Amino Acid Composition in Different Tissues of Iceland Scallop from the Barents Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
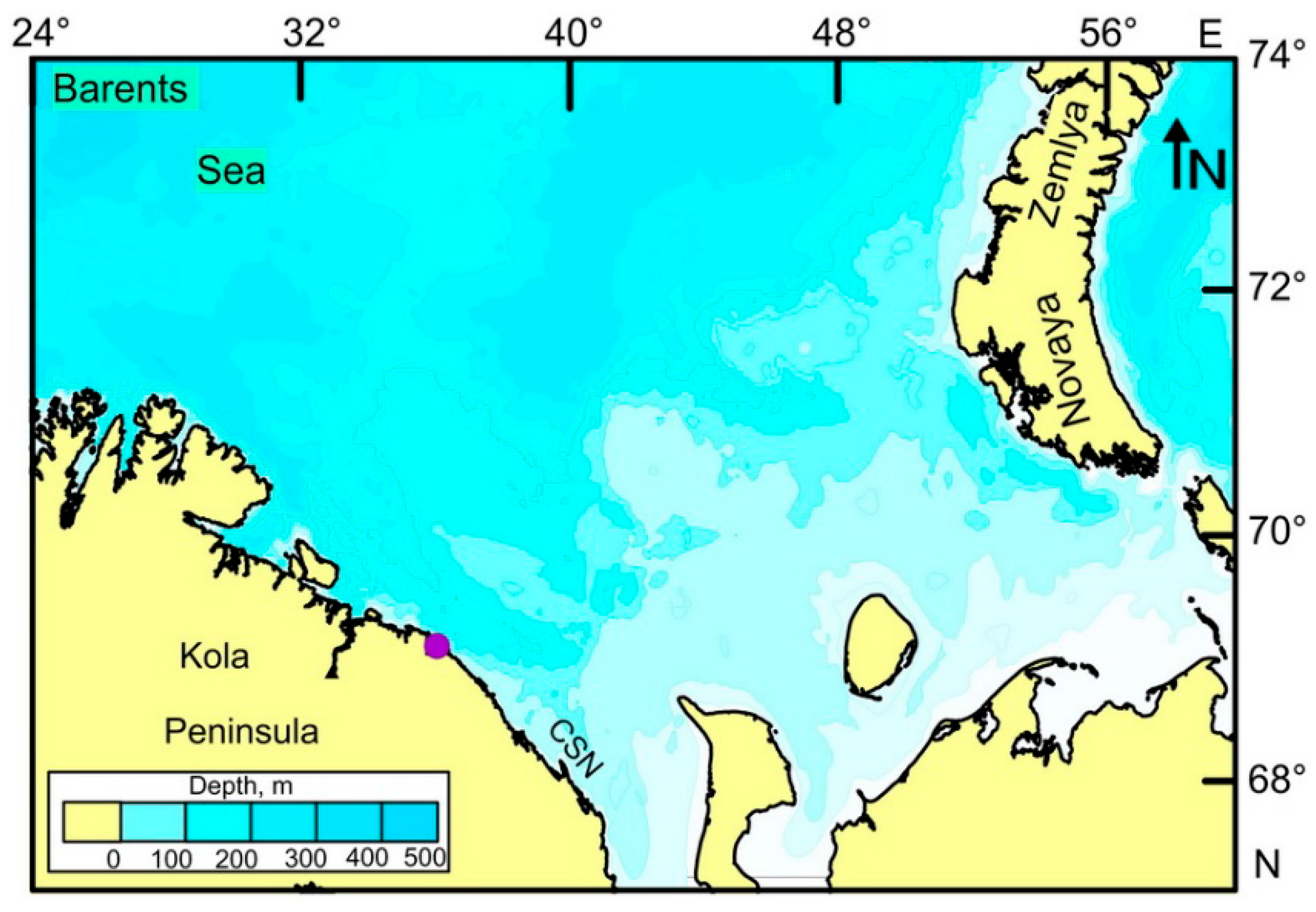
2.1. Study Area
2.2. Sampling and Processing
2.3. Biochemical Assay
- (1)
- Each sample was mechanically homogenized using a universal homogenizer (Ultra-Turrax Tube Driv, IKA, IKA-Werke GmbH & Co. KG, Staufen, Germany) equipped with DT-20-M-gamma tubes at 6000 rpm, and approximately 100 mg of the sample was transferred to a vial for further analysis.
- (2)
- The sample was then hydrolyzed in the nitrogen-filled vial using 10 mL of 6 M HCl at a temperature of 110 °C for a duration of 24 h.
- (3)
- After the hydrolysis process, the sample was cooled to room temperature, followed by filtration through a membrane filter of 2 µm and mixing.
- (4)
- A 0.1 mL aliquot of the sample was pipetted and evaporated under a hot plate at 65 °C.
- (5)
- The evaporated aliquot was then dissolved in a mixture comprising 0.1 mL of a 0.15 M L−1 NaOH solution, 0.35 mL of a solution of phenylisothiocyanate in isopropanol, and 0.05 mL of distilled water.
- (6)
- The resulting mixture was stored for 20 min and evaporated until complete drying.
- (7)
- The dried sample was dissolved in 1 mL of distilled water and centrifuged at 10,000 r.p.m. for 5 min.
- (8)
- Finally, the resulting sample was analyzed using a Shimadzu LC-20AD Prominence high-performance liquid chromatography system equipped with a detector Shimadzu SPD-M20A Prominence (Shimadzu, Japan) and a 250 mm × 4.6 mm × 5 µm column Supelco C18 (Supelco, Bellefonte, PA, USA).
2.4. Statistical Analysis
3. Results
3.1. Scallops
3.2. Amino Acid Composition
3.3. Factors
4. Discussion
4.1. Scallops
4.2. Amino Acid Composition
4.3. Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvoretsky, V.G.; Vodopianova, V.V.; Bulavina, A.S. Effects of climate change on chlorophyll a in the Barents Sea: A long-term assessment. Biology 2023, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Filling knowledge gaps in Arctic marine biodiversity: Environment, plankton, and benthos of Franz Josef Land, Barents Sea. Ocean Coast. Manag. 2024, 249, 106987. [Google Scholar] [CrossRef]
- Matishov, G.G.; Matishov, D.G.; Moiseev, D.V. Inflow of Atlantic-origin waters to the Barents Sea along glacial troughs. Oceanologia 2009, 51, 321–340. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Local variability of Arctic mesozooplankton biomass and production: A case summer study. Environ. Res. 2024, 241, 117416. [Google Scholar] [CrossRef]
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Co-Operation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Marine copepod assemblages in the Arctic: The effect of frontal zones on biomass and productivity. Mar. Environ. Res. 2024, 193, 106250. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Ecology and distribution of red king crab larvae in the Barents Sea: A review. Water 2022, 14, 2328. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: Biodiversity and infestation patterns. Diversity 2022, 14, 6. [Google Scholar] [CrossRef]
- Kuzmin, S.A.; Gudimova, E.N. Introduction of the Kamchatka (Red King) Crab in the Barents Sea: Peculiarities of Biology, Perspectives of Fishery; KSC RAS Press: Apatity, Russia, 2002. (In Russian) [Google Scholar]
- Sundet, J.H.; Bakanev, S. Snow Crab (Chionoecetes opilio)—A New Invasive Crab Species Becoming an Important Player in the Barents Sea Ecosystem. 2014. Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/CM-2014/Theme%20Session%20F%20contributions/F0414.pdf (accessed on 8 January 2024).
- Dvoretsky, A.G.; Dvoretsky, V.G. Aquaculture of green sea urchin in the Barents Sea: A brief review of Russian studies. Rev. Aquac. 2020, 12, 1280–1290. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Cucumaria in Russian waters of the Barents Sea: Biological aspects and aquaculture potential. Front. Mar. Sci. 2021, 8, 613453. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Zuyev, Y.A.; Dvoretsky, A.G. Shallow-water benthic communities on soft bottoms of a sub-Arctic fjord (Southern Barents Sea, Russia) along a gradient of ecological factors. Diversity 2023, 15, 84. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Dvoretsky, A.G.; Frolov, A.A.; Zimina, O.L.; Evseeva, O.Y.; Dikaeva, D.R.; Rumyantseva, Z.Y.; Panteleeva, N.N. The impact of sea ice loss on benthic communities of the Makarov Strait (northeastern Barents Sea). Animals 2023, 13, 2320. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, S.G.; Denisenko, N.V. Experiment on cultivation of the Iceland scallop Chlamys islandica (Muller) and possibilities of its industrial reproduction. In Modern Technologies and Forecasting in the Polar Oceanography and Biology; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 1999; pp. 157–165. (In Russian) [Google Scholar]
- Zolotarev, P.N. Biology and Fishery of the Icelandic Scallop Chlamys Islandica in the Barents and White Seas; PINRO: Murmansk, Russia, 2016. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Shellfish as biosensors in online monitoring of aquatic ecosystems: A review of Russian studies. Fishes 2023, 8, 102. [Google Scholar] [CrossRef]
- Coleman, S.; Morse, D.; Brayden, W.C.; Brady, D.C. Developing a bioeconomic framework for scallop culture optimization and product development. Aquac. Econ. Manag. 2023, 27, 25–49. [Google Scholar] [CrossRef]
- Subra-Paternault, P.; ThongDeng, H.; Grélard, A.; Cansell, M. Extraction of phospholipids from scallop by-product using supercritical CO2/alcohol mixtures. LWT 2015, 60, 990–998. [Google Scholar] [CrossRef]
- Duncan, P.F.; Brand, A.R.; Strand, Ø.; Foucher, E. The European Scallop Fisheries for Pecten maximus, Aequipecten opercularis, Chlamys islandica, and Mimachlamys varia. In Scallops Biology, Ecology, Aquaculture, and Fisheries; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 781–858. [Google Scholar]
- Eiríksson, H. The Molluscan Fisheries of Iceland. In The History, Present Condition, and Future of the Molluscan Fisheries of North America and Europe; MacKenzie, C.L., Jr., Burrell, V., Rosenfield, A., Hobart, W.L., Eds.; NOAA Technical Report NMFS 129; US Department of Commerce: Washington, DC, USA, 1997; pp. 39–47. [Google Scholar]
- Denisenko, S.G. Ecology and Resources of the Iceland Scallop in the Barents Sea; KSC RAS Press: Apatity, Russia, 1989; p. 140. (In Russian) [Google Scholar]
- Rzhavsky, A.V.; Buyanovsky, A.I.; Britaev, T.A. Biology and spatial-temporal organization of the Iceland Scallop (Chlamys islandica) populations in fjords of the Eastern Murman (the Barents Sea). Adv. Curr. Biol. 2010, 130, 63–79. [Google Scholar]
- Tastesen, H.S.; Keenan, A.H.; Madsen, L.; Kristiansen, K.; Liaset, B. Scallop protein with endogenous high taurine and glycine content prevents high-fat, high-sucrose-induced obesity and improves plasma lipid profile in male C57BL/6J mice. Amino Acids 2014, 46, 1659–1671. [Google Scholar] [CrossRef]
- Tastesen, H.S.; Rønnevik, A.K.; Borkowski, K.; Madsen, L.; Kristiansen, K.; Liaset, B. A mixture of cod and scallop protein reduces adiposity and improves glucose tolerance in high-fat fed male C57BL/6J mice. PLoS ONE 2014, 9, e112859. [Google Scholar] [CrossRef]
- Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental drivers of an intertidal bryozoan community in the Barents Sea: A case study. Animals 2022, 12, 552. [Google Scholar] [CrossRef]
- Beninger, P.G.; Le Pennec, M. Scallop Structure and Function. In Scallops Biology, Ecology, Aquaculture, and Fisheries; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–160. [Google Scholar]
- Beltrán-Lugo, A.I.; Maeda-Martínez, A.N.; Pacheco-Aguilar, R.; Nolasco-Soria, H.G. Seasonal variations in chemical, physical, textural, and microstructural properties of adductor muscles of Pacific lions-paw scallop (Nodipecten subnodosus). Aquaculture 2006, 258, 619–632. [Google Scholar] [CrossRef]
- Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Rehbein, H.; Schröder, U. Meat composition and quality assessment of king scallops (Pecten maximus) and Frozen Atlantic sea scallops (Placopecten magellanicus) on a retail level. Foods 2015, 4, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Vural, P.; Acarlı, S. Monthly variations of protein and amino acid composition of the smooth scallop Flexopecten glaber (Linnaeus 1758) in the Çardak Lagoon (Lapseki-Çanakkale). Cah. Biol. Mar. 2021, 62, 195–204. [Google Scholar]
- Xu, Q.; Gao, F.; Wang, H.; Yang, H. Quality indices as potential markers indicating the origin of cultured scallop (Argopecten irradians) in the north china sea. J. Shellfish Res. 2015, 34, 743–750. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, Y.Y.; Zheng, J.; Chen, J.N.; Huang, X.H.; Dong, X.P.; Zhu, B.W.; Qin, L. Seasonal variations in free amino acid, 5′-nucleotide, and lipid profiles of scallop (Patinopecten yessoensis) revealed by targeted and untargeted metabolomic approaches. LWT 2022, 154, 112881. [Google Scholar] [CrossRef]
- Veske, E.; Çankiriligil, E.C.; Yavuzcan, H. Seasonal proximate composition, amino acid and trace metal contents of the great Mediterranean scallop (Pecten jacobaeus) collected from the Gulf of Antalya. J. Anatol. Environ. Anim. Sci. 2016, 7, 358–366. [Google Scholar] [CrossRef]
- Song, D.; Peng, J.; Zhao, X.; Wu, H.; Zheng, G.; Zhao, Y.; Jiang, Y.; Sheng, X.; Guo, M.; Tan, Z. Quality and safety profiles of Chlamys farreri cultured in the Shandong peninsula: Analysis of nutritional content, flavor, and hazards. J. Food Compos. Anal. 2023, 118, 105193. [Google Scholar] [CrossRef]
- Tan, K.S.; Leng, X.; Zhao, Y.; Hongxing, L.; Cheng, D.; Ma, H.; Li, S.; Zheng, H. Amino acid variations in polymorphic noble scallops, Chlamys nobilis. J. Food Process. Preserv. 2019, 43, e14262. [Google Scholar] [CrossRef]
- Han, J.R.; Tang, Y.; Li, Y.; Shang, W.H.; Yan, J.N.; Du, Y.N.; We, H.T.; Zhu, B.W.; Xiong, Y.L. Physiochemical properties and functional characteristics of protein isolates from the scallop (Patinopecten yessoensis) gonad. J. Food Sci. 2019, 84, 1023–1034. [Google Scholar] [CrossRef]
- Jancso, A.; Szent-Gyorgyi, A.G. Regulation of scallop myosin by the regulatory light chain depends on a single glycine residue. Proc. Natl. Acad. Sci. USA 1994, 91, 8762–8766. [Google Scholar] [CrossRef]
- Chantler, P.D. Scallop Adductor Muscles: Structure and Function. In Scallops Biology, Ecology, Aquaculture, and Fisheries; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 161–218. [Google Scholar]
- Tremblay, I.; Guderley, H.E. Scallops show that muscle metabolic capacities reflect locomotor style and morphology. Physiol. Biochem. Zool. 2014, 87, 231–244. [Google Scholar] [CrossRef]
- Enomoto, T.; Nakao, C.; Ohyama, H. Regulation of glycolysis during acclimation of scallops (Patinopecten yessoensis Jay) to anaerobiosis. Comp. Biochem. Physiol. B 2000, 127, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bubel, A. An electron microscopic study of the periostracum formation in some marine bivalves. I. The origin of the periostracum. Mar. Biol. 1973, 20, 213–221. [Google Scholar] [CrossRef]
- Bubel, A. An electron microscopic study of the periostracum formation in some marine bivalves. II. The cell lining in the periostracal groove. Mar. Biol. 1973, 20, 222–234. [Google Scholar] [CrossRef]
- Wilkens, L.A. Neurobiology and behaviour of the scallop. In Scallops: Biology, Ecology and Aquaculture; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 35, pp. 317–356. [Google Scholar]
- De Zwaan, A.; Thompson, R.J.; Livingstone, D.R. Physiological and biochemical aspects of the valve snap and valve closure responses in the giant scallop Placopecten magellanicus. J. Comp. Physiol. 1980, 137, 105–114. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization Technical Report Series 935; United Nations University: Geneva, Switzerland, 2007.
- Meyer, R.O.; Johnson, D.J.; Otwell, W.S.; Walker, W.R. Potential Utilization of Scallop Viscera for Solid Waste Management and as Feedstuff for Swine; Technical Paper No 48; Florida Sea Grant College: Gainesville, FL, USA, 1987. [Google Scholar]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Ri, S.X.; Hideyuki, K.; Koretaro, T. Characterization of molecular species of collagen in scallop mantle. Food Chem. 2007, 102, 1187–1191. [Google Scholar]
- Guo, Y.; Wen, S.; Ni, C.; Ou, X.; Qu, T.; Wu, Y.; He, S.W.; Li, H.H.; Cui, B.; Cheng, Y.H.; et al. Analysis of protein extraction and antioxidant activity of enzymatic hydrolysates from scallop processing by-products. Food Mach. 2022, 38, 176–183. [Google Scholar]
- Telahigue, K.; Hajji, T.; Rabeh, I. The Effect of starvation on the biochemical composition of the digestive gland, the gonads and the adductor muscle of the scallop Flexopecten glaber. Food Nutr. Sci. 2013, 4, 29748. [Google Scholar]
- Carroll, J.M.; Peterson, B.J. Ecological trade-offs in seascape ecology: Bay scallop survival and growth across a seagrass seascape. Landsc. Ecol. 2013, 28, 1401–1413. [Google Scholar] [CrossRef]
- Gerasimova, O.V.; Manushin, I.E. Some features of the diet and digestion of the iceland scallop Chlamys islandica in the south-eastern Barents Sea. In Studies of Commercial Invertebrates in the Barents Sea; Barenboim, B.I., Ed.; PINRO: Murmansk, Russia, 1997; pp. 65–71. [Google Scholar]
- Debashish, G.; Malay, S.; Barindra, S.; Joydeep, M. Marine Enzymes. In Marine Biotechnology I; Ulber, R., Le Gal, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 96, pp. 189–218. [Google Scholar]
- Hao, Z.; Yang, L.; Zhan, Y.; Tian, Y.; Ding, J.; Pang, Y.; Chang, Y. Biochemical components of different colored strains of cultured japanese scallop (Mizuhopecten yessoensis) under different cultivation systems. Isr. J. Aquac.-Bamidgeh 2015, 67, 1189. [Google Scholar] [CrossRef]


| Parameter | Male | Female | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | X | SE | Min | Max | X | SE | Min | Max | X | SE | |
| SL | 76.5 | 105.7 | 90.1 | 3.8 | 72.2 | 96.0 | 85.4 | 3.0 | 72.2 | 105.7 | 87.6 | 2.4 |
| TW | 83.0 | 158.0 | 119.4 | 11.7 | 55.0 | 149.0 | 98.7 | 12.2 | 55.0 | 158.0 | 108.3 | 8.7 |
| MW | 2.0 | 6.7 | 4.9 | 0.7 | 1.2 | 23.9 | 5.9 | 3.0 | 1.2 | 23.9 | 5.4 | 1.6 |
| GW | 6.0 | 21.0 | 14.0 | 2.5 | 7.3 | 14.6 | 11.8 | 1.0 | 6.0 | 21.0 | 12.8 | 1.3 |
| MW | 6.3 | 17.0 | 11.1 | 1.5 | 4.2 | 9.8 | 7.4 | 0.9 | 4.2 | 17.0 | 9.1 | 1.0 |
| GI | 2.1 | 5.7 | 4.1 | 0.5 | 2.2 | 16.0 | 4.9 | 1.9 | 2.1 | 16.0 | 4.5 | 1.0 |
| MI | 6.5 | 14.6 | 11.3 | 1.2 | 8.5 | 14.3 | 12.4 | 0.8 | 6.5 | 14.6 | 11.8 | 0.7 |
| MNI | 7.6 | 10.8 | 9.2 | 0.6 | 6.5 | 8.3 | 7.5 | 0.2 | 6.5 | 10.8 | 8.3 | 0.4 |
| Amino Acid | Muscle | Gonad | Mantle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | X | SE | Min | Max | X | SE | Min | Max | X | SE | |
| Aspartic acid | 1.4 | 20.9 | 9.6 | 2.1 | 1.4 | 13.1 | 5.7 | 1.2 | 1.2 | 12.3 | 6.5 | 1.2 |
| Glutamic acid | 2.0 | 4.8 | 3.6 | 0.2 | 1.6 | 4.6 | 3.3 | 0.3 | 1.7 | 4.3 | 3.1 | 0.2 |
| Hydroxyproline | 0.3 | 0.9 | 0.6 | 0.1 | 0.3 | 1.1 | 0.7 | 0.1 | 0.3 | 3.7 | 0.8 | 0.2 |
| Serine | 1.1 | 6.8 | 3.5 | 0.6 | 1.3 | 7.7 | 3.6 | 0.6 | 1.4 | 18.5 | 4.6 | 1.2 |
| Glycine | 1.4 | 24.7 | 11.8 | 2.5 | 0.7 | 21.6 | 11.5 | 2.2 | 1.4 | 17.5 | 9.6 | 1.6 |
| Histidine | 0.8 | 15.1 | 4.4 | 1.2 | 0.7 | 10.6 | 2.8 | 0.7 | 1.2 | 8.7 | 3.2 | 0.6 |
| Arginine | 5.2 | 21.6 | 11.2 | 1.1 | 2.0 | 16.7 | 8.3 | 1.3 | 3.3 | 8.6 | 5.8 | 0.4 |
| Threonine | 1.1 | 16.3 | 6.0 | 1.3 | 1.1 | 9.4 | 4.6 | 0.7 | 1.4 | 9.8 | 4.7 | 0.8 |
| Alanine | 2.3 | 11.7 | 5.6 | 0.9 | 0.7 | 9.6 | 4.1 | 0.8 | 1.3 | 7.4 | 3.2 | 0.5 |
| Proline | 0.3 | 10.4 | 3.2 | 0.9 | 0.3 | 26.4 | 6.7 | 2.1 | 0.4 | 20.0 | 5.8 | 1.4 |
| Tyrosine | 1.3 | 8.3 | 4.0 | 0.5 | 1.6 | 7.9 | 3.9 | 0.6 | 1.1 | 5.4 | 2.9 | 0.4 |
| Valine | 1.2 | 34.4 | 9.5 | 2.8 | 1.1 | 19.6 | 6.7 | 1.8 | 1.5 | 18.5 | 5.8 | 1.5 |
| Isoleucine | 1.0 | 13.7 | 4.6 | 1.0 | 0.8 | 6.8 | 3.5 | 0.6 | 1.3 | 7.9 | 3.8 | 0.6 |
| Leucine | 1.8 | 9.8 | 5.9 | 0.8 | 1.0 | 9.0 | 4.3 | 0.7 | 1.2 | 6.9 | 3.3 | 0.5 |
| Phenylalanine | 0.4 | 10.7 | 3.7 | 0.9 | 0.4 | 7.4 | 2.3 | 0.6 | 0.6 | 4.9 | 2.5 | 0.4 |
| Lysine | 0.6 | 13.6 | 3.6 | 1.3 | 0.5 | 1.9 | 1.2 | 0.1 | 0.6 | 9.5 | 2.8 | 0.8 |
| Amino Acid | Av.Diss | Diss/SD | Contrib% | Cum.% |
|---|---|---|---|---|
| Muscle vs. Gonad, Dissimilarity 40.79% | ||||
| Glycine | 6.06 | 1.35 | 14.87 | 14.87 |
| Valine | 5.44 | 0.87 | 13.35 | 28.22 |
| Asparagine | 4.33 | 1.37 | 10.62 | 38.84 |
| Proline | 3.52 | 0.88 | 8.64 | 47.48 |
| Arginine | 3.45 | 1.19 | 8.46 | 55.94 |
| Muscle vs. Mantle, Dissimilarity 41.10% | ||||
| Glycine | 5.59 | 1.5 | 13.61 | 13.61 |
| Valine | 5.44 | 0.88 | 13.24 | 26.86 |
| Asparagine | 4.43 | 1.45 | 10.78 | 37.63 |
| Arginine | 3.67 | 1.52 | 8.92 | 46.55 |
| Proline | 2.92 | 1.14 | 7.1 | 53.66 |
| Gonad vs. Mantle, Dissimilarity 37.33% | ||||
| Glycine | 5.9 | 1.43 | 15.79 | 15.79 |
| Valine | 4.37 | 0.86 | 11.7 | 27.49 |
| Proline | 4.35 | 1.03 | 11.65 | 39.14 |
| Asparagine | 3.3 | 1.32 | 8.84 | 47.98 |
| Arginine | 2.8 | 1.34 | 7.51 | 55.49 |
| Variable | Muscle | Variable | Mantle | ||||
|---|---|---|---|---|---|---|---|
| EV | F | p | EV | F | p | ||
| GI | 19 | 2.64 | 0.009 | T | 26 | 4.71 | 0.002 |
| D | 16 | 2.69 | 0.041 | SL | 15 | 1.99 | 0.109 |
| T | 11 | 1.56 | 0.193 | D | 9 | 1.10 | 0.320 |
| MI | 8 | 1.34 | 0.233 | MNI | 6 | 1.03 | 0.365 |
| MNI | 5 | 0.87 | 0.488 | GI | 5 | 0.92 | 0.428 |
| Sed | 4 | 0.58 | 0.679 | MI | 7 | 1.43 | 0.230 |
| SL | 2 | 0.37 | 0.832 | Sed | 4 | 0.72 | 0.581 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretsky, A.G.; Obluchinskaya, E.D.; Gorshenina, E.V.; Dvoretsky, V.G. Amino Acid Composition in Different Tissues of Iceland Scallop from the Barents Sea. Animals 2024, 14, 230. https://doi.org/10.3390/ani14020230
Dvoretsky AG, Obluchinskaya ED, Gorshenina EV, Dvoretsky VG. Amino Acid Composition in Different Tissues of Iceland Scallop from the Barents Sea. Animals. 2024; 14(2):230. https://doi.org/10.3390/ani14020230
Chicago/Turabian StyleDvoretsky, Alexander G., Ekaterina D. Obluchinskaya, Elena V. Gorshenina, and Vladimir G. Dvoretsky. 2024. "Amino Acid Composition in Different Tissues of Iceland Scallop from the Barents Sea" Animals 14, no. 2: 230. https://doi.org/10.3390/ani14020230
APA StyleDvoretsky, A. G., Obluchinskaya, E. D., Gorshenina, E. V., & Dvoretsky, V. G. (2024). Amino Acid Composition in Different Tissues of Iceland Scallop from the Barents Sea. Animals, 14(2), 230. https://doi.org/10.3390/ani14020230









