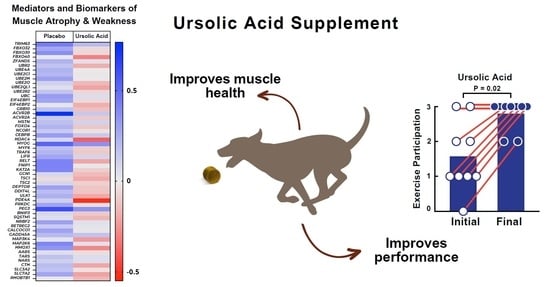
Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Dietary Supplementation with Ursolic Acid Is Well Tolerated and Safe in Dogs
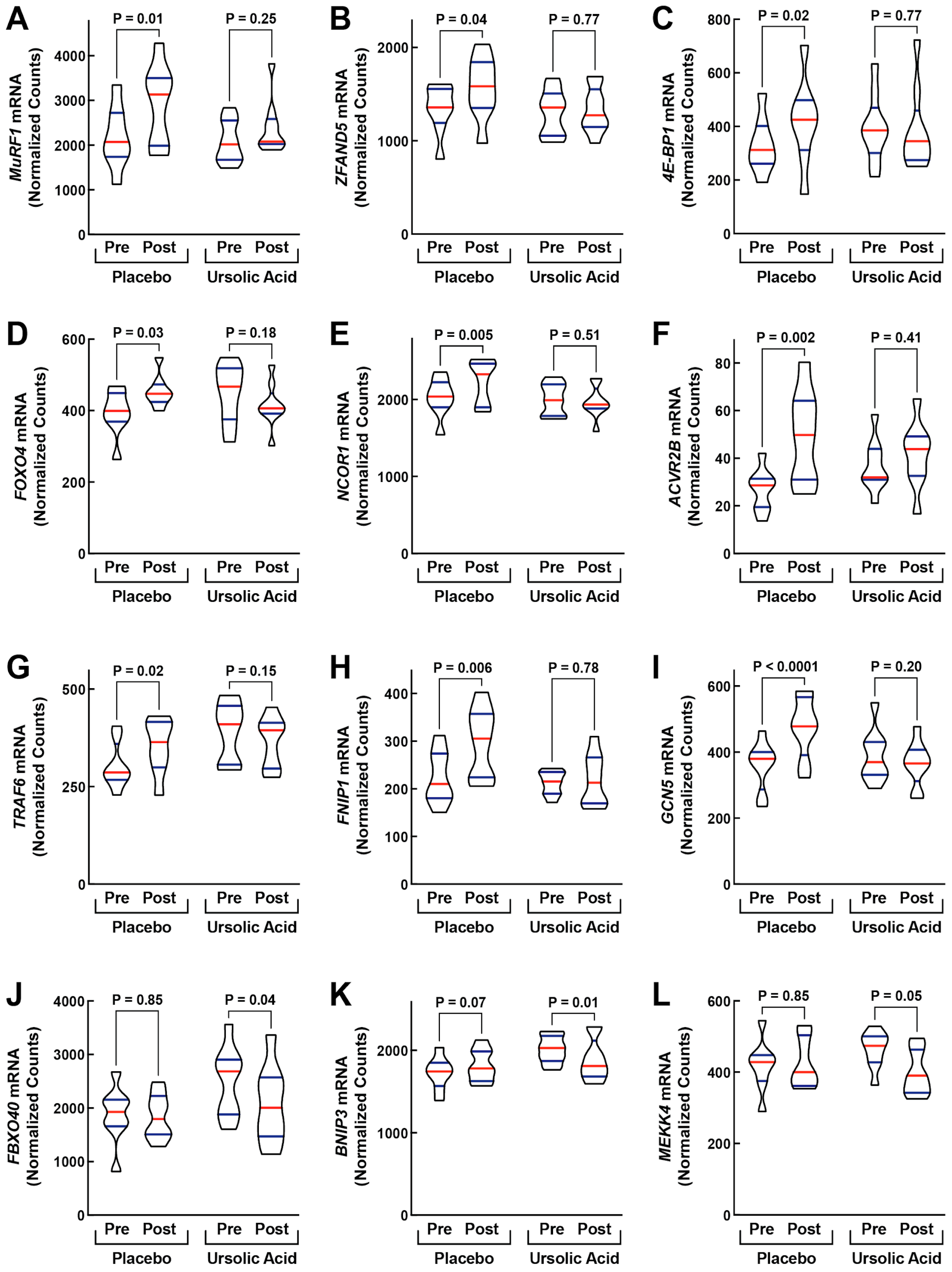
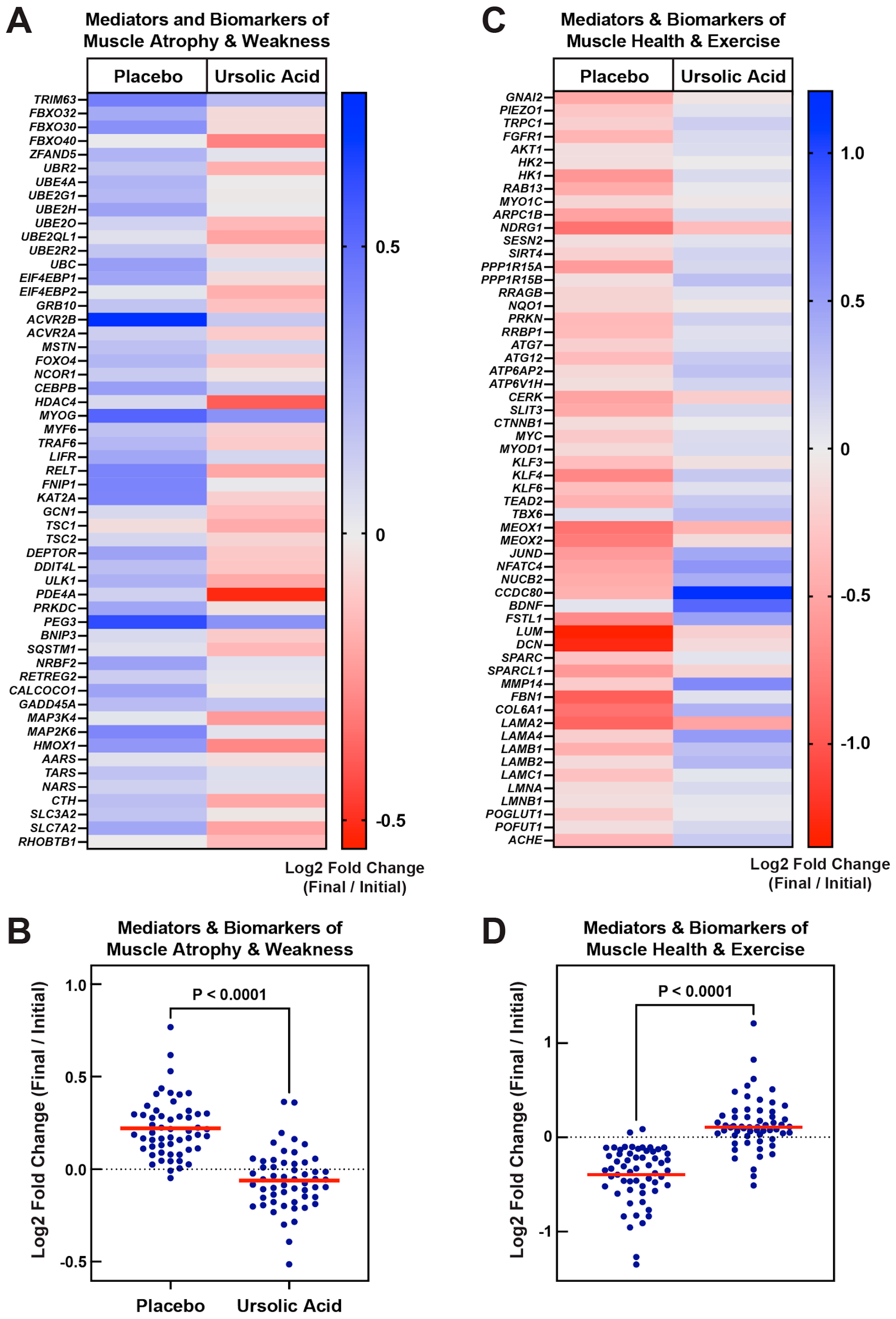
3.2. Dietary Supplementation with Ursolic Acid Inhibits Atrophy-Associated mRNA Expression in Skeletal Muscle of Older Dogs with Age-Related Skeletal Muscle Atrophy
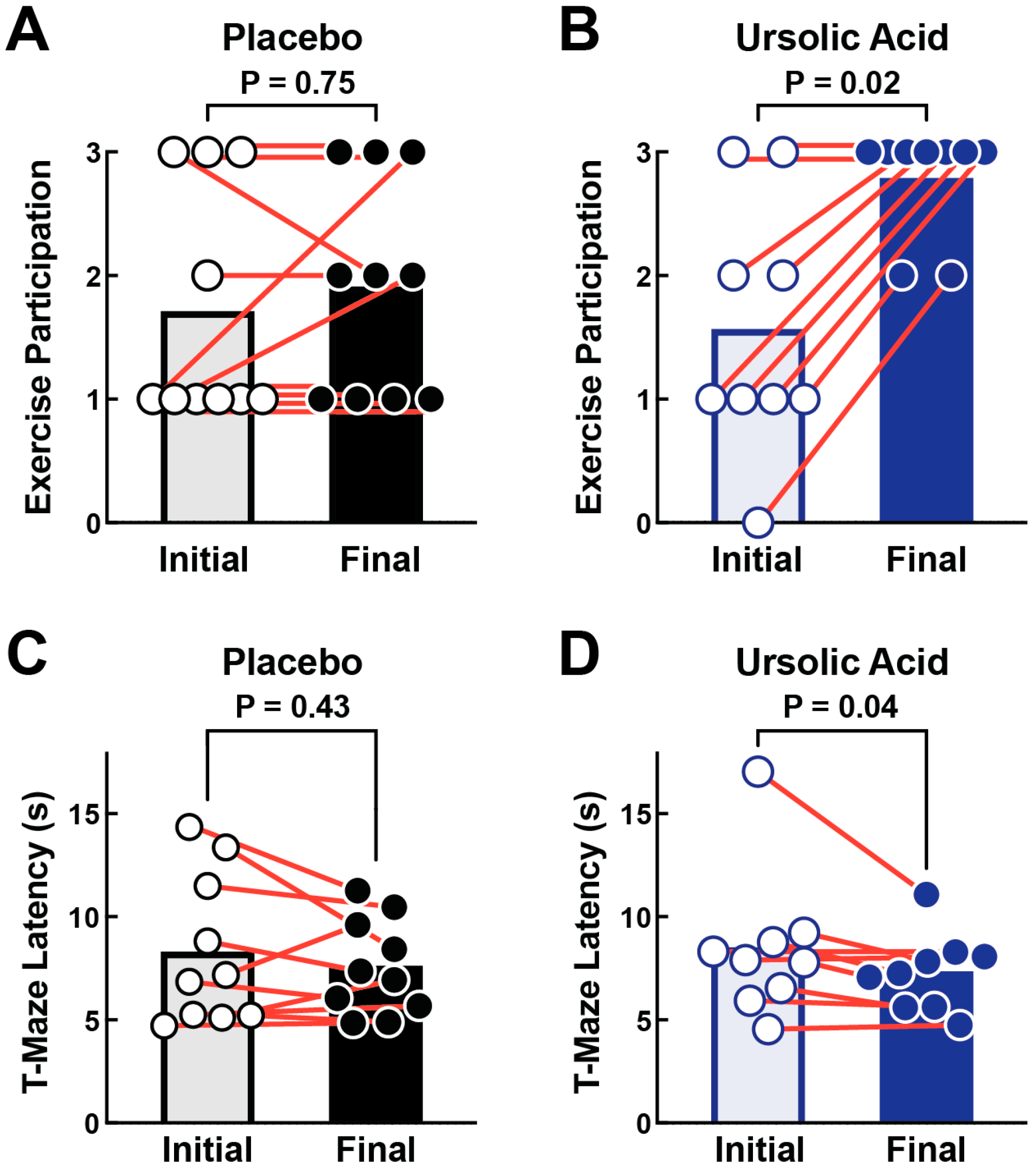
3.3. Dietary Supplementation with Ursolic Acid Increases Exercise Participation and T-Maze Performance in Older Dogs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, L.M. Cachexia and Sarcopenia: Emerging Syndromes of Importance in Dogs and Cats. J. Vet. Intern. Med. 2012, 26, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M. Cachexia and Sarcopenia in Companion Animals: An under-Utilized Natural Animal Model of Human Disease. JCSM Rapid Commun. 2018, 1, 1–17. [Google Scholar] [CrossRef]
- Frye, C.; Carr, B.J.; Lenfest, M.; Miller, A. Canine Geriatric Rehabilitation: Considerations and Strategies for Assessment, Functional Scoring, and Follow Up. Front. Vet. Sci. 2022, 9, 842458. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Hyatt, J.-P.K.; Raffaello, A.; Jagoe, R.T.; Roy, R.R.; Edgerton, V.R.; Lecker, S.H.; Goldberg, A.L. Rapid Disuse and Denervation Atrophy Involve Transcriptional Changes Similar to Those of Muscle Wasting during Systemic Diseases. FASEB J. 2007, 21, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.M.; Al-Zougbi, A.; Bodine, S.C.; Adams, C.M. Skeletal Muscle Atrophy: Discovery of Mechanisms and Potential Therapies. Physiology 2019, 34, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Ebert, S.M.; Rasmussen, B.B.; Judge, A.R.; Judge, S.M.; Larsson, L.; Wek, R.C.; Anthony, T.G.; Marcotte, G.R.; Miller, M.J.; Yorek, M.A.; et al. Biology of Activating Transcription Factor 4 (ATF4) and Its Role in Skeletal Muscle Atrophy. J. Nutr. 2022, 152, 926–938. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA Expression Signatures of Human Skeletal Muscle Atrophy Identify a Natural Compound That Increases Muscle Mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef]
- Adams, C.M.; Ebert, S.M.; Dyle, M.C. Use of mRNA Expression Signatures to Discover Small Molecule Inhibitors of Skeletal Muscle Atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 263–268. [Google Scholar] [CrossRef]
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of Ursolic Acid from Apple Peels by High Speed Counter-Current Chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic Acid Increases Skeletal Muscle and Brown Fat and Decreases Diet-Induced Obesity, Glucose Intolerance and Fatty Liver Disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.M.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Murry, D.J.; Fox, D.K.; Bongers, K.S.; Lira, V.A.; Meyerholz, D.K.; Talley, J.J.; et al. Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-Dependent Pathway to Age-Related Skeletal Muscle Weakness and Atrophy. J. Biol. Chem. 2015, 290, 25497–25511. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, J.-A.; Xu, J.; Cao, J.; Wang, Y.; Thomas, S.S.; Hu, Z. Suppression of Muscle Wasting by the Plant-Derived Compound Ursolic Acid in a Model of Chronic Kidney Disease. J. Cachexia Sarcopenia Muscle 2017, 8, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ouyang, Z.; Liao, Z.; Li, L.; Zhang, Y.; Gao, J.; Ma, L.; Yu, S. Ursolic Acid Alleviates Cancer Cachexia and Prevents Muscle Wasting via Activating SIRT1. Cancers 2023, 15, 2378. [Google Scholar] [CrossRef]
- Bigford, G.E.; Darr, A.J.; Bracchi-Ricard, V.C.; Gao, H.; Nash, M.S.; Bethea, J.R. Effects of Ursolic Acid on Sub-Lesional Muscle Pathology in a Contusion Model of Spinal Cord Injury. PLoS ONE 2018, 13, e0203042. [Google Scholar] [CrossRef] [PubMed]
- Negi, H.; Shukla, A.; Khan, F.; Pandey, R. 3β-Hydroxy-Urs-12-En-28-Oic Acid Prolongs Lifespan in C. Elegans by Modulating JNK-1. Biochem. Biophys. Res. Commun. 2016, 480, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Wagner, A.E.; Lüersen, K.; Künstner, A.; Meyer, T.; Kahns, A.K.; Derer, S.; Graspeuntner, S.; Rupp, J.; Busch, H.; et al. Dietary Ursolic Acid Improves Health Span and Life Span in Male Drosophila Melanogaster. Biofactors 2019, 45, 169–186. [Google Scholar] [CrossRef]
- Bang, H.S.; Seo, D.Y.; Chung, Y.M.; Oh, K.-M.; Park, J.J.; Arturo, F.; Jeong, S.-H.; Kim, N.; Han, J. Ursolic Acid-Induced Elevation of Serum Irisin Augments Muscle Strength during Resistance Training in Men. Korean J. Physiol. Pharmacol. 2014, 18, 441–446. [Google Scholar] [CrossRef]
- Swimmer, R.A.; Rozans, E.A. Evaluation of the 6-minute walk test in pet dogs. J. Vet. Intern. Med. 2011, 25, 405–406. [Google Scholar] [CrossRef]
- Cerda-Gonzalez, S.; Talarico, L.; Todhunter, R. Noninvasive Assessment of Neuromuscular Disease in Dogs: Use of the 6-minute Walk Test to Assess Submaximal Exercise Tolerance in Dogs with Centronuclear Myopathy. J. Vet. Intern. Med. 2016, 30, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Hercock, C.A.; Pinchbeck, G.; Giejda, A.; Clegg, P.D.; Innes, J.F. Validation of a client-based clinical metrology instrument for the evaluation of canine elbow osteoarthritis. J. Small Anim. Pract. 2009, 50, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal Muscle Atrophy and the E3 Ubiquitin Ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Hishiya, A.; Iemura, S.-I.; Natsume, T.; Takayama, S.; Ikeda, K.; Watanabe, K. A Novel Ubiquitin-Binding Protein ZNF216 Functioning in Muscle Atrophy. EMBO J. 2006, 25, 554–564. [Google Scholar] [CrossRef]
- Lee, D.; Takayama, S.; Goldberg, A.L. ZFAND5/ZNF216 Is an Activator of the 26S Proteasome That Stimulates Overall Protein Degradation. Proc. Natl. Acad. Sci. USA 2018, 115, E9550–E9559. [Google Scholar] [CrossRef]
- Le Bacquer, O.; Combe, K.; Patrac, V.; Ingram, B.; Combaret, L.; Dardevet, D.; Montaurier, C.; Salles, J.; Giraudet, C.; Guillet, C.; et al. 4E-BP1 and 4E-BP2 Double Knockout Mice Are Protected from Aging-Associated Sarcopenia. J. Cachexia Sarcopenia Muscle 2019, 10, 696–709. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of Autophagy and the Ubiquitin-Proteasome System by the FoxO Transcriptional Network during Muscle Atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Yamamoto, H.; Williams, E.G.; Mouchiroud, L.; Cantó, C.; Fan, W.; Downes, M.; Héligon, C.; Barish, G.D.; Desvergne, B.; Evans, R.M.; et al. NCoR1 Is a Conserved Physiological Modulator of Muscle Mass and Oxidative Function. Cell 2011, 147, 827–839. [Google Scholar] [CrossRef]
- Lee, S.-J. Targeting the Myostatin Signaling Pathway to Treat Muscle Loss and Metabolic Dysfunction. J. Clin. Investig. 2021, 131, JCI148372. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.K.; Gupta, S.K.; Bhatnagar, S.; Panguluri, S.K.; Darnay, B.G.; Choi, Y.; Kumar, A. Targeted Ablation of TRAF6 Inhibits Skeletal Muscle Wasting in Mice. J. Cell Biol. 2010, 191, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, J.; Sun, Z.; Yin, Y.; Mao, Y.; Xu, D.; Liu, L.; Xu, Z.; Guo, Q.; Ding, C.; et al. AMPK-Dependent and -Independent Coordination of Mitochondrial Function and Muscle Fiber Type by FNIP1. PLoS Genet. 2021, 17, e1009488. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Goldberg, A.L. Muscle Wasting in Fasting Requires Activation of NF-κB and Inhibition of AKT/Mechanistic Target of Rapamycin (mTOR) by the Protein Acetylase, GCN5. J. Biol. Chem. 2015, 290, 30269–30279. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, L.; Eash, J.; Ibebunjo, C.; Glass, D.J. The SCF-Fbxo40 Complex Induces IRS1 Ubiquitination in Skeletal Muscle, Limiting IGF1 Signaling. Dev. Cell 2011, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 Controls Autophagy in Skeletal Muscle in Vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Bullard, S.A.; Seo, S.; Schilling, B.; Dyle, M.C.; Dierdorff, J.M.; Ebert, S.M.; DeLau, A.D.; Gibson, B.W.; Adams, C.M. Gadd45a Protein Promotes Skeletal Muscle Atrophy by Forming a Complex with the Protein Kinase MEKK4. J. Biol. Chem. 2016, 291, 17496–17509. [Google Scholar] [CrossRef]
- Marcotte, G.R.; Miller, M.J.; Kunz, H.E.; Ryan, Z.C.; Strub, M.D.; Vanderboom, P.M.; Heppelmann, C.J.; Chau, S.; Von Ruff, Z.D.; Kilroe, S.P.; et al. GADD45A Is a Mediator of Mitochondrial Loss, Atrophy, and Weakness in Skeletal Muscle. JCI Insight 2023, 2023, 171772. [Google Scholar] [CrossRef]
- Miller, M.J.; Marcotte, G.R.; Basisty, N.; Wehrfritz, C.; Ryan, Z.C.; Strub, M.D.; McKeen, A.T.; Stern, J.I.; Nath, K.A.; Rasmussen, B.B.; et al. The Transcription Regulator ATF4 Is a Mediator of Skeletal Muscle Aging. Geroscience 2023, 45, 2525–2543. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, Y.; Tweardy, D.J.; Mitch, W.E. Stat3 Activation Induces Insulin Resistance via a Muscle-Specific E3 Ubiquitin Ligase Fbxo40. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E625–E635. [Google Scholar] [CrossRef]
- Tiira, K.; Lohi, H. Early Life Experiences and Exercise Associate with Canine Anxieties. PLoS ONE 2015, 10, e0141907. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.E.; Raichlen, D.A.; Forsyth, K.K.; Promislow, D.E.L.; Alexander, G.E.; MacLean, E.L. Dog Aging Project Consortium Associations between Physical Activity and Cognitive Dysfunction in Older Companion Dogs: Results from the Dog Aging Project. Geroscience 2023, 45, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.N.; Kanazono, S.; Coates, J.R.; Johnson, G.S.; Johnson, G.C.; Narfstrom, K.; O’Brien, D.P.; Katz, M.L. Cognitive decline in a dog model for an inherited neurodegenerative disease using t-maze performance. J. Vet. Behav. 2010, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.; Bonagura, J.; Ettinger, S.; Fox, P.; Gordon, S.; Haggstrom, J.; Hamlin, R.; Keene, B.; Luis-Fuentes, V.; Stepien, R. Guidelines for the Diagnosis and Treatment of Canine Chronic Valvular Heart Disease. J. Vet. Intern. Med. 2009, 23, 1142–1150. [Google Scholar] [CrossRef]
| Control (No Chew) | Ursolic Acid (5 Chews/Day) | Reference Range | |||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| Food consumption (g) | 244 ± 55 | 243 ± 68 | 275 ± 49 | 248 ± 77 | N/A |
| Body weight (kg) | 8.8 ± 2.8 | 9.12 ± 2.9 * | 9.82 ± 2.8 | 10.14 ± 3.0 * | N/A |
| Body temperature (°C) | 38.3 ± 0.3 | 38.4 ± 0.3 | 38.4 ± 0.3 | 38.5 ± 0.4 | N/A |
| Complete Blood Counts | |||||
| Hemoglobin (g/dL) | 16.7 ± 1.6 | 16.4 ± 1.4 | 16.1 ± 0.9 | 16.2 ± 1.2 | 13.1–20.5 |
| Hematocrit (%) | 47.2 ± 4.5 | 46.2 ± 4.1 | 45.4 ± 3.3 | 45.7 ± 3.5 | 37.3–61.7 |
| Red blood cells (M/µL) | 7.45 ± 0.66 | 7.21 ± 0.64 | 7.07 ± 0.28 | 7.09 ± 0.49 | 5.65–8.87 |
| MCV (fl) | 63.3 ± 3.6 | 64.1 ± 3.5 * | 64.1 ± 2.6 | 64.4 ± 1.9 | 61.6–73.5 |
| MCH (pg) | 22.4 ± 1.1 | 22.8 ± 1.3 | 22.7 ± 0.7 | 22.9 ± 1.0 | 21.2–25.9 |
| MCHC (g/dL) | 35.5 ± 0.5 | 35.5 ± 0.6 | 35.5 ± 0.8 | 35.5 ± 0.8 | 32.0–37.9 |
| Reticulocytes (K/µL) | 21 ± 9 | 30 ± 8 | 25 ± 12 | 28 ± 17 | 10–110 |
| Platelets (K/µL) | 305 ± 69 | 326 ± 116 | 277 ± 81 | 288 ± 90 | 148–484 |
| White blood cells (K/µL) | 8.78 ± 2.10 | 9.10 ± 3.65 | 9.16 ± 1.19 | 8.73 ± 1.56 | 5.05–16.76 |
| Neutrophils (K/µL) | 5.35 ± 1.18 | 5.90 ± 2.68 | 5.73 ± 0.81 | 5.40 ± 1.07 | 2.95–11.64 |
| Lymphocytes (K/µL) | 2.23 ± 0.68 | 1.96 ± 0.49 | 2.39 ± 0.67 | 2.20 ± 0.43 | 1.05–5.10 |
| Monocytes (K/µL) | 0.68 ± 0.27 | 0.78 ± 0.45 | 0.69 ± 0.19 | 0.71 ± 0.31 | 0.16–1.12 |
| Eosinophils (K/µL) | 0.52 ± 0.59 | 0.46 ± 0.38 | 0.34 ± 0.35 | 0.40 ± 0.20 | 0.06–1.23 |
| Basophils (K/µL) | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.03 | 0.00–0.10 |
| Blood Chemistry | |||||
| Glucose (g/L) | 0.85 ± 0.09 | 0.95 ± 0.08 * | 0.85 ± 0.12 | 0.86 ± 0.09 | 0.74–1.43 |
| Sodium (mmol/L) | 155 ± 2 | 155 ± 2 | 155 ± 2 | 155 ± 2 | 144–160 |
| Potassium (mmol/L) | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 | 3.5–5.8 |
| Chloride (mmol/L) | 115 ± 2 | 114 ± 2 | 115 ± 3 | 114 ± 2 | 109–122 |
| Calcium (mg/L) | 99 ± 4 | 99 ± 3 | 100 ± 5 | 100 ± 3 | 79–120 |
| Magnesium (mg/L) | 18 ± 1 | 18 ± 1 | 18 ± 1 | 17 ± 1 | 14–24 |
| Phosphate (mg/L) | 38 ± 12 | 42 ± 4.5 | 42 ± 8.7 | 41 ± 8.3 | 25–68 |
| BUN (g/L) | 0.25 ± 0.04 | 0.26 ± 0.06 | 0.25 ± 0.07 | 0.24 ± 0.04 | 0.15–0.57 |
| Creatinine (mg/L) | 6.1 ± 1.3 | 5.8 ± 1.1 | 6.6 ± 1.7 | 6.0 ± 0.9 | 5.0–18.0 |
| Total protein (g/L) | 58 ± 3 | 59 ± 3 | 58 ± 2 | 58 ± 2 | 52–82 |
| Albumin (g/L) | 32 ± 4 | 33 ± 3 | 32 ± 1 | 32 ± 2 | 23–40 |
| Globulin (g/L) | 25 ± 1 | 26 ± 1 | 26 ± 1 | 26 ± 1 | 25–45 |
| AST (IU/L) | 30 ± 8 | 32 ± 6 | 31 ± 11 | 35 ± 9 * | 0–50 |
| ALT (IU/L) | 48 ± 27 | 41 ± 11 | 46 ± 19 | 46 ± 26 | 10–125 |
| Alkaline phosphatase (IU/L) | 73 ± 25 | 77 ± 28 | 104 ± 61 | 87 ± 68 | 23–212 |
| SDMA (μg/L) | 7 ± 2 | 7 ± 2 | 8 ± 2 | 7 ± 1 | 0–14 |
| Urine Chemistry | |||||
| pH | 6.1 ± 0.6 | 5.7 ± 0.4 | 6.0 ± 0.4 | 5.9 ± 0.4 | 5.3–7.0 |
| Specific gravity | 1.040 ± 0.014 | 1.037 ± 0.010 | 1.037 ± 0.008 | 1.032 ± 0.014 | 1.016–1.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebert, S.M.; Nicolas, C.S.; Schreiber, P.; Lopez, J.G.; Taylor, A.T.; Judge, A.R.; Judge, S.M.; Rasmussen, B.B.; Talley, J.J.; Rème, C.A.; et al. Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy. Animals 2024, 14, 186. https://doi.org/10.3390/ani14020186
Ebert SM, Nicolas CS, Schreiber P, Lopez JG, Taylor AT, Judge AR, Judge SM, Rasmussen BB, Talley JJ, Rème CA, et al. Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy. Animals. 2024; 14(2):186. https://doi.org/10.3390/ani14020186
Chicago/Turabian StyleEbert, Scott M., Celine S. Nicolas, Paul Schreiber, Jaime G. Lopez, Alan T. Taylor, Andrew R. Judge, Sarah M. Judge, Blake B. Rasmussen, John J. Talley, Christophe A. Rème, and et al. 2024. "Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy" Animals 14, no. 2: 186. https://doi.org/10.3390/ani14020186
APA StyleEbert, S. M., Nicolas, C. S., Schreiber, P., Lopez, J. G., Taylor, A. T., Judge, A. R., Judge, S. M., Rasmussen, B. B., Talley, J. J., Rème, C. A., & Adams, C. M. (2024). Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy. Animals, 14(2), 186. https://doi.org/10.3390/ani14020186