Renal Sugar Metabolites and mRNA Expression of Glucose Transporters in Meat-Type Chickens with Differing Residual Water Intake
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds
2.3. Sampling
2.4. Sugar Metabolites
2.5. RNA Extraction, cDNA Synthesis, and RT-qPCR
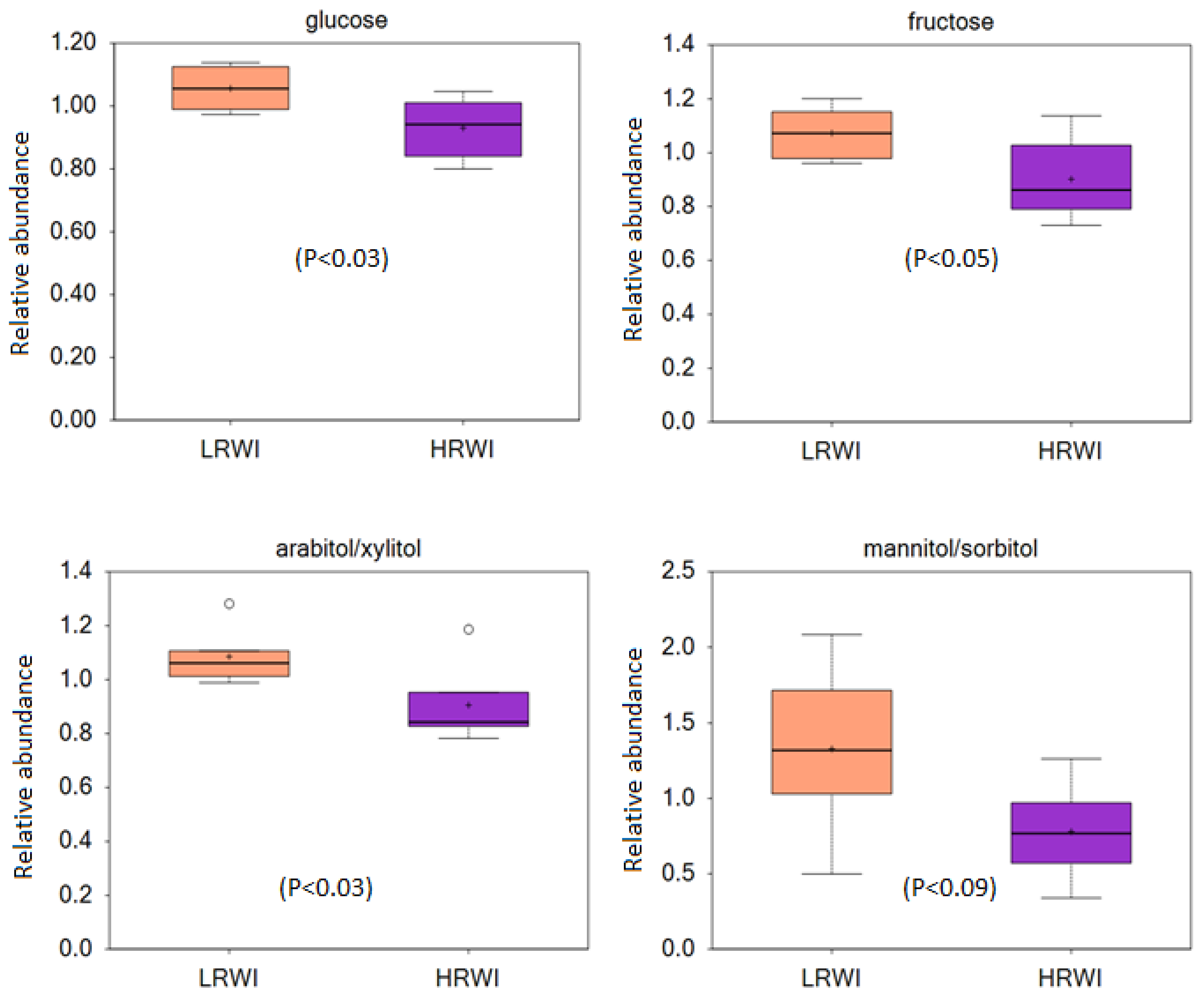
3. Results
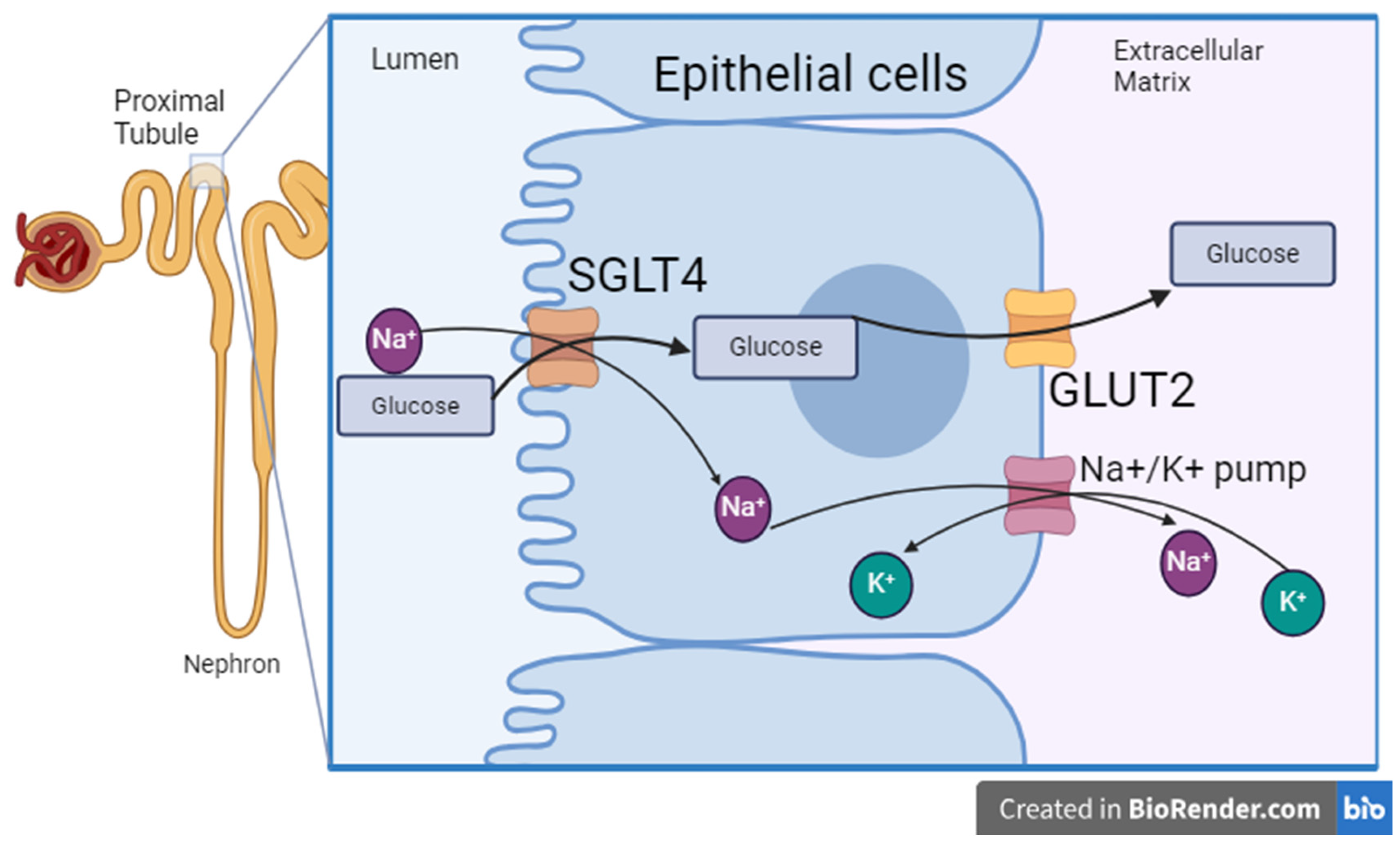
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koncagül, E.; Connor, R. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; Facts, Figures and Action Examples. 2023. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000384655 (accessed on 12 March 2024).
- Bank, W. High and Dry: Climate Change, Water, and the Economy; The World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Dohlman, E.; Hansen, J.; Boussios, D. USDA Agricultural Projections to 2031. 2022. Available online: https://www.usda.gov/sites/default/files/documents/USDA-Agricultural-Projections-to-2031.pdf (accessed on 12 March 2024).
- Aggrey, S.E.; Ghareeb, A.F.A.; Milfort, M.C.; Ariyo, O.W.; Aryal, B.; Hartono, E.; Kwakye, J.; Sovi, S.; Hipple, S.A.; Stevenson, C.; et al. Quantitative and molecular aspects of water intake in meat-type chickens. Poult. Sci. 2023, 102, 102973. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Kumar, K.; Kumar, P. Anatomical and Physiological Similarities of Kidney in Different Experimental Animals Used for Basic Studies. J. Clin. Exp. Nephrol. 2018, 3, 9. [Google Scholar] [CrossRef]
- Braun, E.J. Comparative renal function in reptiles, birds, and mammals. Semin. Avian Exot. Pet Med. 1998, 7, 62–71. [Google Scholar] [CrossRef]
- Dantzler, W.H.; Braun, E.J. Comparative nephron function in reptiles, birds, and mammals. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1980, 239, R197–R213. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Middleton, J.L.; Ernest, B.; Saxton, A.M.; Lamont, S.J.; Campagna, S.R.; Voy, B.H. Molecular and metabolic profiles suggest that increased lipid catabolism in adipose tissue contributes to leanness in domestic chickens. Physiol. Genom. /Physiol. Genom. 2014, 46, 315–327. [Google Scholar] [CrossRef]
- Sweaza, K.L.; Tsosie, K.S.; Beckman, E.J.; Benham, P.M.; Witt, C.C. Seasonal and elevational variation in glucose and glycogen-in two songbird species. Comp. Biochem. Physiol. 2020, 245A, 110703. [Google Scholar] [CrossRef]
- Vágási, C.I.; Vincze, O.; Adámková, M.; Kauzálová, T.; Lendvai, Á.Z.; Pătraş, L.I.; Pénzes, J.; Pap, P.L.; Albrecht, T.; Tomášek, O. Songbirds avoid the oxidative stress costs of high blood glucose levels: A comparative study. J. Exp. Biol. 2024, 227, jeb246848. [Google Scholar] [CrossRef]
- Sun, B.; Chen, H.; Xue, J.; Li, P.; Fu, X. The role of GLUT2 in glucose metabolism in multiple organs and tissues. Mol. Biol. Rep. 2023, 50, 6963–6974. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Aviagen. Ross Broiler Management Handbook. 2018. Available online: https://aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 12 March 2024).
- Aggrey, S.E.; Milfort, M.C.; Fuller, A.L.; Yuan, J.; Rekaya, R. Effect of host genotype and Eimeria acervulina infection on the metabolome of meat-type chickens. PLoS ONE 2019, 14, e0223417. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- SAS Publishing. SAS/IML Studio 3. 3 for SAS/STAT Users; SAS Institute: Singapore, 2010. [Google Scholar]
- Maldonado, K.A.; Mohiuddin, S.S. Biochemistry, Hypertonicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Akiba, Y.; Chida, Y.; Takahashi, T.; Ohtomo, Y.; Sato, K.; Takahashi, K. Persistent hypoglycemia induced by continuous insulin infusion in broiler chickens. Br. Poult. Sci. 1999, 40, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Saneyasu, T.; Fukuzo, S.; Kitashiro, A.; Nagata, K.; Honda, K.; Kamisoyama, H. Central administration of insulin and refeeding lead to the phosphorylation of AKT, but not FOXO1, in the hypothalamus of broiler chicks. Physiol. Behav. 2019, 210, 112644. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Dagou, C.; Derouet, M.; Simon, J.; Taouis, M. Early steps of insulin receptor signaling in chicken and rat: Apparent refractoriness in chicken muscle. Domest. Anim. Endocrinol. 2004, 26, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Freychet, P.; Rosselin, G. Chicken Insulin: Radioimmunological Characterization and Enhanced Activity in Rat Fat Cells and Liver Plasma Membranes. Endocrinology 1974, 95, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Cheng, Q.; Cai, Z.; Zhang, L.; Xing, T.; Xu, X.; Gao, F. Gas chromatography-mass spectrometry-based untargeted metabolomics analysis reveals circulating biomarkers related to wooden breast myopathy in broilers: A preliminary study. Poult. Sci. 2023, 102, 102718. [Google Scholar] [CrossRef]
- Ito, K.; Miyamoto, H.; Matsuura, M.; Ishii, C.; Tsuboi, A.; Tsuji, N.; Nakaguma, T.; Nakanishi, Y.; Kato, T.; Suda, W.; et al. Noninvasive fecal metabolic profiling for the evaluation of characteristics of thermostable lactic acid bacteria, Weizmannia coagulans SANK70258, for broiler chickens. J. Biosci. Bioeng. 2022, 134, 105–115. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M. Production of arabitol by yeasts: Current status and future prospects. J. Appl. Microbiol. 2015, 119, 303–314. [Google Scholar] [CrossRef]
- Yeagle, P.L.; Elsevier. The Membranes of Cells; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Mogyorósi, A.; Ziyadeh, F.N. GLUT1 and TGF-β: The link between hyperglycaemia and diabetic nephropathy. Nephrol. Dial. Transplant. 1999, 14, 2827–2829. [Google Scholar] [CrossRef]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Cordeiro, L.M.d.S.; Bainbridge, L.; Devisetty, N.; McDougal, D.H.; Peters, D.J.M.; Chhabra, K.H. Loss of function of renal Glut2 reverses hyperglycaemia and normalises body weight in mouse models of diabetes and obesity. Diabetologia 2022, 65, 1032–1047. [Google Scholar] [CrossRef]
- Dominguez, J.H.; Camp, K.; Maianu, L.; Feister, H.; Garvey, W.T. Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am. J. Physiol. 1994, 266, F283–F290. [Google Scholar] [CrossRef] [PubMed]
- Sugawara-Yokoo, M.; Suzuki, T.; Matsuzaki, T.; Naruse, T.; Takata, K. Presence of fructose transporter GLUT5 in the S3 proximal tubules in the rat kidney. Kidney Int. 1999, 56, 1022–1028. [Google Scholar] [CrossRef]
- Song, A.; Mao, Y.; Wei, H. GLUT5: Structure, functions, diseases and potential applications. Acta Biochim. Et Biophys. Sin. 2023, 55, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Dobson, H.; Mason, S.; Cullingham, F.; Shakibaei, M.; Moley, J.; Moley, K. Expression of the GLUT1 and GLUT9 facilitative glucose transporters in embryonic chondroblasts and mature chondrocytes in ovine articular cartilage. Cell Biol. Int. 2005, 29, 249–260. [Google Scholar] [CrossRef]
- Preitner, F.; Bonny, O.; Laverrière, A.; Rotman, S.; Firsov, D.; da Costa, A.; Metref, S.; Thorens, B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. USA 2009, 106, 15501–15506. [Google Scholar] [CrossRef] [PubMed]
- Byers, M.; Bohannon-Stewart, A.; Khwatenge, C.; Alqureish, E.; Alhathlol, A.; Nahashon, S.; Wang, X. Absolute quantification of tissue specific expression of glucose transporters in chickens. J. Mol. Cell. Biol. Forecast 2018, 1, 1009. [Google Scholar]
- Ding, X.; Peng, C.; Li, S.; Li, M.; Li, X.; Wang, Z.; Li, Y.; Wang, X.; Li, J.; Wu, J. Chicken serum uric acid level is regulated by glucose transporter 9. Anim. Biosci. 2021, 34, 670–679. [Google Scholar] [CrossRef]
- Sabolić, I.; Vrhovac, I.; Eror, D.B.; Gerasimova, M.; Rose, M.R.; Breljak, D.; Ljubojević, M.; Brzica, H.; Sebastiani, A.; Thal, S.C.; et al. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. American journal of physiology. Cell Physiol. 2012, 302, C1174–C1188. [Google Scholar] [CrossRef]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 161–174. [Google Scholar] [CrossRef]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1345–1370. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S.C. Renal Function in Diabetic Disease Models: The Tubular System in the Pathophysiology of the Diabetic Kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, S.; Yamato, T.; Fujikura, H.; Hiratochi, M.; Itoh, F.; Tomae, M.; Takemura, Y.; Maruyama, H.; Sugiyama, T.; Wakamatsu, A.; et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci. 2005, 76, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, T.; Fukazawa, M.; Ueda, O.; Shimada, H.; Kito, A.; Kakefuda, M.; Kawase, Y.; Wada, N.A.; Goto, C.; Fukushima, N.; et al. SGLT5 Reabsorbs Fructose in the Kidney but Its Deficiency Paradoxically Exacerbates Hepatic Steatosis Induced by Fructose. PLoS ONE 2013, 8, e56681. [Google Scholar] [CrossRef] [PubMed]
- Baader-Pagler, T.; Eckhardt, M.; Himmelsbach, F.; Sauer, A.; Stierstorfer, B.E.; Hamilton, B.S. SGLT6—A pharmacological target for the treatment of obesity? Adipocyte 2018, 7, 277–284. [Google Scholar] [CrossRef]
- Santer, R. Molecular Analysis of the SGLT2 Gene in Patients with Renal Glucosuria. J. Am. Soc. Nephrol. 2003, 14, 2873–2882. [Google Scholar] [CrossRef]
- Seki, Y.; Sato, K.; Kono, T.; Abe, H.; Akiba, Y. Broiler chickens (Ross strain) lack insulin-responsive glucose transporter GLUT4 and have GLUT8 cDNA. Gen. Comp. Endocrinol. 2003, 133, 80–87. [Google Scholar] [CrossRef]

| Gene Symbol | Accession Number | Product Size | Primer Sequence |
|---|---|---|---|
| GLUT1 | NM_205209.1 | 105 bp | Fwd 5′ CTTCTGCATACACTCCTTCTCC 3′ |
| (SLC2A1) | Rev 5′ TGGACGTGAAACCAGCTAAA 3′ | ||
| GLUT2 | NM_207178.1 | 150 bp | Fwd 5′ TCATTGTAGCTGAGCTGTTCAGCC 3′ |
| (SLC2A2) | Rev 5′ CGGCGAAGACAACGAACACATAC 3′ | ||
| GLUT5 | XM_417596.6 | 108 bp | Fwd 5′ AGGCTGATCTCTGCCTTTG 3′ |
| (SLC2A5) | Rev 5′ GTCGATGTAGGTTCGGTTGTAG 3′ | ||
| GLUT9 | XM_420789.8 | 88 bp | Fwd 5′ GTGTCAGTCCTTCAGCTCCTTAGA 3′ |
| (SLC2A9) | Rev 5′ CACAAAGCTGGTAGCATCCCATAG 3′ | ||
| SGLT1 | NM_001293240 | 97 bp | Fwd 5′ GAGGAGAAACCCGATGAAAGAG 3′ |
| (SLC5A1) | Rev 5′ CTAAGCCACAGAACCAGTTGTA3 3′ | ||
| SGLT4 | XM_040678521.2 | 104 bp | Fwd 5′ GAGAGCATGACTTGGTCGAAAGTG 3′ |
| (SLC5A9) | Rev 5′ GGACAAACCACAGAACCACAGATAC 3′ | ||
| SGLT5 | XM_046927938 | 89 bp | Fwd 5′ TGAGGGCTCAGGGCTCTTTAT 3′ |
| (SLC5A10) | Rev 5′ CGTACGTCGCATTCCACTCAAA 3′ | ||
| SGLT6 | XM_414862.8 | 102 bp | Fwd 5′ GCTGCTACTTACGGTGGTCTCTAT 3′ |
| (SLC5A11) | Rev 5′ TGCAGGTAGGAGCTGATGGATT 3′ | ||
| βActin | NM_205518.2 | 125 bp | Fwd 5′ AGACATCAGGGTGTGATGGTTGGT 3′ |
| Rev 5′ TCCCAGTTGGTGACAATACCGTGT 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milfort, M.C.; Ghareeb, A.F.A.; Ariyo, O.W.; Kwakye, J.; Hartono, E.; Sovi, S.; Aryal, B.; Fuller, A.L.; El Sabry, M.I.; Stino, F.; et al. Renal Sugar Metabolites and mRNA Expression of Glucose Transporters in Meat-Type Chickens with Differing Residual Water Intake. Animals 2024, 14, 2912. https://doi.org/10.3390/ani14192912
Milfort MC, Ghareeb AFA, Ariyo OW, Kwakye J, Hartono E, Sovi S, Aryal B, Fuller AL, El Sabry MI, Stino F, et al. Renal Sugar Metabolites and mRNA Expression of Glucose Transporters in Meat-Type Chickens with Differing Residual Water Intake. Animals. 2024; 14(19):2912. https://doi.org/10.3390/ani14192912
Chicago/Turabian StyleMilfort, Marie C., Ahmed F. A. Ghareeb, Oluwatomide W. Ariyo, Josephine Kwakye, Evan Hartono, Selorm Sovi, Bikash Aryal, Alberta L. Fuller, Mohamed I. El Sabry, Farid Stino, and et al. 2024. "Renal Sugar Metabolites and mRNA Expression of Glucose Transporters in Meat-Type Chickens with Differing Residual Water Intake" Animals 14, no. 19: 2912. https://doi.org/10.3390/ani14192912
APA StyleMilfort, M. C., Ghareeb, A. F. A., Ariyo, O. W., Kwakye, J., Hartono, E., Sovi, S., Aryal, B., Fuller, A. L., El Sabry, M. I., Stino, F., Rekaya, R., & Aggrey, S. E. (2024). Renal Sugar Metabolites and mRNA Expression of Glucose Transporters in Meat-Type Chickens with Differing Residual Water Intake. Animals, 14(19), 2912. https://doi.org/10.3390/ani14192912





