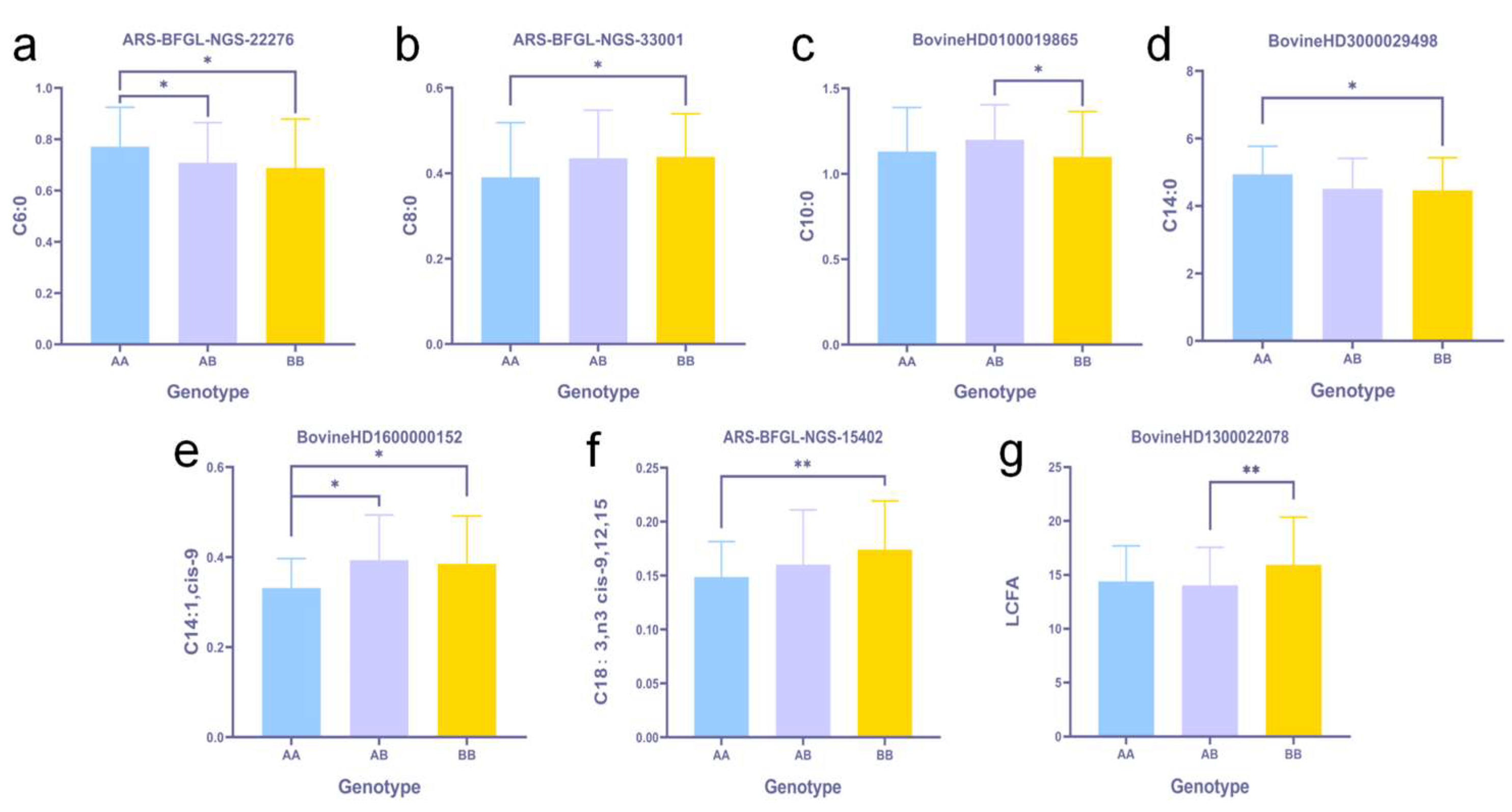
| Trait | * Fatty Acids Included |
|---|---|
| TFA | SFA, UFA/SCFA, MCFA, LCFA |
| SFA | C4:0, C6:0, C8:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C23:0, C24:0 |
| UFA | MUFA, PUFA |
| MUFA | C14:1, C15:1, C16:1, C17:1, C18:1, C20:1, C22:1, C24:1 |
| PUFA | C18:2, C18:3, C20:2, C20:3, C20:4, C20:5, C22:2, C22:6 |
| SCFA | C4:0, C6:0, C8:0, C10:0 |
| MCFA | C11:0, C12:0, C13:0, C14:0, C14:1, C15:0, C15:1,C16:0, C16:1 |
| LCFA | C17:0, C17:1, C18:0, C18:1, C18:2, C18:3, C20:0, C20:1, C20:2, C20:3, C20:4, C20:5, C21:0, C22:0, C22:1, C22:2, C22:6, C23:0, C24:0, C24:1 |
| Trait | 1 g/100 g of Total Fat | 1 Percentage of Total Fatty Acid, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 Mean | 2 SD | 2 Max | 2 Min | 2 CV, % | 2 Mean | 2 SD | 2 Max | 2 Min | 2 CV, % | |
| C4:0 | 1.18 | 1.06 | 7.59 | 0.09 | 90.55 | 3.36 | 3.09 | 32.44 | 1.14 | 92.01 |
| C6:0 | 0.59 | 0.32 | 1.58 | 0.08 | 54.22 | 1.66 | 0.31 | 2.32 | 0.60 | 18.84 |
| C8:0 | 0.38 | 0.21 | 1.06 | 0.05 | 54.38 | 1.06 | 0.21 | 1.54 | 0.34 | 19.76 |
| C10:0 | 1.00 | 0.57 | 3.26 | 0.09 | 57.47 | 2.80 | 0.73 | 5.02 | 0.81 | 26.00 |
| C12:0 | 1.13 | 0.65 | 3.72 | 0.15 | 56.97 | 3.21 | 0.80 | 5.88 | 1.07 | 24.80 |
| C14:0 | 3.77 | 1.95 | 9.49 | 0.52 | 51.56 | 10.72 | 1.69 | 15.22 | 4.99 | 15.78 |
| C14:1,cis-9 | 0.31 | 0.20 | 1.21 | 0.03 | 62.42 | 0.89 | 0.32 | 2.17 | 0.20 | 36.13 |
| C15:0 | 0.38 | 0.24 | 1.69 | 0.05 | 61.68 | 1.08 | 0.33 | 2.58 | 0.38 | 30.36 |
| C16:0 | 12.74 | 6.19 | 31.06 | 1.61 | 48.60 | 36.37 | 3.53 | 49.21 | 24.89 | 9.69 |
| C17:0 | 0.18 | 0.10 | 0.62 | 0.02 | 55.50 | 0.51 | 0.11 | 0.97 | 0.27 | 20.98 |
| C18:0 | 3.92 | 2.10 | 11.50 | 0.52 | 53.58 | 11.15 | 2.35 | 22.36 | 6.19 | 21.09 |
| C18:1,n9 cis-9 | 6.53 | 4.29 | 30.72 | 0.05 | 65.79 | 17.98 | 6.73 | 37.94 | 0.45 | 37.42 |
| C18:2,n6cis-9,12 | 1.00 | 0.55 | 2.84 | 0.12 | 55.02 | 2.79 | 0.55 | 4.37 | 1.42 | 19.56 |
| C18:3,n3cis-9,12,15 | 0.10 | 0.07 | 0.34 | 0.00 | 72.92 | 0.26 | 0.13 | 0.88 | 0.04 | 50.98 |
| 3 TFA | 35.22 | 17.14 | 80.97 | 4.52 | 48.67 | —— | —— | —— | —— | —— |
| 3 SFA | 25.39 | 12.33 | 59.80 | 3.46 | 48.55 | 72.30 | 4.50 | 90.89 | 51.57 | 6.23 |
| 3 MUFA | 8.52 | 4.65 | 34.66 | 0.48 | 54.65 | 24.04 | 4.44 | 43.55 | 4.89 | 18.46 |
| 3 PUFA | 1.33 | 0.72 | 3.69 | 0.15 | 54.53 | 3.71 | 0.68 | 6.71 | 2.14 | 18.42 |
| 3 UFA | 9.85 | 5.28 | 37.04 | 0.89 | 53.66 | 27.77 | 4.52 | 48.44 | 9.11 | 16.26 |
| 3 SCFA | 3.14 | 1.82 | 10.14 | 0.35 | 58.07 | 8.88 | 3.35 | 37.73 | 4.72 | 37.76 |
| 3 MCFA | 18.94 | 9.32 | 47.66 | 2.52 | 49.20 | 53.97 | 5.53 | 69.22 | 36.73 | 10.25 |
| 3 LCFA | 13.14 | 6.93 | 44.67 | 1.62 | 52.74 | 37.16 | 5.79 | 57.04 | 22.94 | 15.58 |
| Trait | 1 Preprocessing Methods | Number of Characteristic Wavelengths | 2 Rcv2 | 2 RMSEcv | 2 Rt2 | 2 RMSEt | 2 RPDcv |
|---|---|---|---|---|---|---|---|
| C4:0 | D2 + D2 | 585 | 0.76 | 0.48 | 0.65 | 0.74 | 2.04 |
| C6:0 | D1 + D1 | 657 | 0.64 | 0.19 | 0.57 | 0.22 | 1.67 |
| C8:0 | D2 + D2 | 532 | 0.76 | 0.10 | 0.72 | 0.12 | 2.03 |
| C10:0 | D1 + D1 | 647 | 0.68 | 0.31 | 0.65 | 0.37 | 1.77 |
| C12:0 | D1 + D1 | 564 | 0.67 | 0.36 | 0.62 | 0.44 | 1.74 |
| C14:0 | D1 + D1 | 603 | 0.64 | 1.10 | 0.58 | 1.44 | 1.68 |
| C14:1,cis-9 | D1 + D1 | 576 | 0.67 | 0.11 | 0.62 | 0.14 | 1.73 |
| C15:0 | D1 + D2 | 688 | 0.71 | 0.11 | 0.60 | 0.19 | 1.85 |
| C16:0 | D1 + D1 | 709 | 0.64 | 3.71 | 0.52 | 4.25 | 1.67 |
| C17:0 | D2 + D2 | 456 | 0.64 | 0.06 | 0.49 | 0.07 | 1.67 |
| C18:0 | D1 + D1 | 510 | 0.59 | 1.30 | 0.60 | 1.45 | 1.57 |
| C18:1,n9 cis-9 | D1 + D2 | 561 | 0.67 | 2.32 | 0.58 | 3.21 | 1.74 |
| C18:2,n6 cis-9,12 | D1 + D1 | 612 | 0.65 | 0.32 | 0.50 | 0.39 | 1.69 |
| C18:3,n3 cis-9,12,15 | D1 + D2 | 693 | 0.69 | 0.04 | 0.44 | 0.06 | 1.81 |
| 3 TFA | D2 + D2 | 511 | 0.70 | 9.24 | 0.66 | 10.39 | 1.82 |
| 3 SFA | D2 + D2 | 511 | 0.71 | 6.50 | 0.67 | 7.52 | 1.86 |
| 3 UFA | D2 + D2 | 469 | 0.65 | 3.13 | 0.65 | 3.07 | 1.69 |
| 3 MUFA | D2 + D2 | 459 | 0.65 | 2.78 | 0.65 | 2.68 | 1.68 |
| 3 PUFA | D2 + D2 | 491 | 0.68 | 0.40 | 0.62 | 0.47 | 1.77 |
| 3 SCFA | D2 + D2 | 561 | 0.72 | 0.97 | 0.74 | 0.90 | 1.90 |
| 3 MCFA | D2 + D2 | 472 | 0.66 | 5.18 | 0.67 | 5.88 | 1.73 |
| 3 LCFA | D2 + D2 | 568 | 0.62 | 4.12 | 0.62 | 4.67 | 1.62 |
| Fatty Acid | Variance Component Estimates | Estimated Breeding Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 σu2 | 1 σc2 | 1 σe2 | 1 σT2 | 1 h2 | 1 t | 2 Mean | 2 Min | 2 Max | 2 Acc | |
| C4:0 | 0.14 | 0.20 | 0.17 | 0.51 | 0.28 | 0.67 | −0.03 | −0.90 | 0.89 | 0.73 |
| C6:0 | 0.02 | 0.02 | 0.03 | 0.07 | 0.31 | 0.59 | −0.01 | −0.31 | 0.59 | 0.71 |
| C8:0 | 0.01 | 0.01 | 0.02 | 0.03 | 0.23 | 0.49 | −0.01 | −0.18 | 0.23 | 0.67 |
| C10:0 | 0.08 | 0.06 | 0.10 | 0.24 | 0.33 | 0.59 | −0.02 | −0.60 | 1.10 | 0.72 |
| C12:0 | 0.09 | 0.07 | 0.11 | 0.27 | 0.34 | 0.59 | −0.03 | −0.65 | 0.76 | 0.73 |
| C14:0 | 0.54 | 0.69 | 0.66 | 1.89 | 0.29 | 0.65 | −0.05 | −1.95 | 2.25 | 0.76 |
| C14:1,cis-9 | 0.01 | 0.01 | 0.02 | 0.04 | 0.25 | 0.48 | 0.00 | −0.20 | 0.18 | 0.54 |
| C15:0 | 0.01 | 0.01 | 0.02 | 0.04 | 0.29 | 0.54 | 0.00 | −0.23 | 0.27 | 0.70 |
| C16:0 | 4.01 | 6.26 | 4.80 | 15.06 | 0.27 | 0.68 | −0.24 | −5.73 | 9.13 | 0.79 |
| C17:0 | 0.00 | 0.00 | 0.00 | 0.01 | 0.20 | 0.33 | 0.00 | −0.10 | 0.08 | 0.63 |
| C18:0 | 0.43 | 0.70 | 0.52 | 1.65 | 0.26 | 0.69 | −0.04 | −2.00 | 3.15 | 0.78 |
| C18:1,n9 cis-9 | 2.08 | 2.87 | 2.48 | 7.42 | 0.28 | 0.67 | −0.08 | −3.33 | 3.65 | 0.69 |
| C18:2,n6 cis-9,12 | 0.07 | 0.08 | 0.09 | 0.24 | 0.31 | 0.62 | −0.02 | −0.63 | 0.67 | 0.70 |
| C18:3,n3 cis-9,12,15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 0.50 | 0.00 | −0.05 | 0.06 | 0.57 |
| 3 TFA | 29.02 | 35.99 | 37.39 | 102.39 | 0.28 | 0.64 | −0.26 | −16.06 | 26.69 | 0.82 |
| 3 SFA | 18.91 | 18.77 | 24.27 | 61.94 | 0.31 | 0.61 | −0.21 | −10.20 | 23.65 | 0.82 |
| 3 UFA | 4.00 | 3.97 | 5.54 | 13.51 | 0.30 | 0.59 | −0.11 | −5.60 | 7.00 | 0.78 |
| 3 MUFA | 3.30 | 3.27 | 4.61 | 11.17 | 0.30 | 0.59 | −0.10 | −5.35 | 6.79 | 0.78 |
| 3 PUFA | 0.07 | 0.07 | 0.09 | 0.22 | 0.30 | 0.60 | −0.01 | −0.64 | 0.79 | 0.74 |
| 3 SCFA | 0.47 | 0.65 | 0.55 | 1.68 | 0.28 | 0.67 | −0.06 | −1.54 | 2.45 | 0.76 |
| 3 MCFA | 8.78 | 13.79 | 10.14 | 32.71 | 0.27 | 0.69 | −0.20 | −8.18 | 9.77 | 0.74 |
| 3 LCFA | 5.81 | 8.78 | 6.73 | 21.33 | 0.27 | 0.68 | −0.19 | −7.47 | 9.66 | 0.74 |
| Fat | Protein | Lactose | Total Solids | Urea | C4:0 | C6:0 | C8:0 | C10:0 | C12:0 | C14:0 | C14:1, cis-9 | C15:0 | C16:0 | C17:0 | C18:0 | C18:1,n9 cis-9 | C18:2,n6 cis-9,12 | C18:3,n3 cis-9,12,15 | 1 TFA | 1 SFA | 1 UFA | 1 MUFA | 1 PUFA | 1 SCFA | 1 MCFA | 1 LCFA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat | 0.42 | −0.06 | 0.93 | 0.08 | 0.09 | 0.22 | 0.18 | 0.20 | 0.20 | 0.14 | 0.17 | 0.15 | 0.27 | 0.27 | 0.23 | 0.18 | 0.15 | 0.16 | 0.19 | 0.18 | 0.17 | 0.18 | 0.09 | 0.21 | 0.20 | 0.22 | |
| Protein | 0.66 | −0.14 | 0.66 | 0.14 | −0.07 | 0.12 | 0.09 | 0.21 | 0.28 | 0.16 | 0.22 | 0.23 | 0.19 | 0.11 | 0.00 | −0.16 | 0.00 | 0.01 | 0.03 | 0.08 | −0.04 | −0.03 | −0.10 | 0.12 | 0.23 | −0.07 | |
| Lactose | 0.13 | −0.11 | 0.08 | 0.05 | 0.02 | −0.23 | −0.10 | −0.17 | −0.18 | −0.04 | −0.17 | −0.03 | −0.14 | −0.03 | −0.08 | −0.14 | −0.28 | −0.22 | −0.09 | −0.08 | −0.10 | −0.10 | −0.14 | −0.11 | −0.17 | −0.06 | |
| Total solids | 0.98 | 0.74 | 0.18 | 0.14 | 0.10 | 0.21 | 0.18 | 0.25 | 0.26 | 0.19 | 0.23 | 0.21 | 0.28 | 0.24 | 0.20 | 0.11 | 0.09 | 0.13 | 0.16 | 0.16 | 0.12 | 0.13 | 0.02 | 0.23 | 0.22 | 0.16 | |
| Urea | 0.20 | 0.23 | 0.10 | 0.26 | 0.20 | −0.15 | 0.03 | 0.02 | 0.02 | −0.02 | −0.02 | −0.16 | −0.12 | −0.17 | −0.02 | −0.10 | −0.17 | 0.01 | −0.14 | −0.09 | −0.14 | −0.16 | −0.09 | 0.07 | −0.05 | −0.21 | |
| C4:0 | 0.21 | 0.07 | −0.02 | 0.24 | 0.37 | 0.09 | 0.40 | 0.24 | 0.14 | 0.14 | 0.01 | 0.01 | 0.10 | 0.03 | 0.33 | 0.18 | 0.06 | 0.25 | 0.22 | 0.27 | 0.13 | 0.12 | 0.17 | 0.55 | 0.24 | 0.11 | |
| C6:0 | 0.26 | 0.12 | −0.10 | 0.28 | −0.24 | 0.07 | 0.38 | 0.75 | 0.76 | 0.71 | 0.59 | 0.64 | 0.67 | 0.21 | 0.50 | 0.51 | 0.75 | 0.57 | 0.28 | 0.29 | 0.17 | 0.19 | 0.21 | 0.27 | 0.41 | 0.41 | |
| C8:0 | 0.32 | 0.23 | −0.16 | 0.35 | 0.06 | 0.68 | 0.43 | 0.46 | 0.42 | 0.38 | 0.24 | 0.32 | 0.35 | 0.21 | 0.46 | 0.28 | 0.30 | 0.40 | 0.46 | 0.50 | 0.29 | 0.28 | 0.37 | 0.59 | 0.45 | 0.25 | |
| C10:0 | 0.30 | 0.24 | −0.09 | 0.34 | −0.05 | 0.29 | 0.87 | 0.60 | 0.87 | 0.72 | 0.63 | 0.54 | 0.72 | 0.10 | 0.50 | 0.40 | 0.59 | 0.65 | 0.33 | 0.39 | 0.17 | 0.17 | 0.31 | 0.46 | 0.54 | 0.30 | |
| C12:0 | 0.26 | 0.28 | −0.06 | 0.32 | −0.05 | 0.16 | 0.86 | 0.51 | 0.94 | 0.75 | 0.64 | 0.54 | 0.70 | 0.12 | 0.43 | 0.31 | 0.58 | 0.55 | 0.26 | 0.32 | 0.11 | 0.10 | 0.24 | 0.32 | 0.50 | 0.22 | |
| C14:0 | 0.28 | 0.24 | −0.02 | 0.34 | −0.10 | 0.27 | 0.88 | 0.61 | 0.93 | 0.92 | 0.61 | 0.70 | 0.61 | 0.11 | 0.48 | 0.36 | 0.55 | 0.54 | 0.31 | 0.35 | 0.16 | 0.16 | 0.19 | 0.32 | 0.51 | 0.32 | |
| C14:1,cis−9 | 0.25 | 0.20 | −0.10 | 0.28 | −0.06 | −0.01 | 0.80 | 0.35 | 0.82 | 0.84 | 0.81 | 0.60 | 0.65 | 0.15 | 0.31 | 0.42 | 0.55 | 0.43 | 0.27 | 0.27 | 0.20 | 0.21 | 0.16 | 0.22 | 0.39 | 0.31 | |
| C15:0 | 0.22 | 0.28 | 0.01 | 0.26 | −0.24 | 0.10 | 0.65 | 0.42 | 0.64 | 0.66 | 0.80 | 0.65 | 0.53 | 0.27 | 0.31 | 0.40 | 0.56 | 0.43 | 0.37 | 0.37 | 0.23 | 0.24 | 0.21 | 0.25 | 0.56 | 0.43 | |
| C16:0 | 0.40 | 0.21 | −0.04 | 0.42 | −0.27 | 0.24 | 0.89 | 0.61 | 0.88 | 0.84 | 0.94 | 0.79 | 0.79 | 0.28 | 0.47 | 0.42 | 0.60 | 0.52 | 0.37 | 0.38 | 0.25 | 0.25 | 0.29 | 0.29 | 0.45 | 0.41 | |
| C17:0 | 0.32 | 0.15 | −0.03 | 0.31 | −0.25 | 0.16 | 0.30 | 0.34 | 0.26 | 0.25 | 0.33 | 0.32 | 0.38 | 0.49 | 0.27 | 0.19 | 0.22 | 0.09 | 0.68 | 0.61 | 0.61 | 0.60 | 0.51 | 0.21 | 0.23 | 0.37 | |
| C18:0 | 0.38 | 0.09 | 0.01 | 0.39 | 0.00 | 0.50 | 0.74 | 0.68 | 0.74 | 0.68 | 0.77 | 0.66 | 0.51 | 0.82 | 0.53 | 0.41 | 0.45 | 0.61 | 0.44 | 0.44 | 0.41 | 0.41 | 0.35 | 0.44 | 0.35 | 0.35 | |
| C18:1,n9 cis-9 | 0.28 | −0.11 | 0.03 | 0.26 | −0.13 | 0.15 | 0.58 | 0.28 | 0.43 | 0.35 | 0.55 | 0.51 | 0.58 | 0.69 | 0.29 | 0.56 | 0.66 | 0.54 | 0.27 | 0.19 | 0.34 | 0.34 | 0.27 | 0.20 | 0.31 | 0.60 | |
| C18:2,n6 cis-9,12 | 0.17 | −0.04 | −0.11 | 0.17 | −0.25 | 0.04 | 0.86 | 0.35 | 0.70 | 0.68 | 0.78 | 0.79 | 0.68 | 0.83 | 0.24 | 0.68 | 0.80 | 0.61 | 0.25 | 0.20 | 0.21 | 0.21 | 0.30 | 0.20 | 0.37 | 0.51 | |
| C18:3,n3 cis-9,12,15 | 0.27 | 0.03 | −0.16 | 0.29 | −0.02 | 0.40 | 0.77 | 0.69 | 0.84 | 0.72 | 0.85 | 0.75 | 0.58 | 0.87 | 0.34 | 0.82 | 0.70 | 0.82 | 0.35 | 0.38 | 0.26 | 0.25 | 0.41 | 0.42 | 0.48 | 0.41 | |
| 1 TFA | 0.26 | 0.11 | −0.09 | 0.26 | −0.15 | 0.41 | 0.38 | 0.68 | 0.47 | 0.39 | 0.52 | 0.33 | 0.47 | 0.63 | 0.85 | 0.66 | 0.35 | 0.30 | 0.57 | 0.97 | 0.85 | 0.84 | 0.80 | 0.49 | 0.48 | 0.38 | |
| 1 SFA | 0.25 | 0.13 | −0.09 | 0.26 | −0.13 | 0.42 | 0.36 | 0.68 | 0.47 | 0.39 | 0.51 | 0.31 | 0.45 | 0.60 | 0.84 | 0.65 | 0.29 | 0.26 | 0.55 | 1.00 | 0.75 | 0.73 | 0.77 | 0.56 | 0.52 | 0.29 | |
| 1 UFA | 0.18 | −0.05 | −0.06 | 0.15 | −0.15 | 0.28 | 0.22 | 0.49 | 0.24 | 0.16 | 0.29 | 0.20 | 0.28 | 0.44 | 0.87 | 0.56 | 0.34 | 0.21 | 0.42 | 0.94 | 0.93 | 1.00 | 0.75 | 0.29 | 0.33 | 0.41 | |
| 1 MUFA | 0.17 | −0.07 | −0.06 | 0.14 | −0.16 | 0.26 | 0.21 | 0.47 | 0.22 | 0.14 | 0.27 | 0.20 | 0.27 | 0.43 | 0.87 | 0.56 | 0.35 | 0.21 | 0.41 | 0.93 | 0.92 | 1.00 | 0.71 | 0.29 | 0.32 | 0.42 | |
| 1 PUFA | 0.11 | −0.05 | −0.14 | 0.11 | −0.11 | 0.31 | 0.24 | 0.54 | 0.36 | 0.27 | 0.37 | 0.21 | 0.32 | 0.47 | 0.77 | 0.49 | 0.28 | 0.19 | 0.49 | 0.92 | 0.92 | 0.91 | 0.89 | 0.39 | 0.41 | 0.38 | |
| 1 SCFA | 0.33 | 0.26 | −0.09 | 0.37 | 0.11 | 0.81 | 0.31 | 0.85 | 0.52 | 0.40 | 0.55 | 0.19 | 0.43 | 0.55 | 0.48 | 0.63 | 0.23 | 0.20 | 0.59 | 0.75 | 0.76 | 0.55 | 0.52 | 0.62 | 0.47 | 0.26 | |
| 1 MCFA | 0.35 | 0.25 | −0.07 | 0.38 | −0.15 | 0.47 | 0.58 | 0.74 | 0.68 | 0.59 | 0.78 | 0.46 | 0.77 | 0.82 | 0.56 | 0.64 | 0.58 | 0.55 | 0.76 | 0.79 | 0.78 | 0.59 | 0.56 | 0.67 | 0.82 | 0.44 | |
| 1 LCFA | 0.31 | 0.01 | −0.01 | 0.29 | −0.30 | 0.19 | 0.56 | 0.42 | 0.46 | 0.37 | 0.61 | 0.44 | 0.71 | 0.76 | 0.44 | 0.53 | 0.92 | 0.73 | 0.70 | 0.55 | 0.49 | 0.47 | 0.47 | 0.45 | 0.43 | 0.80 |
| Name | SNP | Chrom | Position | Genetic Variation | Effect | Raw p-Value | p-Value Bonferroni | Gene | Functional Region |
|---|---|---|---|---|---|---|---|---|---|
| C4:0 | BTB-01865288 | 6 | g.39109137 | A > C | −0.091298053 | 0.873 | LCORL (dist = 1,552,031), SLIT2 (dist = 670,758) | Intergenic | |
| C4:0 | BovineHD2200013291 | 22 | g.45609641 | T > C | 0.109639913 | 0.114 | WNT5A (dist = 51,524), CACNA2D3 (dist = 314,894) | Intergenic | |
| C6:0 | BovineHD0100039519 | 1 | g.156602608 | T > G | 0.034016364 | 0.141 | KCNH8 | Intronic | |
| C6:0 | BovineHD1400023113 | 14 | g.79488283 | A > G | 0.04046719 | 0.439 | ENSBTAG00000046821 (dist = 838,481), \SNX16 (dist = 1,274,216) | Intergenic | |
| C6:0 | ARS-BFGL-NGS-22276 | 17 | g.53567893 | A > G | 0.03801704 | TMEM120B | Upstream | ||
| C6:0 | BovineHD2100014553 | 21 | g.50065194 | A > G | 0.056103731 | 0.607 | FBXO33 (dist = 755,051), LRFN5 (dist = 1,263,846) | Intergenic | |
| C8:0 | BovineHD0100039519 | 1 | g.156602608 | T > G | 0.018626629 | 0.758 | KCNH8 | Intronic | |
| C8:0 | ARS-BFGL-NGS-33001 | 6 | g.103480813 | A > G | −0.017966404 | EVC | Exonic | ||
| C8:0 | BovineHD0800031399 | 8 | g.103616676 | T > G | 0.021357656 | 0.892 | AKNA (dist = 11,743), WHRN (dist = 4323) | Intergenic | |
| C8:0 | BovineHD1200017499 | 12 | g.63379103 | A > C | 0.018193801 | 0.104 | ENSBTAG00000051320 (dist = 61,739), NONE (dist = NONE) | Intergenic | |
| C8:0 | BovineHD2900002136 | 29 | g.7493345 | A > C | −0.0197566 | 0.146 | CTSC (dist = 76,111), RAB38 (dist = 7945) | Intergenic | |
| C10:0 | BovineHD0100019865 | 1 | g.69045145 | T > C | −0.053724787 | ENSBTAG00000053238 | Intronic | ||
| C10:0 | Hapmap48014-BTA-59805 | 5 | g.15293572 | T > C | 0.048419799 | 0.413 | ALX1 (dist = 354,357), RASSF9 (dist = 174,821) | Intergenic | |
| C10:0 | BovineHD2900009379 | 29 | g.30870766 | A > G | −0.053948553 | 0.793 | ENSBTAG00000052770 (dist = 293,780), ETS1 (dist = 942,420) | Intergenic | |
| C10:0 | BovineHD3000029498 | X | g.102101013 | A > G | −0.05687289 | 0.971 | CASK (dist = 28,036), NYX (dist = 6763) | Intergenic | |
| C12:0 | BovineHD0100019865 | 1 | g.69045145 | T > C | −0.067241259 | ENSBTAG00000053238 | Intronic | ||
| C14:0 | BovineHD1500018842 | 15 | g.64979528 | T > C | 0.202541955 | 0.997 | CAT | Intronic | |
| C14:0 | BovineHD3000029498 | X | g.102101013 | A > G | −0.199760831 | CASK (dist = 28,036), NYX (dist = 6763) | Intergenic | ||
| C14:1,cis-9 | BovineHD1300014447 | 13 | g.49614540 | T > C | 0.023699371 | 0.351 | BMP2 (dist = 428,961), ENSBTAG00000007199 (dist = 932,496) | Intergenic | |
| C14:1,cis-9 | ARS-BFGL-NGS-75890 | 14 | g.65908412 | A > G | 0.558449636 | — | —— | —— | |
| C14:1,cis-9 | BovineHD1600000152 | 16 | g.896630 | T > C | −0.019590243 | ENSBTAG00000050274 (dist = 75,663), ADORA1 (dist = 18,476) | Intergenic | ||
| C14:1,cis-9 | Hapmap27983-BTA-163473 | 16 | g.17975275 | T > C | 0.194892692 | —— | —— | —— | |
| C14:1,cis-9 | BovineHD3000036571 | X | g.121649547 | T > C | 0.012453536 | 0.781 | CNKSR2 | Intronic | |
| C15:0 | BovineHD0400022351 | 4 | g.80194206 | A > C | 0.04267447 | 0.801 | —— | —— | |
| C15:0 | BovineHD2200010872 | 22 | g.38049578 | A > G | 0.025775523 | 0.392 | SYNPR | Intronic | |
| C15:0 | BovineHD3000019134 | X | g.60227886 | T > C | 0.024999374 | 0.124 | TRPC5 | Intronic | |
| C16:0 | BovineHD0400022351 | 4 | g.80194206 | A > C | 0.04267447 | 0.650 | —— | —— | |
| C16:0 | BovineHD2200010872 | 22 | g.38049578 | A > G | 0.025775523 | 0.771 | SYNPR | Intronic | |
| C16:0 | BovineHD3000019134 | X | g.60227886 | T > C | 0.024999374 | 0.984 | TRPC5 | Intronic | |
| C18:0 | BovineHD2200008234 | 22 | g.28238616 | A > G | 0.268793511 | 0.247 | —— | —— | |
| C18:0 | BovineHD3000036571 | X | g.121649547 | T > C | 0.217017144 | 0.397 | CNKSR2 | intronic | |
| C18:1,n9 cis-9 | ARS-BFGL-NGS-100563 | 5 | g.98601044 | T > G | 0.663309395 | 0.761 | ENSBTAG00000001336 (dist = 4735), ENSBTAG00000054460 (dist = 18,399) | Intergenic | |
| C18:1,n9 cis-9 | ARS-USDA-AGIL-chr26-44746016-000548 | 26 | g.44408705 | T > G | 0.552863575 | 0.902 | —— | —— | |
| C18:1,n9 cis-9 | BTB-01892890 | 29 | g.6982262 | T > C | 0.332464096 | 0.374 | TYR (dist = 546,774), GRM5 (dist = 25,615) | Intergenic | |
| C18:2,n6 cis-9,12 | BTB-00387033 | 9 | g.28842247 | T > C | 0.071584274 | 0.454 | HSF2 (dist = 39,233), ENSBTAG00000053171 (dist = 266,891) | Intergenic | |
| C18:2,n6 cis-9,12 | BovineHD1200006970 | 12 | g.23185693 | T > C | −0.102781399 | 0.839 | —— | —— | |
| C18:2,n6 cis-9,12 | BovineHD3000011570 | X | g.35028719 | A > G | 0.079096988 | 0.230 | ENSBTAG00000048998 (dist = 65,578), GABRQ (dist = 17,494) | Intergenic | |
| C18:3,n3 cis-9,12,15 | ARS-BFGL-NGS-34990 | 8 | g.11469311 | T > C | 0.006830786 | 0.754 | TMEM215 (dist = 133,759), NDUFB6 (dist = 35,314) | Intergenic | |
| C18:3,n3 cis-9,12,15 | AX-27555724 | 8 | g.83221868 | T > G | −0.006388075 | 0.714 | CDC14B | Exonic | |
| C18:3,n3 cis-9,12,15 | ARS-BFGL-NGS-15402 | 17 | g.11368599 | T > C | 0.007664341 | TTC29 | Intronic | ||
| 1 UFA | ARS-BFGL-NGS-27018 | X | g.29070096 | T > C | 0.671700039 | 0.293 | —— | —— | |
| 1 MUFA | ARS-BFGL-NGS-27018 | X | g.29070096 | T > C | 0.671700039 | 0.330 | —— | —— | |
| 1 SCFA | BovineHD0300028970 | 3 | g.100601230 | A > G | −0.185365212 | 0.465 | TESK2 | Intronic | |
| SCFA | BovineHD0700028128 | 7 | g.94179851 | A > G | −0.27438426 | 0.530 | MCTP1 | Intronic | |
| 1 LCFA | BovineHD1300022078 | 13 | g.75585136 | A > G | 0.580890257 | EYA2 | Intronic | ||
| LCFA | ARS-USDA-AGIL-chr24-46907845-000534 | 24 | g.46441847 | A > C | −0.961849131 | 0.267 | ST8SIA5 | Intronic | |
| LCFA | ARS-BFGL-NGS-93595 | 24 | g.52964581 | A > G | 2.190506381 | 0.197 | —— | —— |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Fan, Y.; Wang, D.; Chu, C.; Shen, X.; Wang, H.; Luo, X.; Nan, L.; Ren, X.; Chen, S.; et al. The Genetic Characteristics of FT-MIRS-Predicted Milk Fatty Acids in Chinese Holstein Cows. Animals 2024, 14, 2901. https://doi.org/10.3390/ani14192901
Li C, Fan Y, Wang D, Chu C, Shen X, Wang H, Luo X, Nan L, Ren X, Chen S, et al. The Genetic Characteristics of FT-MIRS-Predicted Milk Fatty Acids in Chinese Holstein Cows. Animals. 2024; 14(19):2901. https://doi.org/10.3390/ani14192901
Chicago/Turabian StyleLi, Chunfang, Yikai Fan, Dongwei Wang, Chu Chu, Xiong Shen, Haitong Wang, Xuelu Luo, Liangkang Nan, Xiaoli Ren, Shaohu Chen, and et al. 2024. "The Genetic Characteristics of FT-MIRS-Predicted Milk Fatty Acids in Chinese Holstein Cows" Animals 14, no. 19: 2901. https://doi.org/10.3390/ani14192901





