Differences in Growth Performance and Meat Quality between Male and Female Juvenile Nile Tilapia (Oreochromis niloticus) during Separate Rearing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Fish
2.3. Sex Determination
2.4. Experimental Design
2.5. Fish Feed
2.6. Sample Collections and Analyses of Growth Parameters
2.7. Sample Collections for Blood Serum Analyses
2.8. Meat Sample Collection
2.9. Analysis of Meat Proximate Composition
2.9.1. Moisture Content
2.9.2. Crude Protein
2.9.3. Crude Fat
2.9.4. Ash Content
2.9.5. Analysis of Amino and Fatty Acids
Amino Acid Analysis
Fatty Acid Analysis
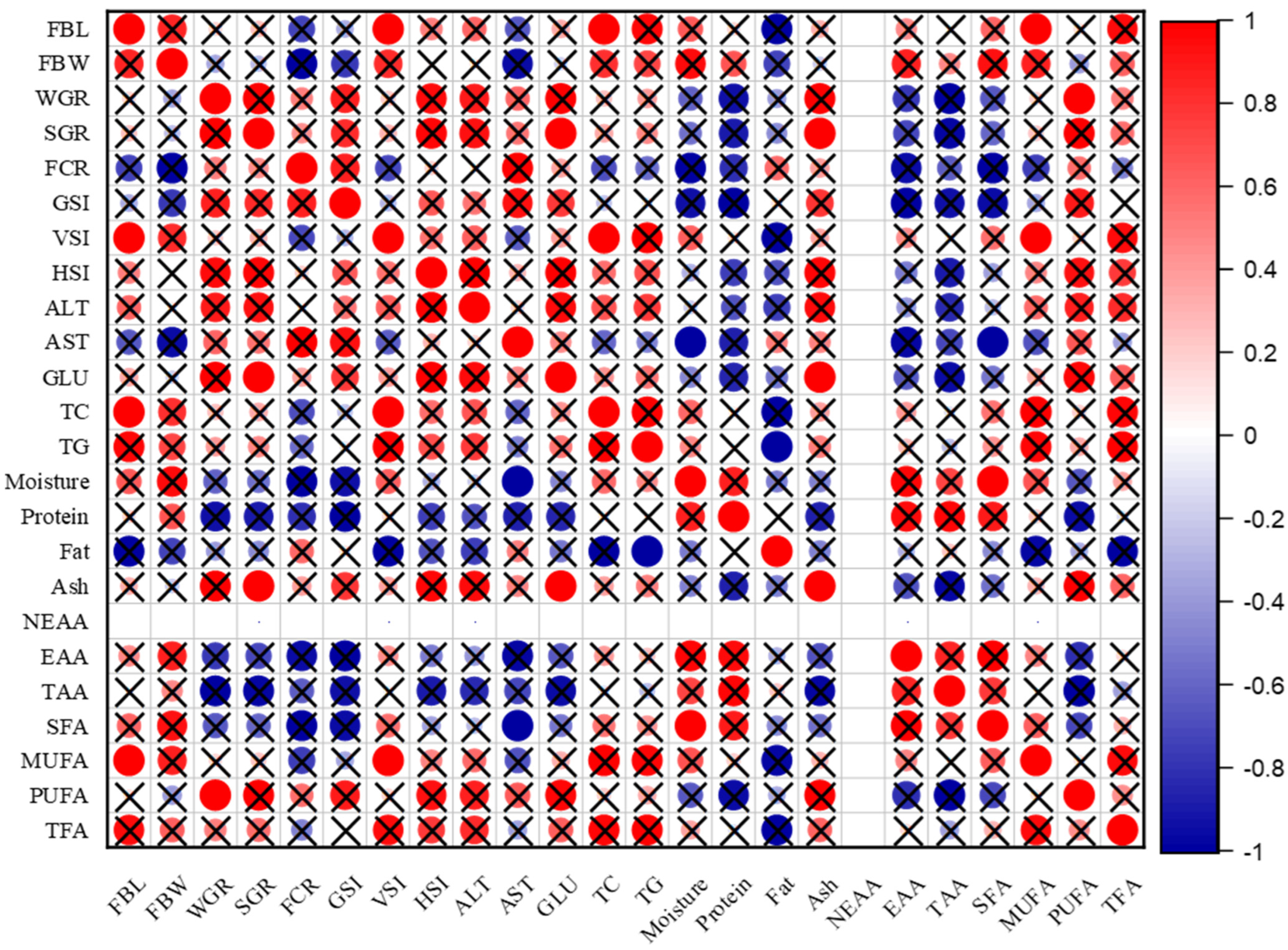
2.10. Correlation Analyses
2.11. Data Analysis
3. Results
3.1. Genetic Sex Determination
3.2. Growth Performance Parameters
3.3. Blood Serum (ALT, AST, GLU, TC, TG)
3.4. Approximate Composition of Meat
3.5. Profile of Amino Acids
3.6. Profile of Fatty Acids
3.7. Correlation Analyses
4. Discussion
4.1. The Growth Performance
4.2. Blood Serum Indices
4.3. Meat Quality (Approximate Composition)
4.3.1. Moisture Content
4.3.2. Crude Protein
4.3.3. Crude Fat
4.3.4. Ash Content
4.4. Profile of Amino Acids
4.5. Profile of Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ackman, R.G. Nutritional composition of fats in seafoods. Prog. Food Nutr. Sci. 1989, 13, 161–289. [Google Scholar] [PubMed]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Masoud, J.A.; Tao, Y.; Li, Y.; Sayouh, M.A.; Lu, S.; Qiang, J.; Xu, P. Modulation of chronic hypoxia on ovarian structure, oxidative stress, and apoptosis in female Nile tilapia (Oreochromis niloticus). Aquaculture 2024, 590, 741081. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia Culture; CAB International: Wallingford, UK, 2006; p. 277. [Google Scholar] [CrossRef]
- Tuan, L.A.; Ha, D.N.; Binh, D.T. Effects of stocking density and rearing systems on growth and flesh quality of Nile tilapia. Fish. Sci. 2020, 86, 803–810. [Google Scholar]
- Maita, M.; Satoh, K.; Fukuda, Y.; Lee, H.K.; Winton, J.R.; Okamoto, N. Correlation between plasma component levels of cultured fish and resistance to bacterial infection. Fish Pathol. 1998, 33, 129–133. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A. Evaluation of fermented date palm seed meal with Aspergillus oryzae on the growth, digestion capacity, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2020, 26, 828–841. [Google Scholar] [CrossRef]
- Abelti, A.L. Minerals content and fatty acids profile of Nile tilapia (Oreochromis niloticus) fillet from Lake Zeway: Effect of endogenous factors. J. Nutr. Food Sci. 2017, 7, 574. [Google Scholar] [CrossRef]
- Mekawy, M.; Abo-State, M.A.; El-Hariry, M. Effect of rearing systems on the quality of Nile tilapia (Oreochromis niloticus) fillets. J. Aquac. Res. Dev. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Gonzalez, D.; Garcia, A. Sexual dimorphism and reproductive strategies in tilapia. Aquaculture 2010, 308, 17–24. [Google Scholar] [CrossRef]
- Herrera, L.C. The Effect of Stocking Density on Growth Rate, Survival and Yield of GIFT Tilapia (Oreochromis niloticus) in Cuba: Case Study Fish Farm La Juventud. United Nations University Fisheries Training Programme, Iceland, 2015. Available online: http://www.unuftp.is/static/fellows/document/lesvia14prf.pdf (accessed on 6 October 2024).
- Kheirallah, M.M.; El-Sayed, A.F.M. Effects of sex on growth and carcass quality of Nile tilapia. Aquac. Res. 2006, 37, 558–566. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A tandem duplicate of anti-Mullerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Makkar, M.; Sahu, N.P.; Sahu, S. Effects of stocking density on growth performance and survival of tilapia (Oreochromis niloticus) in aquaculture. J. Aquac. Res. Dev. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- El-Zaeem, S.Y.; Ahmed, M.M.M.; Salama, M.S.; El-Kader, W.N.A. Flesh quality differentiation of wild and cultured Nile tilapia (Oreochromis niloticus) populations. Afr. J. Biotechnol. 2012, 11, 4085–4089. [Google Scholar]
- Biró, J.; Hancz, C.; Szabó, A.; Molnár, T. Effect of sex on the fillet quality of Nile tilapia fed varying lipid sources. Ital. J. Anim. Sci. 2009, 8, 225–227. [Google Scholar] [CrossRef]
- Doğan, G.; Ertan, Ö.Ö. Determination of amino acid and fatty acid composition of goldband goatfish, Upeneus moluccensis (Bleeker, 1855) from the Gulf of Antalya (Turkey). Int. Aquat. Res. 2017, 9, 313–327. [Google Scholar] [CrossRef]
- Mateen, A.; Ahmed, I. Androgen sex reversal, subsequent growth and meat quality of Nile tilapia (Oreochromis niloticus). Pak. J. Agric. Sci. 2015, 52, 199–202. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- DeLong, D.P.; Losordo, T.M.; Rakocy, J.E. Tank Culture of Tilapia; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2009. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Washington, DC, USA, 1998. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990; p. 117. [Google Scholar]
- Gao, J.; Koshio, S.; Nguyen, B.T.; Wang, W.; Cao, X. Comparative studies on lipid profiles and amino acid composition of wild and cultured Dojo loach Misgurnus anguillicaudatus obtained from southern Japan. Fish. Sci. 2012, 78, 1331–1336. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, S. Simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- El-Greisy, Z.A.; El-Gamal, A.E. Monosex production of tilapia, Oreochromis niloticus using different doses of 17α-methyltestosterone with respect to the degree of sex stability after one year of treatment. Egypt. J. Aquat. Res. 2012, 38, 59–66. [Google Scholar] [CrossRef]
- Schreiber, S.; Focken, U.; Becker, K. Individually reared female Nile tilapia (Oreochromis niloticus) can grow faster than males. J. Appl. Ichthyol. 2007, 14, 43–47. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.; Zhu, J.; Lu, S.; Cao, Z.; Ma, J.; He, J.; Xu, P. Effects of heat stress on follicular development and atresia in Nile tilapia (Oreochromis niloticus) during one reproductive cycle and its potential regulation by autophagy and apoptosis. Aquaculture 2022, 548, 738171. [Google Scholar] [CrossRef]
- Bhatta, S.; Iwai, T.; Miura, T.; Higuchi, M.; Maugars, G.; Miura, C. Differences between male and female growth and sexual maturation in tilapia (Oreochromis mossambicus). Kathmandu Univ. J. Sci. Eng. Technol. 2013, 8, 57–65. [Google Scholar] [CrossRef]
- Osibona, A.O. Comparative study of proximate composition, amino and fatty acids of some economically important fish species in Lagos, Nigeria. Afr. J. Food Sci. 2011, 5, 581–588. [Google Scholar]
- Fleming, I.A. Reproductive strategies of Atlantic salmon: Ecology and evolution. Rev. Fish Biol. Fish. 1996, 6, 379–416. [Google Scholar] [CrossRef]
- Ada, F.B. Ganado-hepato-somatic index of Oreochromis niloticus sub adults exposed to some herbicides. Int. J. Aquac. 2013, 3, 49–54. [Google Scholar] [CrossRef]
- Coutteau, P.; Camara, M.R.; Sorgeloos, P. The effect of different levels and sources of dietary phosphatidylcholine on the growth, survival, stress resistance, and fatty acid composition of postlarval Penaeus vannamei. Aquaculture 1996, 147, 261–273. [Google Scholar] [CrossRef]
- Morais, C.A.R.S.; Santana, T.P.; Santos, C.A.; Passetti, R.A.C.; Melo, J.F.B.; Macedo, F.A.F.; Vieira, J.S.; Teixeira, A.J.C.; Del Vesco, A.P. Effect of slaughter weight on the quality of Nile tilapia fillets. Aquaculture 2020, 520, 734941. [Google Scholar] [CrossRef]
- Heiba, A.A.; Amal, M.Y.; Ebrahimet, A.S.; Reham, M.A.; Medhat, H.H. Biochemical, histopathological, and genetic impacts of River Nile pollutants on the Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish. 2021, 25, 883–899. [Google Scholar] [CrossRef]
- Fagbuaro, O.; Iwalaye, O.A.; Adeyinka, F. Hematological and serum biochemical profile of Nile tilapia, Oreochromis niloticus from Ero Dam in Ikun Ekiti, Ekiti State, Nigeria. Am. J. Res. Commun. 2016, 4, 200–205. [Google Scholar]
- Preena, P.G.; Dharmaratnam, A.; Swaminathan, T.R. Antimicrobial resistance analysis of pathogenic bacteria isolated from freshwater Nile tilapia (Oreochromis niloticus) cultured in Kerala, India. Curr. Microbiol. 2020, 77, 3278–3287. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Amer, H.A.; Sedik, M.F.; Khalafalla, F.A.; El-Ghany, A.H.A. Results of chemical analysis of prawn muscle as influenced by sex variations. Food/Nahrung 1991, 35, 133–138. [Google Scholar] [CrossRef]
- Olopade, O.A.; Taiwo, I.O.; Lamidi, A.A.; Awonaike, O.A. Proximate composition of Nile tilapia (Oreochromis niloticus) (Linnaeus, 1758) and Tilapia hybrid (Red Tilapia) from Oyan Lake, Nigeria. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 19–23. [Google Scholar] [CrossRef]
- Olagunju, A.; Muhammad, A.; Bello, S.; Mohammed, A.; Abdullahi Mohammed, H.; Mahmoud, K.T. Nutrient composition of Tilapia zilli, Hemi-synodontis membranacea, Clupea harengus and Scomber scombrus consumed in Zaria. World J. Life Sci. Med. Res. 2012, 2, 16–19. [Google Scholar]
- Stancheva, M.; Merdzhanova, A.; Petrova-Pavlova, E.; Petrova, D. Heavy metals and proximate composition of Black Sea sprat (Sprattus sprattus) and goby (Neogobius melanostomus). Bulg. J. Agric. Sci. 2013, 19, 35–41. [Google Scholar]
- Alemu, L.A.; Melese, A.Y.; Gulelat, D.H. Effect of endogenous factors on proximate composition of Nile tilapia (Oreochromis niloticus L.) fillet from Lake Zeway. Am. J. Res. Commun. 2013, 1, 405–410. [Google Scholar]
- Cornelia, A.B. Investigation of the Chemical Composition and Nutritional Value of Smoothhound Shark (Mustelus mustelus) Meat. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2012. [Google Scholar]
- Abdel-Tawwab, M.; Ahmad, M.H. Effect of dietary protein regime during the growing period on growth performance, feed utilization and whole-body chemical composition of Nile Tilapia, Oreochromis niloticus (L.). Aquac. Res. 2009, 40, 1532–1537. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Costa, L.A. Influence of fish age and sex on fatty acid composition in Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2017, 6, 74–81. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Standard Methods for the Nutrition and Feeding of Farmed Fish and Shrimp; Argent Laboratories Press: Washington, DC, USA, 1990. [Google Scholar]
- Caponio, F.; Lestingi, A.; Summo, C.; Bilancia, M.T.; Laudadio, V. Chemical characteristics and lipid fraction quality of sardines (Sardina pilchardus W.): Influence of sex and length. J. Appl. Ichthyol. 2004, 20, 530–535. [Google Scholar] [CrossRef]
- Massresha, E.; Mateos, H.; Paul, L.P.; Zewdue, A. Proximate composition and fatty acid content of commercially important fish species from Ethiopian lakes: A review. World J. Food Sci. Technol. 2018, 1, 105–114. [Google Scholar] [CrossRef]
- Rastiannasab, A.; Shiva, A.; Ruhollah, R.; Iman, S. Alternations in the liver enzymatic activity of common carp, Cyprinus carpio in response to parasites, Dactylogyrus spp. and Gyrodactylus spp. J. Parasit. Dis. 2016, 40, 11461149. [Google Scholar] [CrossRef]
- Akpinar, M.A.; Salih, G.; Ali, E.A. A comparative analysis of the fatty acid profiles in the liver and muscles of male and female Salmo trutta macrostigma. Food Chem. 2009, 112, 6–8. [Google Scholar] [CrossRef]
- Sun, F.; Liu, S.; Gao, X.; Jiang, Y.; Perera, D.; Wang, X.; Li, C.; Sun, L.; Zhang, J.; Kaltenboeck, L.; et al. Male-biased genes in catfish as revealed by RNA-Seq analysis of the testis transcriptome. PLoS ONE 2013, 8, e68452. [Google Scholar] [CrossRef]
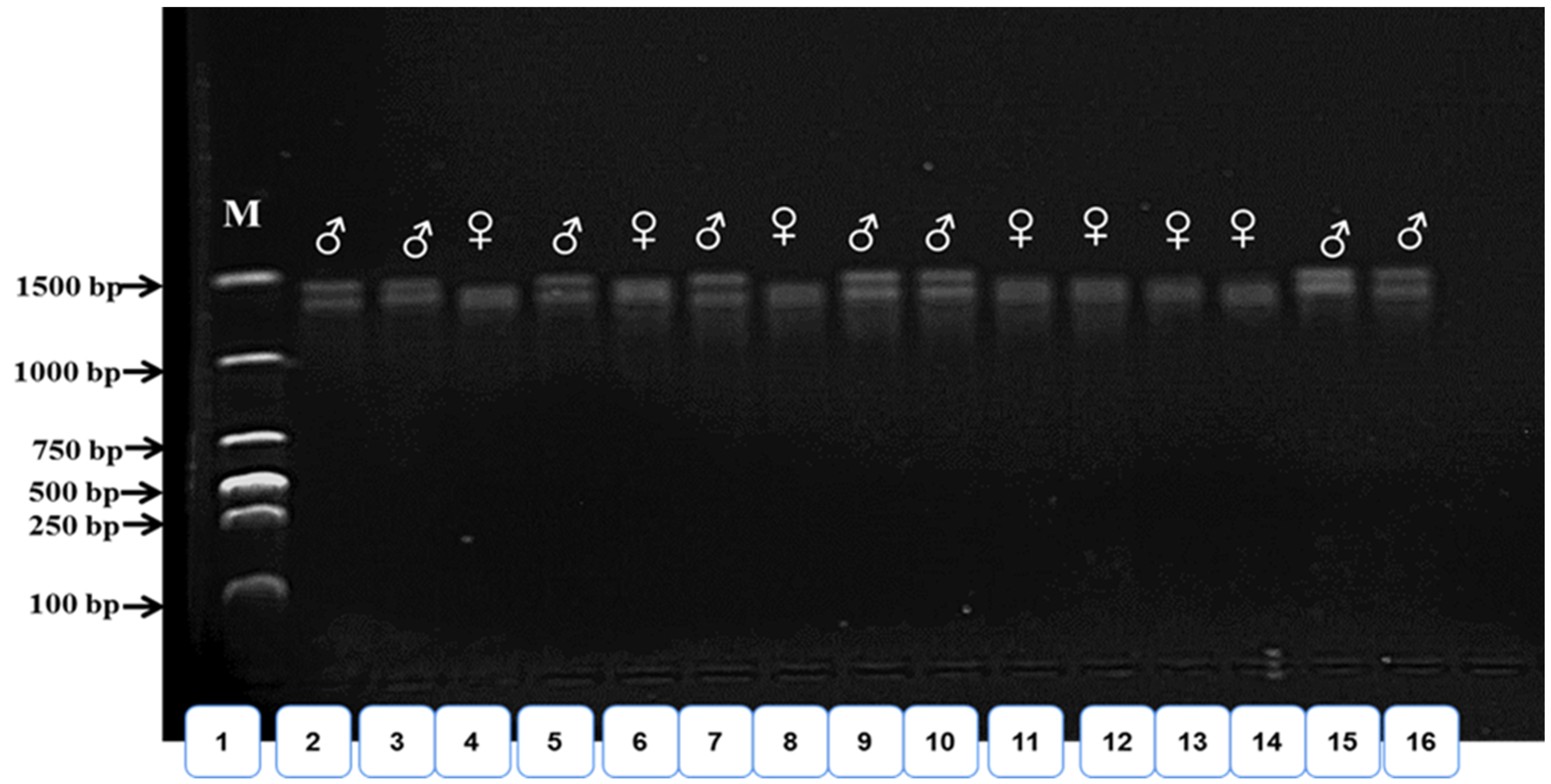

| Parameters | Male | Female |
|---|---|---|
| SR % | 100 ± 0.00 | 100 ± 0.00 |
| FBL (cm) | 17.35 ± 0.14 b | 16.52 ± 0.01 a |
| FBW (g) | 207.35 ± 0.10 b | 173.59 ± 0.01 a |
| WGR % | 88.79 ± 3.95 b | 86.7 ± 0.10 a |
| SGR % | 2.58 ± 1.34 b | 2.37 ± 0.32 a |
| FCR | 1.56 ± 0.57 a | 1.86 ± 1.02 b |
| GSI % | 0.44 ± 0.39 a | 3.35 ± 0.35 b |
| VSI % | 8.14 ± 0.06 a | 11.55 ± 0.31 b |
| HSI % | 1.79 ± 0.03 | 2.18 ± 0.05 |
| Parameters | Male | Female |
|---|---|---|
| ALT (nmol/min/mL) | 47.652 ± 2.462 | 46.688 ± 3.682 |
| AST (nmol/min/mL) | 40.17 ± 2.989 | 40.577 ± 1.417 |
| GLU (mg/mL) | 0.923 ± 0.021 | 0.908 ± 0.004 |
| TC (mmol/L) | 3.207 ± 0.053 | 3.136 ± 0.066 |
| TG (mmol/L) | 1.379 ± 0.107 | 1.377 ± 0.115 |
| Parameters (g/100 g) | Male | Female |
|---|---|---|
| Moisture | 76.90 ± 0.40 | 77 ± 0.05 |
| Protein | 19.76 ± 0.21 | 20.23 ± 0.13 |
| Fat | 1.30 ± 0.17 b | 1 ± 0.10 a |
| Ash | 1.13 ± 0.05 b | 0.76 ± 0.06 a |
| Amino Acids (g/100 g Protein) | Male | Female |
|---|---|---|
| Aspartic acid (Asp) | 1.573 ± 0.024 | 1.686 ± 0.012 |
| Tyrosine (Tyr) | 0.466 ± 0.012 | 0.453 ± 0.008 |
| Serine (Ser) | 0.500 ± 0.000 | 0.510 ± 0.005 |
| Glutamic acid (Glu) | 2.133 ± 0.039 | 2.296 ± 0.008 |
| Arginine (Arg) | 0.983 ± 0.008 | 1.063 ± 0.014 |
| Glycine (Gly) | 0.813 ± 0.008 | 0.893 ± 0.027 |
| Alanine (Ala) | 1.026 ± 0.006 a | 1.097 ± 0.014 b |
| Cystine (Cys) | 0.100 ± 0.005 | 0.108 ± 0.007 |
| Proline (Pro) | 0.566 ± 0.008 | 0.616 ± 0.014 |
| ΣNEAA | 8.163 ± 0.075 a | 8.696 ± 0.075 b |
| Valine (Val) * | 0.889 ± 0.015 | 0.913 ± 0.008 |
| Methionine (Met) * | 0.396 ±0.003 | 0.396 ± 0.014 |
| Isoleucine (IIe) * | 0.783 ± 0.013 | 0.840 ± 0.005 |
| Leucine (Leu) * | 1.370 ± 0.015 | 1.440 ± 0.010 |
| Threonine (Thr) * | 0.720 ± 0.005 | 0.740 ± 0.0100 |
| Phenylalanine (Phe) * | 0.706 ± 0.012 | 0.750 ± 0.010 |
| Lysine (Lys) * | 1.570 ± 0.020 | 1.653 ± 0.008 |
| Histidine (His) * | 0.406 ± 0.006 | 0.443 ± 0.003 |
| ΣEAA | 6.803 ± 0.084 | 7.176 ± 0.038 |
| Total amino acids | 15 ± 0.152 | 15.866 ± 0.120 |
| Fatty Acids (g/100 g Muscle) | Male | Female |
|---|---|---|
| Myristic C14:0 | 0.0159 ± 0.0006 b | 0.009 ± 0.0009 a |
| Palmitic C16:0 | 0.259 ± 0.0106 b | 0.145 ± 0.002 a |
| Stearic C18:0 | 0.107 ± 0.003 b | 0.069 ± 0.0009 a |
| ΣSFAs | 0.381 ± 0.0143 b | 0.224 ± 0.0026 a |
| Palmitoleat C16:1 | 0.0214 ± 0.0014 | 0.0121 ± 0.0013 |
| Cis-11-Eikosanoic, C20:1 | 0.0174 ± 0.0021 | 0.008 ±0.0003 |
| Oleic C18:1n9c | 0.289 ± 0.0272 | 0.147 ± 0.0102 |
| ΣMUFA | 0.327 ± 0.0307 | 0.167 ± 0.0114 |
| Linoleic C18:2n6c | 0.190 ± 0.0103 | 0.117 ±0.009 |
| γ-Linolenic C18:3n6 | 0.011 ± 0.0009 | 0.007 ± 0.0002 |
| Linoleic C18:3n3 | 0.0131 ± 0.0008 | 0.008 ± 0.0007 |
| Cis-11,14-Eicosedienoic C20:2 | 0.0126 ± 0.0007 b | 0.008 ± 0.0008 a |
| Cis-8,11,14-Eicosetrienoic C20:3n6 | 0.023 ± 0.002 b | 0.015 ± 0.0006 a |
| Erucic Acid Methyl Ester, C22:1n9 | 0.008 ± 0.0009 | 0.005 ± 0.0004 |
| Arachidonic C20:4n6 | 0.046 ± 0.0035 | 0.044 ± 0.0035 |
| Docosahexaenoic C22:6n3 | 0.035 ± 0.001 | 0.033 ± 0.003 |
| ΣPUFA | 0.337 ± 0.012 b | 0.237 ± 0.007 a |
| Σn-3 | 0.048 ± 0.002 | 0.041 ± 0.002 |
| Σn-6 | 0.079 ± 0.001 | 0.067 ± 0.004 |
| TFA | 1.05 ± 0.045 b | 0.628 ± 0.015 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayouh, M.; Ali, M.; Li, Y.; Tao, Y.-F.; Lu, S.-Q.; Qiang, J. Differences in Growth Performance and Meat Quality between Male and Female Juvenile Nile Tilapia (Oreochromis niloticus) during Separate Rearing. Animals 2024, 14, 2954. https://doi.org/10.3390/ani14202954
Sayouh M, Ali M, Li Y, Tao Y-F, Lu S-Q, Qiang J. Differences in Growth Performance and Meat Quality between Male and Female Juvenile Nile Tilapia (Oreochromis niloticus) during Separate Rearing. Animals. 2024; 14(20):2954. https://doi.org/10.3390/ani14202954
Chicago/Turabian StyleSayouh, Mohamad, Masoud Ali, Yan Li, Yi-Fan Tao, Si-Qi Lu, and Jun Qiang. 2024. "Differences in Growth Performance and Meat Quality between Male and Female Juvenile Nile Tilapia (Oreochromis niloticus) during Separate Rearing" Animals 14, no. 20: 2954. https://doi.org/10.3390/ani14202954
APA StyleSayouh, M., Ali, M., Li, Y., Tao, Y.-F., Lu, S.-Q., & Qiang, J. (2024). Differences in Growth Performance and Meat Quality between Male and Female Juvenile Nile Tilapia (Oreochromis niloticus) during Separate Rearing. Animals, 14(20), 2954. https://doi.org/10.3390/ani14202954





