Prospective Study of 506 Dogs with Tick Paralysis: Investigating Measures of Severity and Clinical Signs as Predictors of Mortality and Assessing the Benefits of Different Therapeutics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinic and Veterinarian Selection
2.2. Case Selection
2.3. Pre-Trial Tutorial
2.4. Trial Design
2.5. Data Collection
2.6. Data Analyses
3. Results
3.1. Descriptive Data
3.2. Analyses
3.2.1. Animal and Tick Factors
3.2.2. Predictors of Outcome—NMJ and VAS Scores
3.2.3. Predictors of Outcome—Specific Clinical Signs
3.2.4. Predictors of Outcome—Facial Expressions of Distress
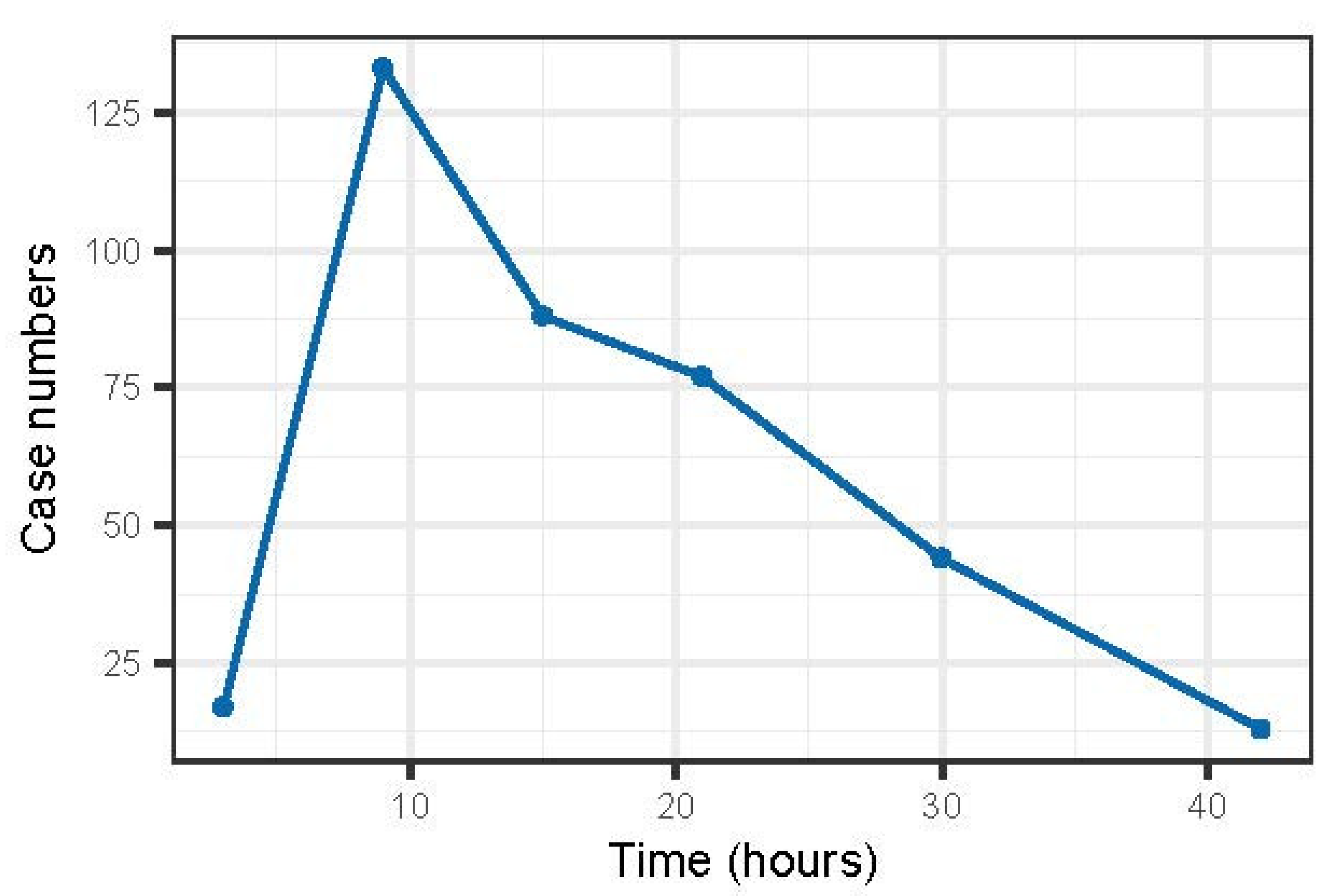
3.2.5. Geographic Variation in Clinical Disease
3.2.6. Pharmaceutical Product Use and Supportive Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, B.; Spence, I. Temperature-dependent inhibition of evoked acetylcholine release in tick paralysis. Nature 1976, 263, 693–695. [Google Scholar] [CrossRef]
- Ilkiw, J.E. A Study of the Effects in the Dog of Ixodes holocyclus. Ph.D. Thesis, University of Sydney, Sydney, Australia, 1979. [Google Scholar]
- Campbell, F.E.; Atwell, R.B. Heart failure in dogs with tick paralysis caused by the Australian paralysis tick, Ixodes holocyclus. Int. J. Appl. Res. Vet. Med. 2003, 1, 148–162. [Google Scholar]
- Fearnley, A. The importance of body temperature in the treatment of tick paralysis. Aust. Vet. Practit 2002, 32, 137–138. [Google Scholar]
- Schull, D.N.; O’Leary, C.A. The use of tick antitoxin serum and associated therapy for the treatment of dogs with Ixodes holocyclus toxicity. Aust. Vet. Practit 2007, 37, 90–97. [Google Scholar]
- Atwell, R.B. Tick Numbers in Different Dogs. Aust. Vet. Practit 2011, 41, 34–37. [Google Scholar]
- Atwell, R.B. Tick Studies—Some Observations. Aust. Vet. Practit 2007, 37, 121–122. [Google Scholar]
- Atwell, R.B. Tick Paralysis. The Merck Veterinary Manual, 10th ed.; Merck & Coy: Whitehouse Station, NJ, USA, 2010. [Google Scholar]
- Atwell, R.B.; Campbell, F.E.; Evans, E.A. Prospective survey of tick paralysis in dogs. Aust. Vet. J. 2008, 79, 412–418. [Google Scholar] [CrossRef]
- Westwood, M.N.; Emery, D.L.; Dhand, N.K. Clinical presentation and treatment of tick paralysis in dogs and cats in Sydney (2001–2010). Aust. Vet. J. 2013, 91, 491–498. [Google Scholar] [CrossRef]
- Atwell, R.B. Technical Bulletin—Tick Anti (Toxin) Serum—An Overview of Knowns and Unknowns; Merial: Sydney, Australia, 2010. [Google Scholar]
- Kunz, M.; Meixner, D.; Lautenbacher, S. Facial muscle movements encoding pain—A systematic review. Pain 2019, 160, 535–549. [Google Scholar] [CrossRef]
- Prkachin, K.M. The consistency of facial expressions of pain: A comparison across modalities. Pain 1992, 51, 297–306. [Google Scholar] [CrossRef]
- McLennan, K.M.; Miller, A.L.; Dalla Costa, E.; Stucke, D.; Corke, M.J.; Broom, D.M.; Leach, M.C. Conceptual and methodological issues relating to pain assessment in mammals: The development and utilisation of pain facial expression scales. Appl. Anim. Behav. Sci. 2019, 217, 1–15. [Google Scholar] [CrossRef]
- Sarolidou, G.; Axelsson, J.; Sundelin, T.; Lasselin, J.; Regenbogen, C.; Sorjonen, K.; Lundström, J.N.; Lekander, M.; Olsson, M.J. Emotional expressions of the sick face. Brain Behav. Immun. 2019, 80, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- McLennan, K.M.; Rebelo Carlos, J.B.; Corke, M.J.; Holmes, M.A.; Leach, M.C.; Constantino-Casas, F. Development of a facial expression scale using footrot and mastitis as models of pain in sheep. Appl. Anim. Behav. Sci. 2016, 176, 19–26. [Google Scholar] [CrossRef]
- Steiner, D.; Norman, G. Health Measurement Scales: A Practical Guide to Their Development and Use, 4th ed.; Oxford University Press: Cambridge, MA, USA, 2008. [Google Scholar] [CrossRef]
- McCormack, H.; Horne, D.; Sheather, S. Clinical applications of visual analogue scales: A critical review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Elera-Fitzcarrald, C.; Vega, K.; Gamboa-Cardenas, R.; Zuniga, K.; Zevallos, F.; Reategui-Sokolova, C.; Pastor-Asurza, C.; Perich-campos, R.; Bellido, Z.; Aranow, C.; et al. Reliability of visual analogue scale and numeric rating scale for the assessment of disease activity in systemic lupus erythematosus. J. Clin. Rheum. 2020, 26, S170–S173. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.E.; Atwell, R.B. Reactions to tick antitoxin serum and the role of atropine in treatment of dogs and cats with tick paralysis caused by Ixodes holocyclus: A pilot survey. Aust. Vet. J. 2001, 79, 394–397. [Google Scholar] [CrossRef]
- Webster, R.A.; Mackie, J.T.; Haskins, S.T. Histopathological changes in the lungs from dogs with tick paralysis: 25 cases (2010–2012). Aust. Vet. J. 2013, 91, 306–311. [Google Scholar] [CrossRef]
- Bloom, T.; Trevathan-Minnis, M.; Atlas, N.; MacDonald, D.A.; Friedman, H.L. Identifying facial expressions in dogs: A replication and extension study. Behav. Process. 2021, 186, 104371. [Google Scholar] [CrossRef]
- Song, S.; Shao, R.; Atwell, R.; Barker, S.; Vankan, D. Phylogenetic and phylogeographic relationships in Ixodes holocyclus and Ixodes cornuatus (Acari: Ixodidae) inferred from COX1 and ITS2 gene sequences. Inter. J. Parasit. 2011, 41, 871–880. [Google Scholar] [CrossRef]
- Cooper, B. Studies on the Pathogenesis of Tick Paralysis. Ph.D. Thesis, University of Sydney, Sydney, Australia, 1976. [Google Scholar]
- Roidriguez-Valle, M.; McAlister, S.; Moolhuijzen, P.; Booth, M.; Agnew, K.; Ellenberger, C.; Knowles, A.; Vanhoff, K.; Bellgard, M.; Tabor, A. Immunomic investigation of Holocyclotoxins to produce the first protective anti-venom vaccine against the Australian paralysis tick, Ixodes holocyclus. Front. Immunol. 2021, 12, 744795. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.; Lee, K.; Lavidis, N.; Rodriguez-Valle, M.; Ijaz, H.; Koehbach, J.; Clark, R.; Lew-Tabor, A.; Noakes, P. Tick holocyclotoxins trigger host paralysis by presynoptic inhibition. Sci. Rep. 2016, 6, 29446. [Google Scholar] [CrossRef] [PubMed]
- Voight, D.; Gorb, S. Functional morphology of tarsal adhesive pads and attachment ability in ticks Ixodes ricinus (Arachnida, Acari. Ixodidae). J. Exp. Biol. 2017, 220, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Schull, D. Findings from Thoracic Radiographs of Dogs and Cats with Tick Toxicity. Aust. Vet. Practit. 2008, 38, 86–90. [Google Scholar]



| Factor | p-Value |
|---|---|
| Tick number | 0.89 |
| Tick size | 0.97 |
| Whether ticks were plucked | 0.46 |
| Whether diagnosis was by presence of crater or tick | 0.63 |
| Whether ticks were alive or dead | 0.32 |
| Adult or non-adult stage of tick | 0.66 |
| Whether tick was located on presentation at clinics or removed at home prior to presentation | 0.18 |
| Whether tick was detected by owner or by clinic | 0.46 |
| Cardiopulmonary disease | 0.37 |
| Other concurrent disease | 0.75 |
| Concurrent (non-tick) drug therapy | 0.77 |
| Scores | OR—A1 (Mortality %) | OR—B2 (Mortality %) | OR—C3 (Mortality %) | OR—D4 (Mortality %) |
|---|---|---|---|---|
| VAS-TOXICITY | 1 (3.1) | 0.6 (2) | 2.6 (7.8) | 19.9 ** (39.1) * |
| NMJ | 1 (3.2) | 0.5 (1.6) | 2.5 (7.6) | 14 (32) |
| VAS-PARALYSIS | 1 (2.7) | 0.6 (1.7) | 2.7 (7) | 15.5 (30.3) |
| VAS-RESPIRATORY | 1 (1.9) | 2.6 (4.7) | 6.6 (11.1) | 30.3 ** (36.4) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atwell, R.; Vankan, D. Prospective Study of 506 Dogs with Tick Paralysis: Investigating Measures of Severity and Clinical Signs as Predictors of Mortality and Assessing the Benefits of Different Therapeutics. Animals 2024, 14, 188. https://doi.org/10.3390/ani14020188
Atwell R, Vankan D. Prospective Study of 506 Dogs with Tick Paralysis: Investigating Measures of Severity and Clinical Signs as Predictors of Mortality and Assessing the Benefits of Different Therapeutics. Animals. 2024; 14(2):188. https://doi.org/10.3390/ani14020188
Chicago/Turabian StyleAtwell, Rick, and Dianne Vankan. 2024. "Prospective Study of 506 Dogs with Tick Paralysis: Investigating Measures of Severity and Clinical Signs as Predictors of Mortality and Assessing the Benefits of Different Therapeutics" Animals 14, no. 2: 188. https://doi.org/10.3390/ani14020188
APA StyleAtwell, R., & Vankan, D. (2024). Prospective Study of 506 Dogs with Tick Paralysis: Investigating Measures of Severity and Clinical Signs as Predictors of Mortality and Assessing the Benefits of Different Therapeutics. Animals, 14(2), 188. https://doi.org/10.3390/ani14020188





