Taurine Protects against Silica Nanoparticle-Induced Apoptosis and Inflammatory Response via Inhibition of Oxidative Stress in Porcine Ovarian Granulosa Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Porcine Ovarian Granulosa Cell Isolation and Culture In Vitro
2.2. Silica Nanoparticle Preparation and Cell Treatment
2.3. Determination of SNP Localization
2.4. Cell Viability Assay
2.5. Determination of Lactate Dehydrogenase (LDH) Level
2.6. Measurement of ROS Production
2.7. Determination of Superoxide Dismutase (SOD) and Catalase (CAT) Enzyme Activities
2.8. FCM Analysis
2.9. RNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
2.10. Western Blot
2.11. Statistical Analysis
3. Results
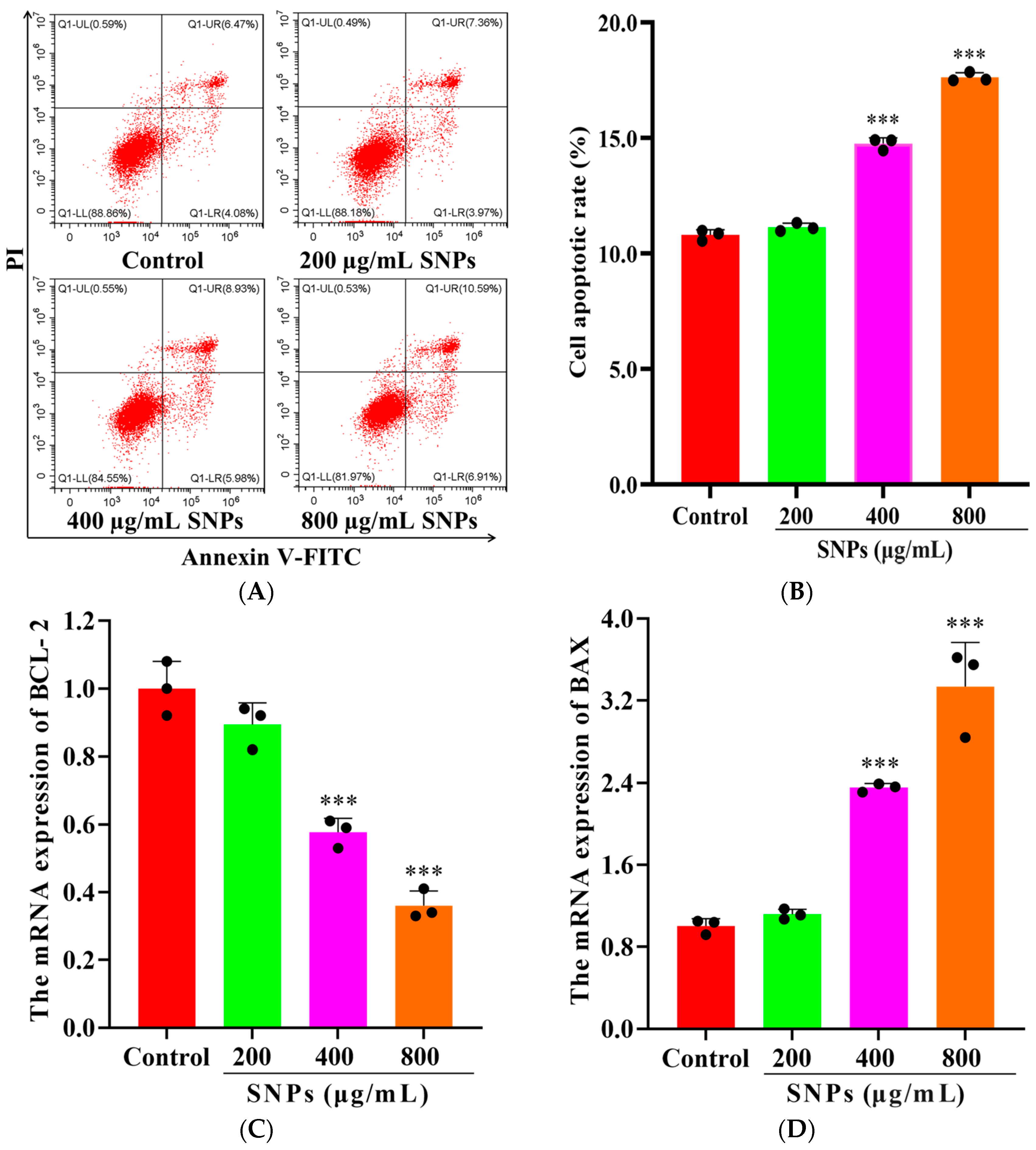
3.1. Effect of SNPs on Cell Viability and Apoptosis
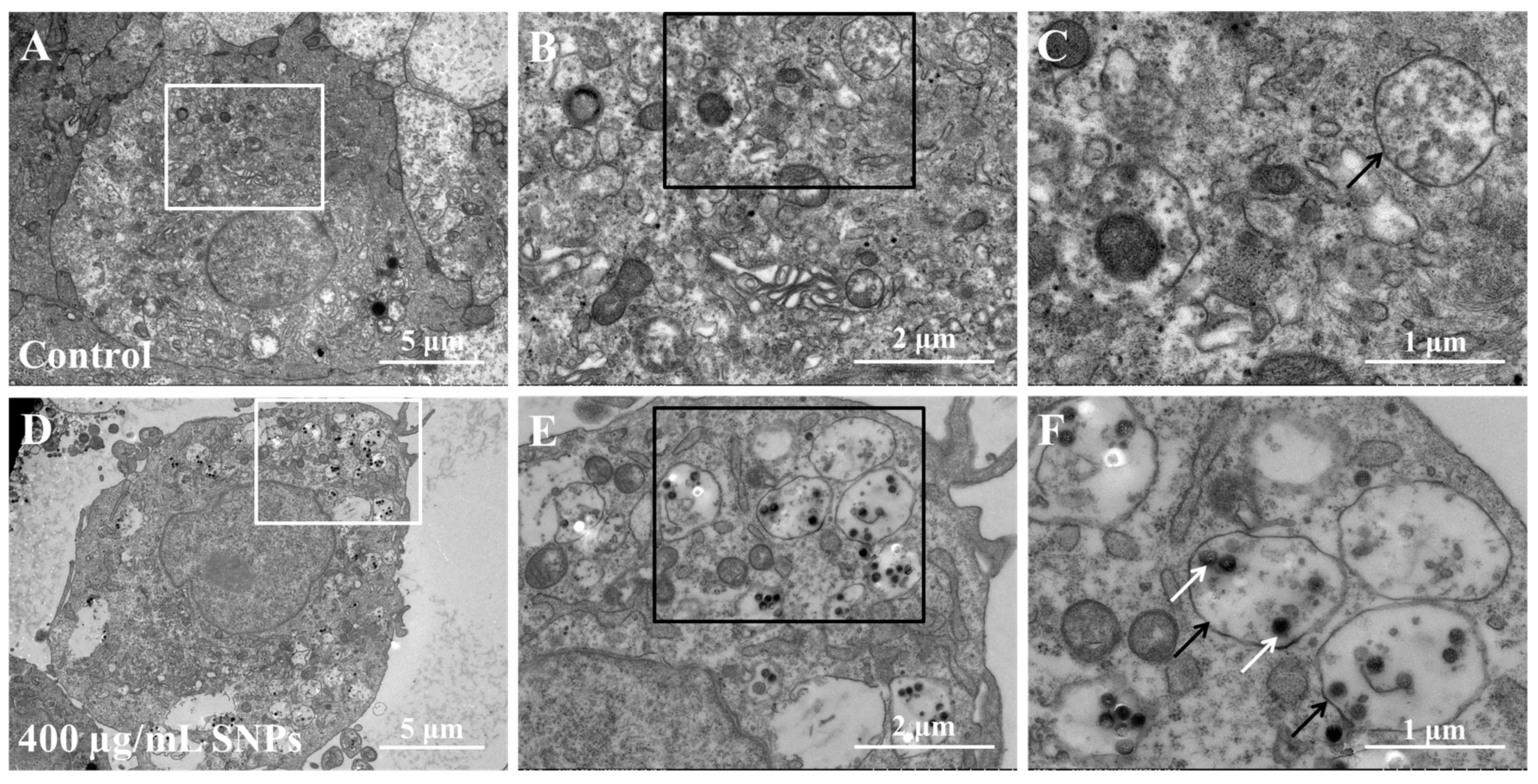
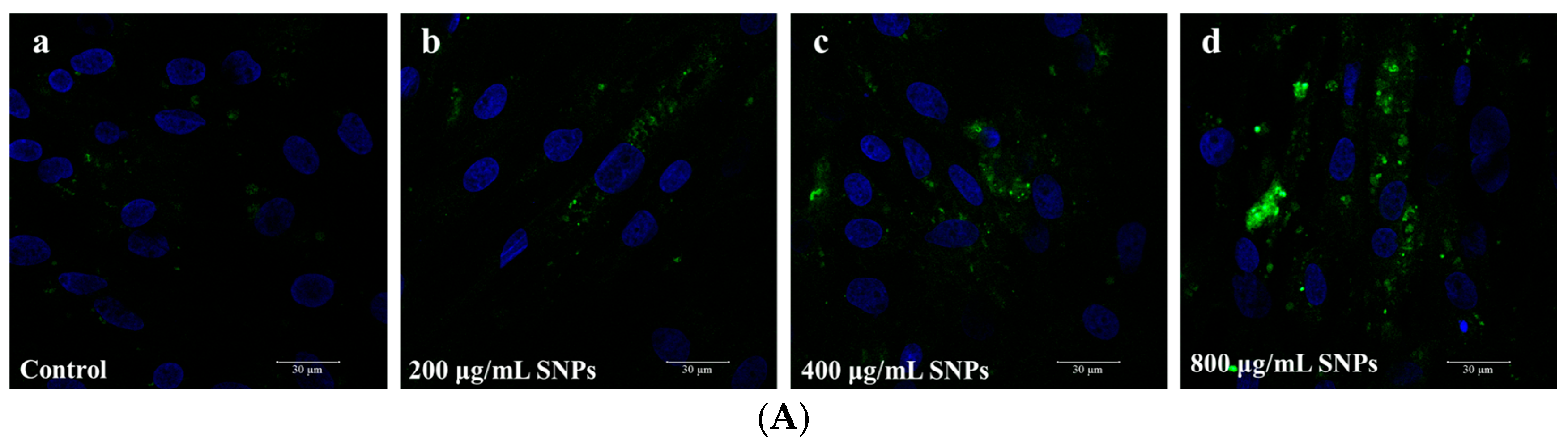
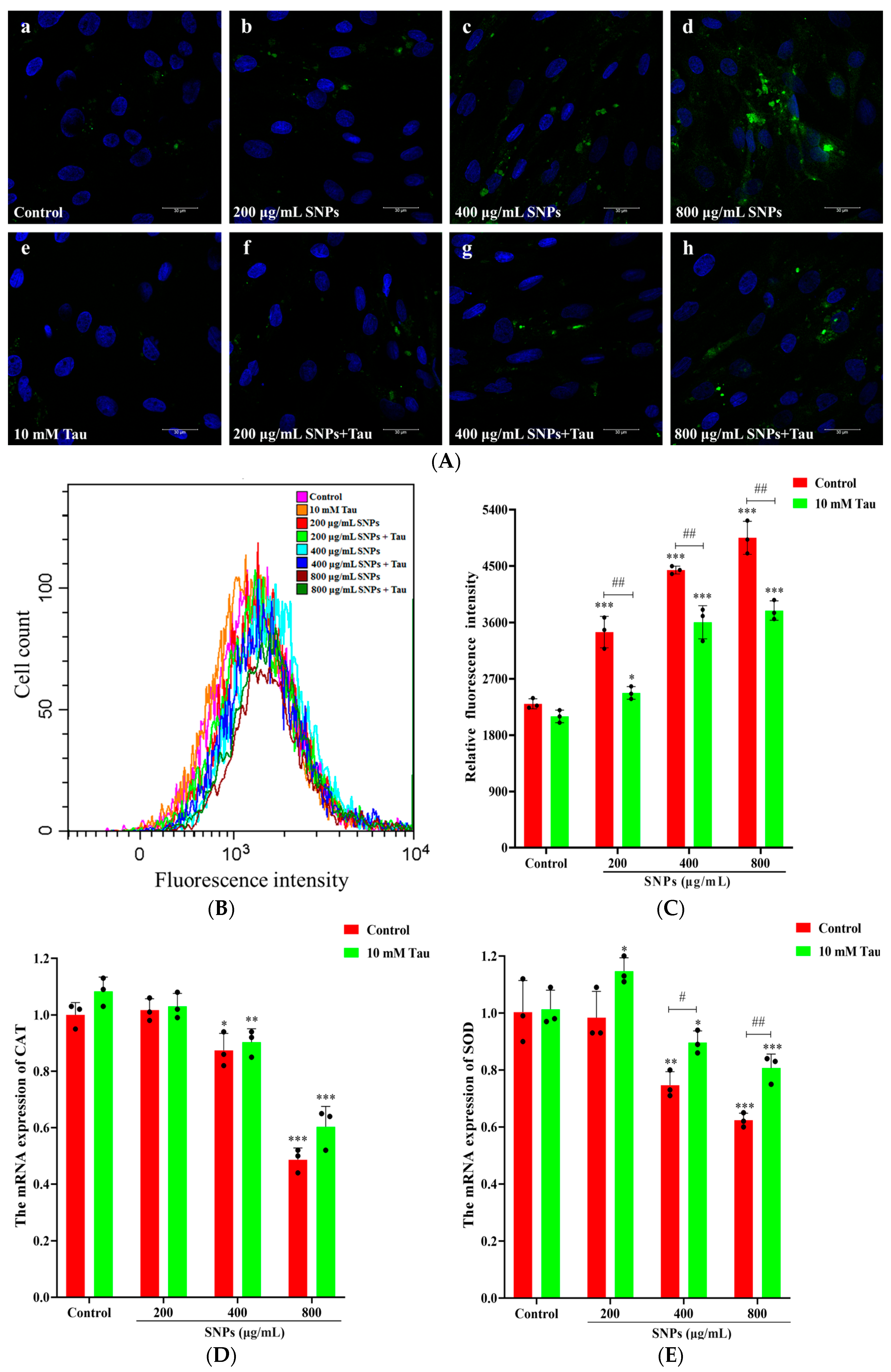
3.2. Cellular Internalization of SNPs in Porcine Ovarian Granulosa Cells
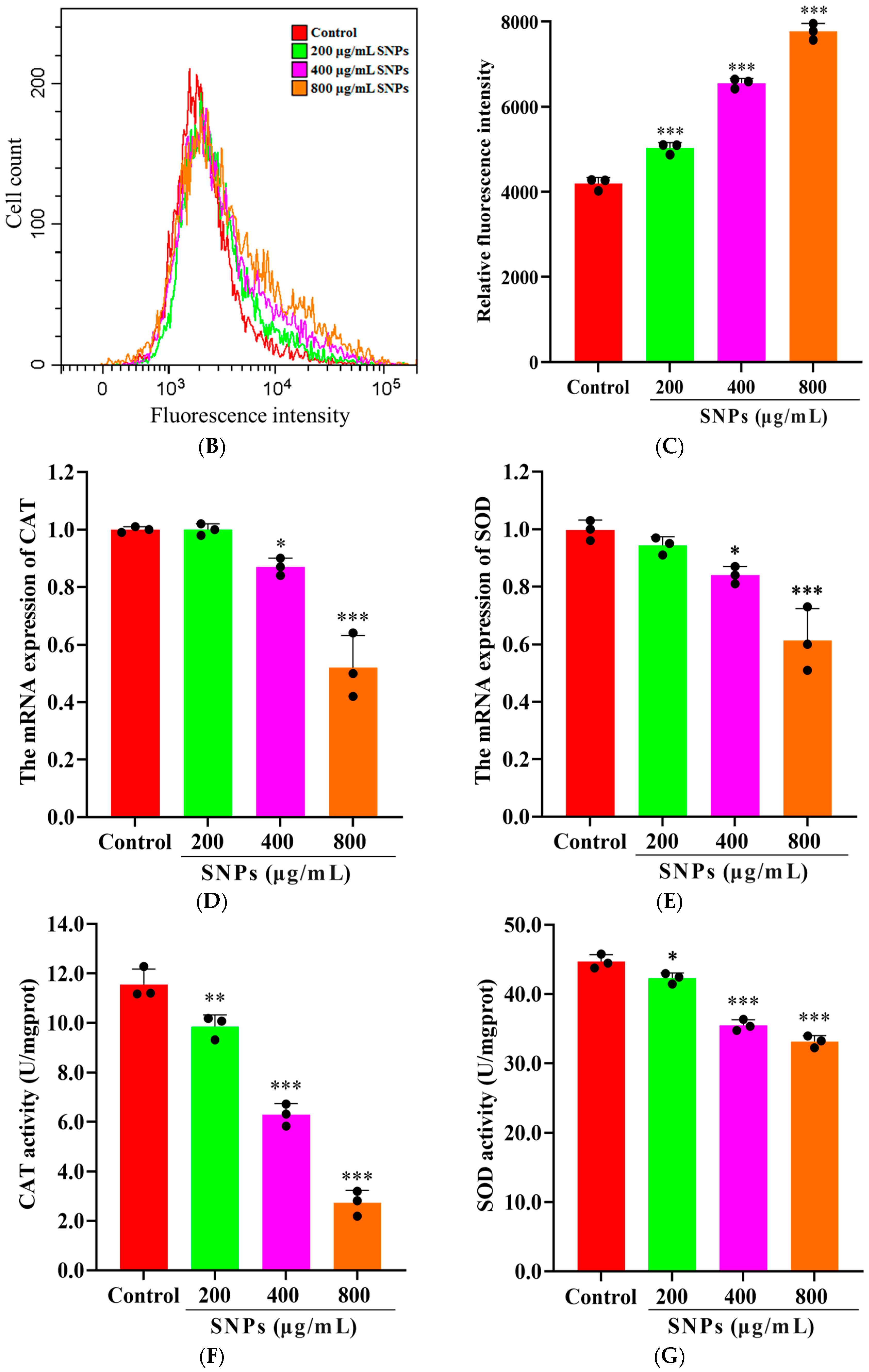
3.3. Effect of SNPs on Oxidative Stress
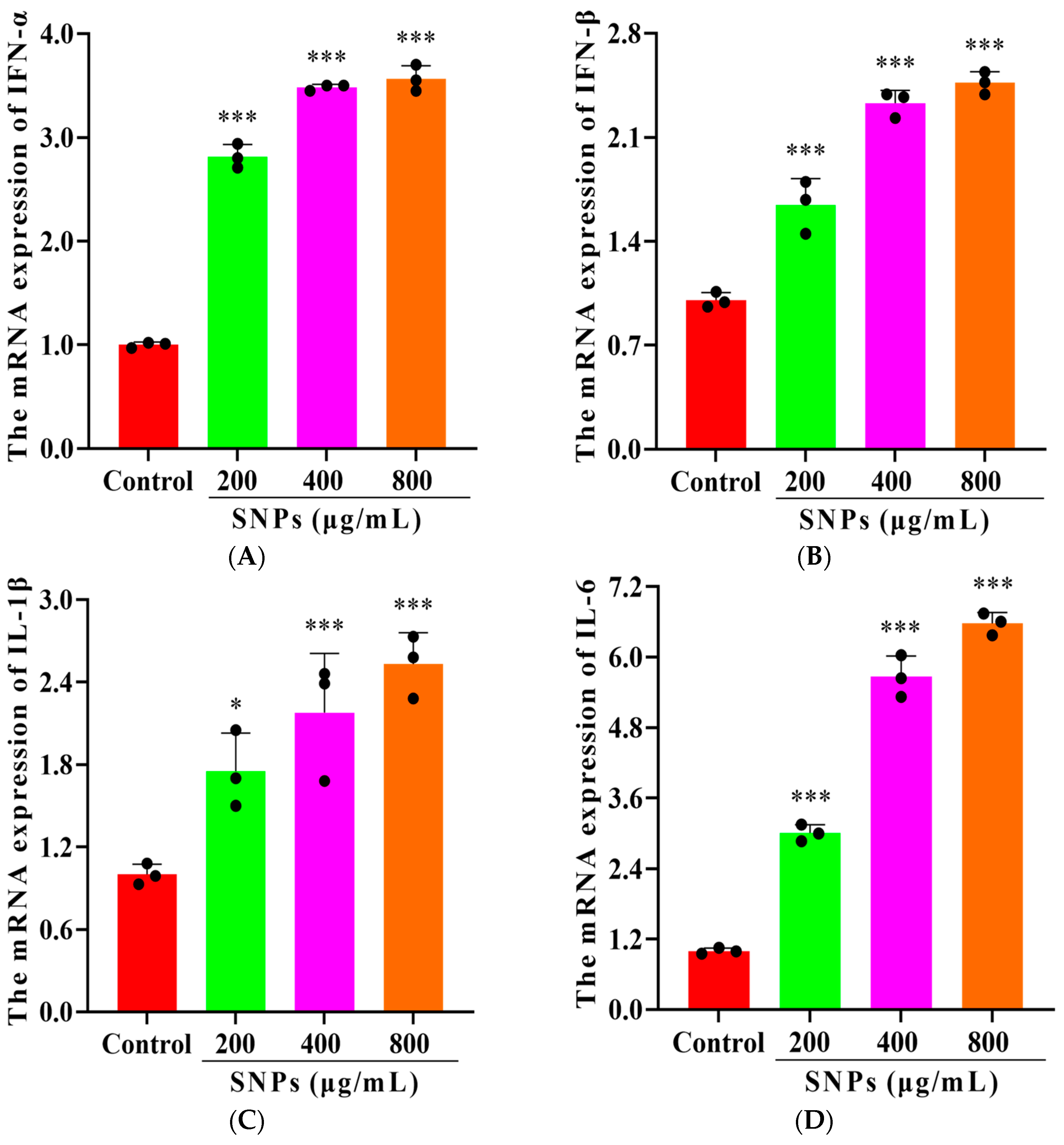
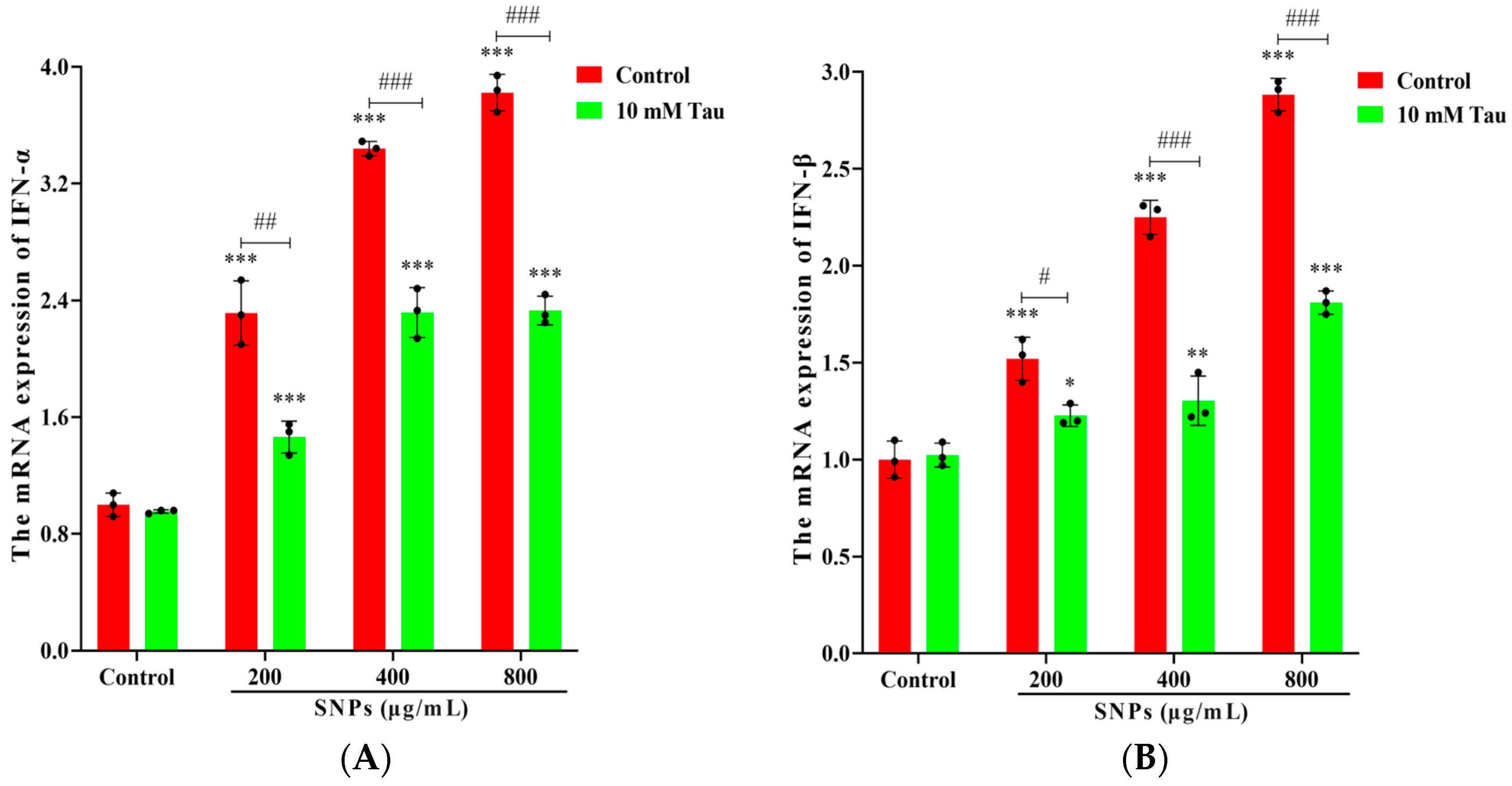
3.4. Effect of SNPs on the Inflammatory Response
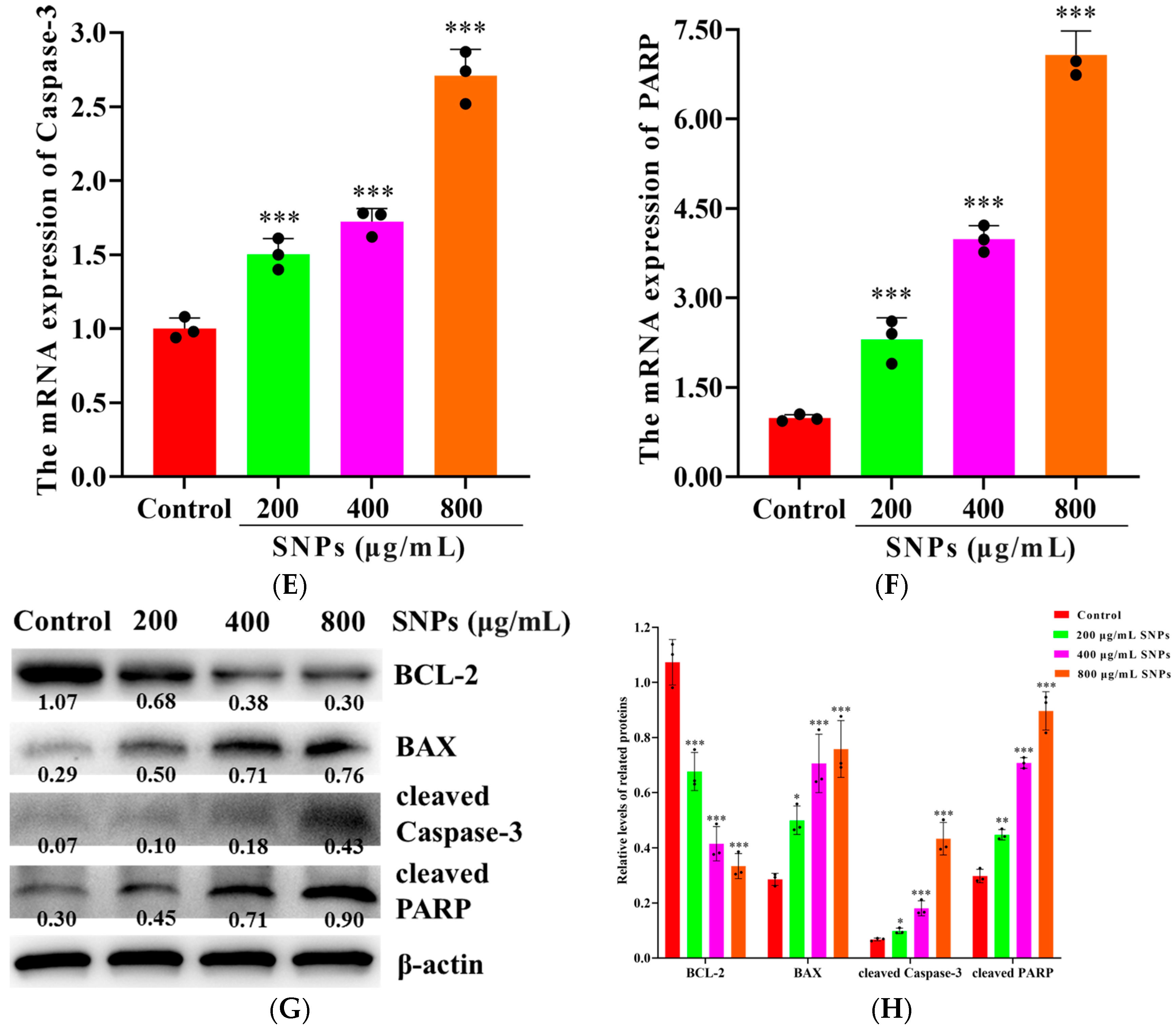
3.5. Effects of SNPs on Cell Apoptosis
3.6. Effects of Tau on SNP-Induced Oxidative Stress
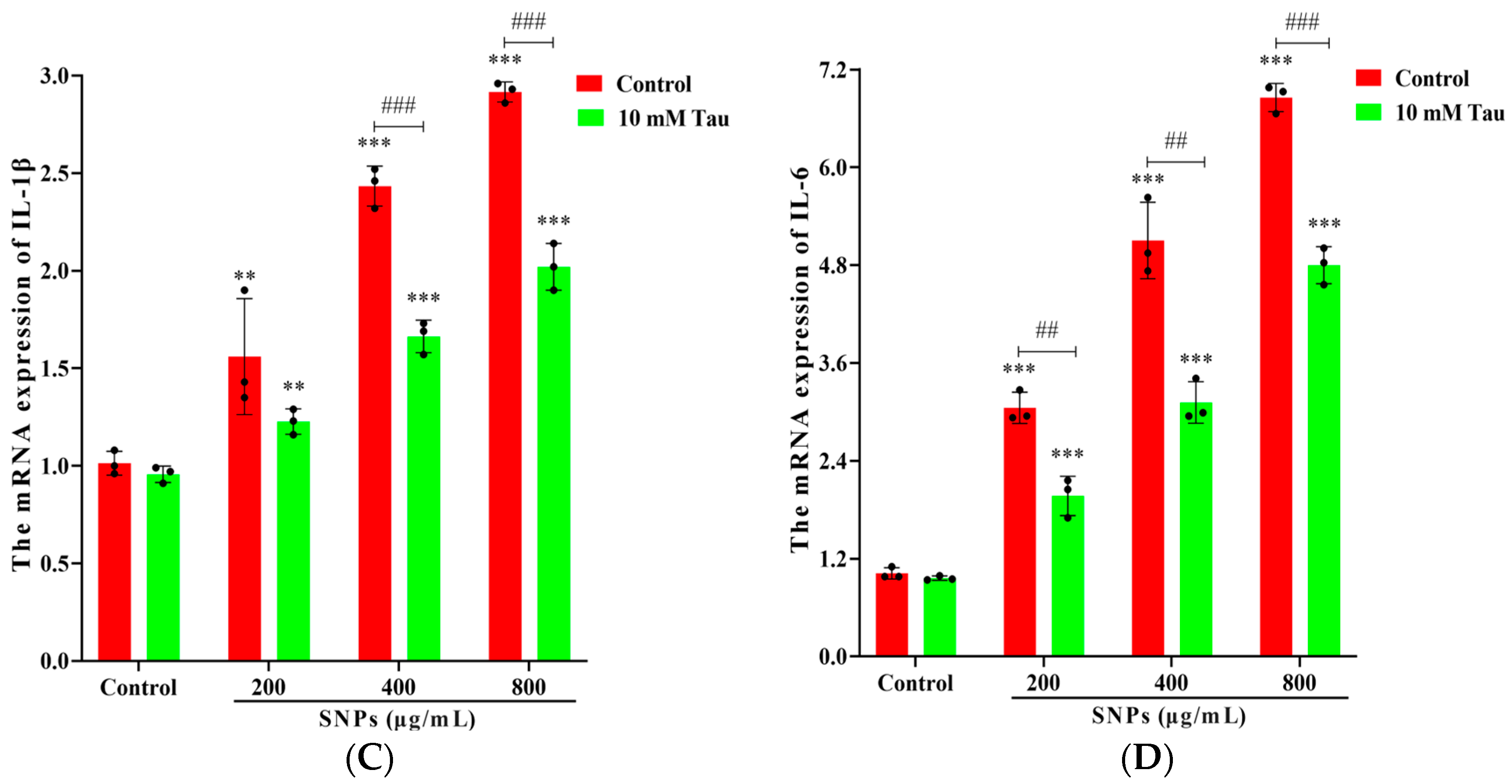
3.7. Effects of Tau on SNP-Induced Inflammatory Response
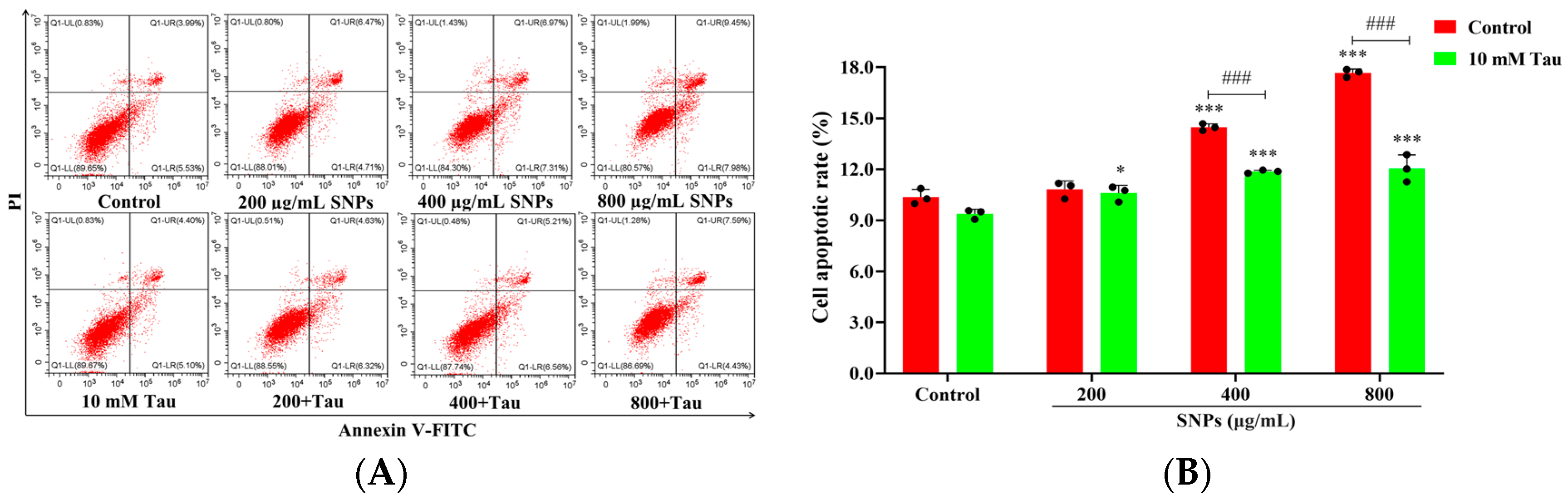
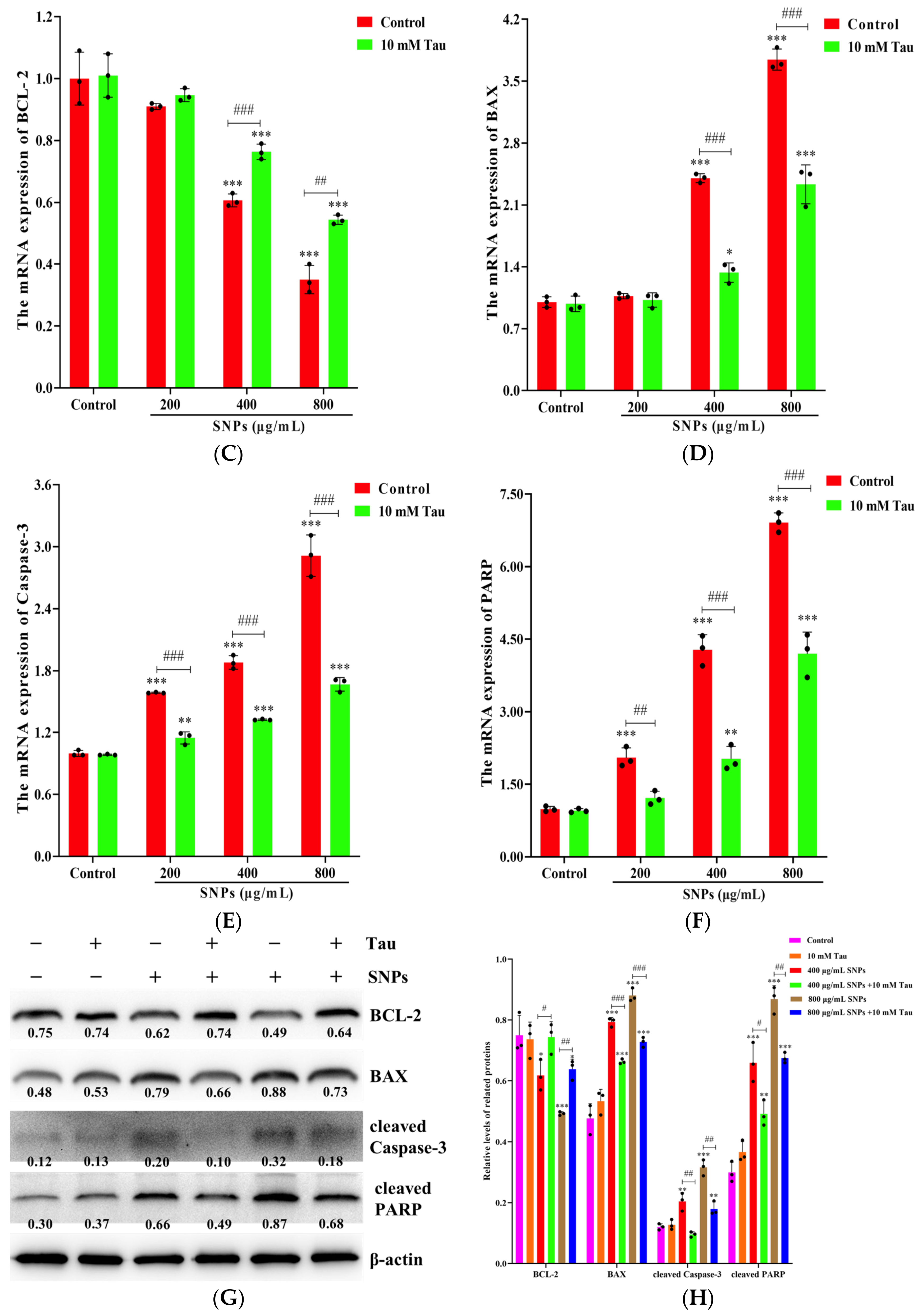
3.8. Effects of Tau on SNP-Induced Cell Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.H.; Zhang, L.; Wang, X.J.; Gu, J.; Song, Z.L.; Wei, S.M.; Guo, H.H.; Xu, L.; Qian, X. Reductions in abundances of intracellular and extracellular antibiotic resistance genes by SiO2 nanoparticles during composting driven by mobile genetic elements. J. Environ. Manag. 2023, 341, 118071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Jin, R.H.; Chen, L.; Dang, M.; Cao, H.; Dong, Y.; Cai, B.L.; Bai, G.; Gooding, J.J.; et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, eabd6740. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; Sanches, T.V.C.; Mechler-Dreibi, M.L.; Almeida, H.M.S.; Storino, G.Y.; Sonalio, K.; Petri, F.A.M.; Martins, T.S.; da Silva, L.C.C.; Montassier, H.J.; et al. Efficacy evaluation of a novel oral silica-based vaccine in inducing mucosal immunity against Mycoplasma hyopneumoniae. Res. Vet. Sci. 2023, 158, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.X.; Chen, Y.; Tao, S.Y.; Du, S.B.; Liang, C.; Teng, Z.D.; Gao, Y. Exploring hollow mesoporous silica nanoparticles as a nanocarrier in the delivery of foot-and-mouth disease virus-like particle vaccines. ACS Appl. Bio Mater. 2024, 7, 1064–1072. [Google Scholar] [CrossRef]
- He, J.; Zhu, T.Y.; Mao, N.N.; Cai, G.F.; Gu, P.F.; Song, Z.C.; Lu, X.Q.; Yang, Y.; Wang, D.Y. Cistanche deserticola polysaccharide-functionalized dendritic fibrous nano-silica as oral vaccine adjuvant delivery enhancing both the mucosal and systemic immunity. Int. J. Biol. Macromol. 2024, 262, 129982. [Google Scholar] [CrossRef]
- Szczurek, P.; Kamyczek, M.; Pierzynowski, S.G.; Goncharova, K.; Michalowski, P.; Westrom, B.; Prykhodko, O.; Grabowski, T.; Pieszka, M. Effects of dietary supplementation with pancreatic-like enzymes of microbial origin (PLEM) and silicon dioxide (SiO2) on the performance of piglets fed creep feed. J. Anim. Sci. 2016, 94, 62–65. [Google Scholar] [CrossRef]
- Szacawa, E.; Dudek, K.; Bednarek, D.; Pieszka, M.; Bederska-Lojewska, D. A Pilot Study on The Effect of a novel feed additive containing exogenous enzymes, acidifiers, sodium butyrate and silicon dioxide nanoparticles on selected cellular immune indices and body weight gains of calves. J. Vet. Res. 2021, 65, 497–504. [Google Scholar] [CrossRef]
- Koskimaki, J.; Tarkia, M.; Ahtola-Satila, T.; Saloranta, L.; Simola, O.; Forsback, A.P.; Laakso, A.; Frantzen, J. Intracranial biodegradable silica-based nimodipine drug release implant for treating vasospasm in subarachnoid hemorrhage in an experimental healthy pig and dog model. Biomed. Res. Int. 2015, 2015, 715752. [Google Scholar] [CrossRef]
- O’Shea, J.P.; Nagarsekar, K.; Wieber, A.; Witt, V.; Herbert, E.; O’Driscoll, C.M.; Saal, C.; Lubda, D.; Griffin, B.T.; Dressman, J.B. Mesoporous silica-based dosage forms improve bioavailability of poorly soluble drugs in pigs: Case example fenofibrate. J. Pharm. Pharmacol. 2017, 69, 1284–1292. [Google Scholar] [CrossRef]
- Fantini, M.C.A.; Oliveira, C.L.P.; Lopes, J.L.S.; Martins, T.D.S.; Akamatsu, M.A.; Trezena, A.G.; Franco, M.T.; Botosso, V.F.; Sant’Anna, O.; Kardjilov, N.; et al. Using crystallography tools to improve vaccine formulations. IUCrJ 2022, 9, 11–20. [Google Scholar] [CrossRef]
- Navarro-Tovar, G.; Palestino, G.; Rosales-Mendoza, S. An overview on the role of silica-based materials in vaccine development. Expert Rev. Vaccines 2016, 15, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Yu, Y.; Li, Y.; Li, Y.B.; Yu, Y.B.; Zhou, X.Q.; Sun, Z.W. Exposure to silica nanoparticles causes reversible damage of the spermatogenic process in mice. PLoS ONE 2014, 9, e101572. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Wnuk, M.; Rattan, S.I.S. Low doses of nanodiamonds and silica nanoparticles have beneficial hormetic effects in normal human skin fibroblasts in culture. Chemosphere 2016, 148, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, R.; Grunberger, J.W.; Jin, J.; Zhang, Q.; Mohammadpour, R.; Khurana, N.; Xu, X.; Ghandehari, H.; Chen, F. Activation of autophagy by low-dose silica nanoparticles enhances testosterone secretion in Leydig cells. Int. J. Mol. Sci. 2022, 23, 3104. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their human hemocompatibility. J. Control Release 2020, 324, 471–481. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, D.W.; Feng, J.Y.; You, H.M.; Bai, Y.; He, J.C.; Cao, H.; Che, Q.S.; Guo, J.; Su, Z.Q. Toxicity evaluation of silica nanoparticles for delivery applications. Drug Deliv. Transl. Res. 2023, 13, 2213–2238. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliver Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Liu, J.; Yang, M.; Jing, L.; Ren, L.; Wei, J.; Zhang, J.; Zhang, F.; Duan, J.; Zhou, X.; Sun, Z. Silica nanoparticle exposure inducing granulosa cell apoptosis and follicular atresia in female Balb/c mice. Environ. Sci. Pollut. Res. Int. 2018, 25, 3423–3434. [Google Scholar] [CrossRef]
- Chen, F.L.; Sun, J.R.; Wang, Y.J.; Grunberger, J.W.; Zheng, Z.; Khurana, N.; Xu, X.Y.; Zhou, X.; Ghandehari, H.; Zhang, J.L. Silica nanoparticles induce ovarian granulosa cell apoptosis via activation of the PERK-ATF4-CHOP-ERO1α pathway-mediated IP3R1-dependent calcium mobilization. Cell Biol. Toxicol. 2023, 39, 1715–1734. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Yazdimamaghani, M.; Cheney, D.L.; Jedrzkiewicz, J.; Ghandehari, H. Subchronic toxicity of silica nanoparticles as a function of size and porosity. J. Control Release 2019, 304, 216–232. [Google Scholar] [CrossRef]
- Zheng, Z.; Zuo, W.L.; Ye, R.R.; Grunberger, J.W.; Khurana, N.; Xu, X.Y.; Ghandehari, H.; Chen, F.L. Silica nanoparticles promote apoptosis in ovarian granulosa cells via autophagy dysfunction. Int. J. Mol. Sci. 2023, 24, 5189. [Google Scholar] [CrossRef] [PubMed]
- An, W.T.; Huang, Z.Q.; Mao, Z.Y.; Qiao, T.L.; Jia, G.; Zhao, H.; Liu, G.M.; Chen, X.L. Dietary Taurine supplementation improves the meat quality, muscle fiber type, and mitochondrial function of finishing pigs. J. Agric. Food Chem. 2023, 71, 15331–15340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, X.B.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.Q.; Chen, D.W. Excessive dietary taurine supplementation reduces growth performance, liver and intestinal health of weaned pigs. Livest. Sci. 2014, 168, 109–119. [Google Scholar] [CrossRef]
- Dinçer, S.; Karakelle, N.A. Role of taurine in the central nervous system and important of dose. Gazi Med. J. 2019, 30, 227–230. [Google Scholar]
- Mu, T.; Yang, J.C.; Li, Z.; Wu, G.F.; Hu, J.M. Effect of taurine on reproductive hormone secretion in female rats. Adv. Exp. Med. Biol. 2015, 803, 449–456. [Google Scholar] [PubMed]
- Lobo, M.V.T.; Alonso, F.J.M.; del Río, R.M. Immunohistochemical localization of taurine in the male reproductive organs of the rat. J. Histochem. Cytochem. 2000, 48, 313–320. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress. Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, S.; Krystek, P.; Peters, R.J.; Lankveld, D.P.; Bokkers, B.G.; van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2011, 5, 393–405. [Google Scholar] [CrossRef]
- Deng, Y.D.; Zhang, X.D.; Yang, X.S.; Huang, Z.L.; Wei, X.; Yang, X.F.; Liao, W.Z. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Jiang, J.; Gao, M.; Wang, W.; Zheng, H.; Xu, S.; Li, R. Molecular Mechanisms, Characterization methods, and utilities of nanoparticle biotransformation in nanosafety assessments. Small 2020, 16, e1907663. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.; Jiang, J.; Xie, Q.; Huang, Y.; Li, G.; Wu, D.; Zheng, H.; Gao, M.; Xu, S.; et al. Engineering the protein corona structure on gold nanoclusters enables red-shifted emissions in the second near-infrared window for gastrointestinal imaging. Angew. Chem. Int. Ed. 2020, 59, 22431–22435. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, H.; Kondoh, M.; Isoda, K.; Tsunoda, S.; Tsutsumi, Y.; Yagi, K. Silica nanoparticles as hepatotoxicants. Eur. J. Pharm. Biopharm. 2009, 72, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Grunberger, J.W.; Khurana, N.; Zhou, X.; Xu, X.; Ghandehari, H.; Chen, F. BECLIN-1-mediated autophagy suppresses silica nanoparticle-induced testicular toxicity via the inhibition of caspase 8-mediated cell apoptosis in Leydig cells. Cells 2022, 11, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.Q.; Lou, H.; Shou, R.S.; Li, A.Y.; Shang, J.X.; Jin, Y.Y.; Li, L.; Zhu, L.D.; Lu, X.Y.; Fan, X.H. Maternal exposure to E 551 during pregnancy leads to genome-wide DNA methylation changes and metabolic disorders in the livers of pregnant mice and their fetuses. J. Hazard. Mater. 2024, 465, 133233. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Costa, C.; Pires, J.; Teixeira, J.P.; Fraga, S. How can exposure to engineered nanomaterials influence our epigenetic code? A review of the mechanisms and molecular targets. Mutation research. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Lu, B.; Fu, J.L.; Zhu, X.K.; Song, E.Q.; Song, Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J. Hazard. Mater. 2021, 404, 124050. [Google Scholar] [CrossRef]
- Marrocco, A.; Frawley, K.; Pearce, L.L.; Peterson, J.; O’Brien, J.P.; Mullett, S.J.; Wendell, S.G.; St Croix, C.M.; Mischler, S.E.; Ortiz, L.A. Metabolic adaptation of macrophages as mechanism of defense against crystalline silica. J. Immunol. 2021, 207, 1627–1640. [Google Scholar] [CrossRef]
- Yao, Y.S.; Zhang, T.; Tang, M. Toxicity mechanism of engineered nanomaterials: Focus on mitochondria. Environ. Pollut. 2024, 343, 123231. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Njoku, C.A.; Ileola-Gold, A.V.; Adelaja, U.A.; Ikeji, C.N.; Owoeye, O.; Adedara, I.A.; Farombi, E.O. Amelioration of neurobehavioral, biochemical, and morphological alterations associated with silver nanoparticles exposure by taurine in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23457. [Google Scholar] [CrossRef]
- Adedara, I.A.; Ileola-Gold, A.V.; Adelaja, U.A.; Njoku, C.A.; Ikeji, C.N.; Owoeye, O.; Farombi, E.O. Exogenous taurine administration abates reproductive dysfunction in male rats exposed to silver nanoparticles. Environ. Toxicol. 2024, 39, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lu, C.Y.; Zhang, D.; Liu, H.; Cui, S. Taurine promotes estrogen synthesis by regulating microRNA-7a2 in mice ovarian granulosa cells. Biochem. Biophys. Res. Commun. 2022, 626, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ochota, M.; Pasieka, A.; Nizanski, W. Superoxide dismutase and taurine supplementation improves blastocyst yield from poor-quality feline oocytes. Theriogenology 2016, 85, 922–927. [Google Scholar] [CrossRef]
- Devreker, F.; Van den Bergh, M.; Biramane, J.; Winston, R.M.L.; Englert, Y.; Hardy, K. Effects of taurine on human embryo development. Hum. Reprod. 1999, 14, 2350–2356. [Google Scholar] [CrossRef]
- Shimada, K.; Jong, C.J.; Takahashi, K.; Schaffer, S.W. Role of ROS production and turnover in the antioxidant activity of taurine. Adv. Exp. Med. Biol. 2015, 803, 581–596. [Google Scholar]
- Ahmad, M.K.; Mahmood, R. Protective effect of taurine against potassium bromate-induced hemoglobin oxidation, oxidative stress, and impairment of antioxidant defense system in blood. Environ. Toxicol. 2016, 31, 304–313. [Google Scholar] [CrossRef]
- Sinha, M.; Manna, P.; Sil, P.C. Taurine protects the antioxidant defense system in the erythrocytes of cadmium treated mice. BMB Rep. 2008, 41, 657–663. [Google Scholar] [CrossRef]
- Schuller-Levis, G.; Gordon, R.E.; Wang, C.H.; Park, S.Y.; Park, E. Protection of bleomycin-induced fibrosis and inflammation by taurine. Int. Immunopharmacol. 2009, 9, 971–977. [Google Scholar] [CrossRef]
- Bhavsar, T.M.; Patel, S.N.; Lau-Cam, C.A. Protective action of taurine, given as a pretreatment or as a posttreatment, against endotoxin-induced acute lung inflammation in hamsters. J. Biomed. Sci. 2010, 17, S19. [Google Scholar] [CrossRef]
- Ramos, C.D.; Campos, K.K.D.; Costa, G.D.; Cangussú, S.D.; Talvani, A.; Bezerra, F.S. Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul. Toxicol. Pharm. 2018, 98, 50–57. [Google Scholar] [CrossRef]
- Niu, X.L.; Zheng, S.M.; Liu, H.T.; Li, S.Y. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol. Med. Rep. 2018, 18, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Azab, S.S.; Kamel, I.; Ismail, N.N.; Hosni, H.E.; Abd El Fatah, M. The defensive role of taurine against gonadotoxicity and testicular apoptosis effects induced by cisplatin in rats. J. Infect. Chemother. 2020, 26, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Obregón, F.; Urbina, M.; Carreira, I.; Baccichet, E.; Peña, S. Taurine concentration in human blood peripheral lymphocytes -: Major depression and treatment with the antidepressant mirtazapine. Adv. Exp. Med. Biol. 2003, 526, 297–304. [Google Scholar] [PubMed]
- Winkler, H.C.; Kornprobst, J.; Wick, P.; von Moos, L.M.; Trantakis, I.; Schraner, E.M.; Bathke, B.; Hochrein, H.; Suter, M.; Naegeli, H. MyD88-dependent pro-interleukin-1β induction in dendritic cells exposed to food-grade synthetic amorphous silica. Part. Fibre Toxicol. 2017, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, J.; Dou, P.Y.; Zhang, X.; Ran, X.K.; Liu, L.L.; Dou, D.Q. The ameliorative effects of arctiin and arctigenin on the oxidative injury of lung induced by silica via TLR-4/NLRP3/TGF-signaling pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5598980. [Google Scholar] [CrossRef]
- El Idrissi, A.; Trenkner, E. Taurine regulates mitochondrial calcium homeostasis. Adv. Exp. Med. Biol. 2003, 526, 527–536. [Google Scholar]
- Takatani, T.; Takahashi, K.; Uozumi, Y.; Shikata, E.; Yamamoto, Y.; Ito, T.; Matsuda, T.; Schaffer, S.W.; Fujio, Y.; Azuma, J. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am. J. Physiol.-Cell Physiol. 2004, 287, C949–C953. [Google Scholar] [CrossRef]
- Ji, X.; Tang, Z.Q.; Zhang, F.; Zhou, F.; Wu, Y.J.; Wu, D. Dietary taurine supplementation counteracts deoxynivalenol-induced liver injury via alleviating oxidative stress, mitochondrial dysfunction, apoptosis, and inflammation in piglets. Ecotoxicol. Environ. Saf. 2023, 253, 114705. [Google Scholar] [CrossRef]


| Gene | Gene Bank No. | Forward (5′-3′) | Reverse (5′-3′) | Product (bp) |
|---|---|---|---|---|
| CAT | NM_214301.2 | CCAGCCAGTGACCAGATGAAG | ACACCTTCGCCTTCGAGAAT | 280 |
| SOD1 | NM_001190422.1 | TGACTGCTGGCAAAGATGGT | TTTCCACCTCTGCCCAAGTC | 133 |
| IFN-α | JQ839262.1 | AGGAGAATCTCTCCCTTCTCC | GAGCCCTCTGTGCTGAAGAG | 155 |
| IFN-β | GQ415073.1 | ACCAACAAAGGAGCAGCAA | TCAGGGACCTCAAAGTTCATC | 103 |
| IL-1β | NM_214055.1 | ACCTGGACCTTGGTTCTCTG | CATCTGCCTGATGCTCTTGT | 83 |
| IL-6 | AF518322.1 | CTGGCAGAAAACAACCTGAACC | TGATTCTCATCAAGCAGGTCTCC | 94 |
| BCL-2 | XM_021099593.1 | TCCAGAACCTCCTTGGTCCT | AACTACAGCGAGGTGCTTCC | 187 |
| BAX | XM_003127290.5 | GCTTCAGGGTTTCATCCAGGATCG | ACTCGCTCAACTTCTTGGTAGATGC | 107 |
| Caspase-3 | NM_214131.1 | AGAATTGGACTGTGGGATTGAGACG | GCCAGGAATAGTAACCAGGTGCTG | 122 |
| PARP | XM_001927325.2 | GATACTGGACTGCTGCTC | ACTCCCATTCAAGGTGAT | 162 |
| GAPDH | NM_001206359.1 | GTCGGTTGTGGATCTGACCT | TTGACGAAGTGGTCGTTGAG | 207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Sun, J.; Ye, R.; Virk, T.L.; Liu, Q.; Yuan, Y.; Xu, X. Taurine Protects against Silica Nanoparticle-Induced Apoptosis and Inflammatory Response via Inhibition of Oxidative Stress in Porcine Ovarian Granulosa Cells. Animals 2024, 14, 2959. https://doi.org/10.3390/ani14202959
Chen F, Sun J, Ye R, Virk TL, Liu Q, Yuan Y, Xu X. Taurine Protects against Silica Nanoparticle-Induced Apoptosis and Inflammatory Response via Inhibition of Oxidative Stress in Porcine Ovarian Granulosa Cells. Animals. 2024; 14(20):2959. https://doi.org/10.3390/ani14202959
Chicago/Turabian StyleChen, Fenglei, Jiarong Sun, Rongrong Ye, Tuba Latif Virk, Qi Liu, Yuguo Yuan, and Xianyu Xu. 2024. "Taurine Protects against Silica Nanoparticle-Induced Apoptosis and Inflammatory Response via Inhibition of Oxidative Stress in Porcine Ovarian Granulosa Cells" Animals 14, no. 20: 2959. https://doi.org/10.3390/ani14202959
APA StyleChen, F., Sun, J., Ye, R., Virk, T. L., Liu, Q., Yuan, Y., & Xu, X. (2024). Taurine Protects against Silica Nanoparticle-Induced Apoptosis and Inflammatory Response via Inhibition of Oxidative Stress in Porcine Ovarian Granulosa Cells. Animals, 14(20), 2959. https://doi.org/10.3390/ani14202959






