Dietary Thymol Supplementation Promotes Antioxidant Responses and Thermal Stress Resistance in Rainbow Trout, Oncorhynchus mykiss
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Feeding Trial
2.2. Growth Performance Calculations
Specific growth rate (SGR; %/d) = 100 × {[ln(final weight) − ln(initial weight)]/[days]}
Feed efficiency (%) = 100 × [(final biomass − initial biomass)/feed intake]
2.3. Thermal Stress
2.4. Sample Collection and Processing
2.5. Analytical Procedures
2.5.1. Proximate Composition of Diets
2.5.2. Plasma Analysis
2.5.3. Liver and Erythrocyte Sample Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Humoral Immunological Parameters
3.3. Post-Stress Survival
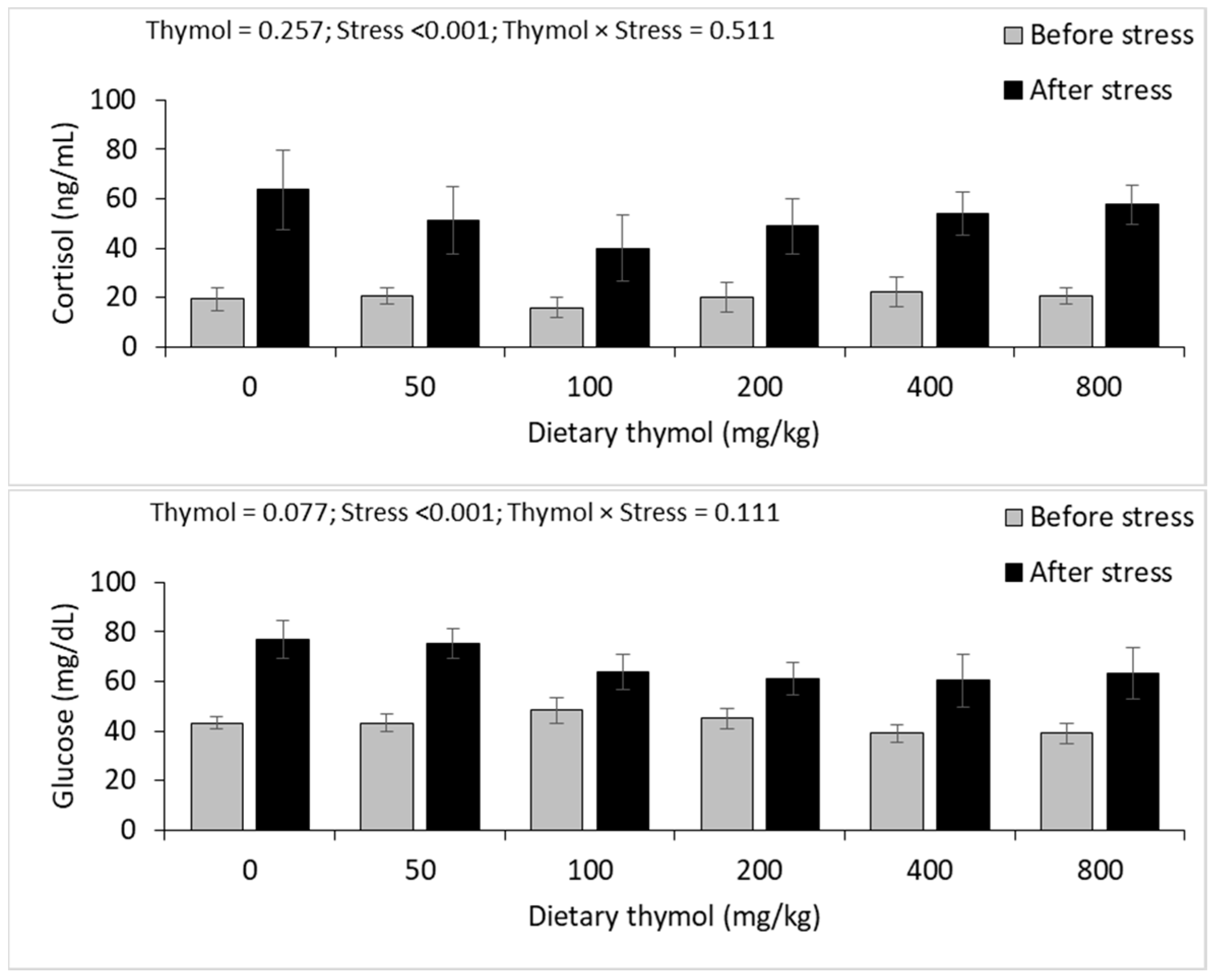
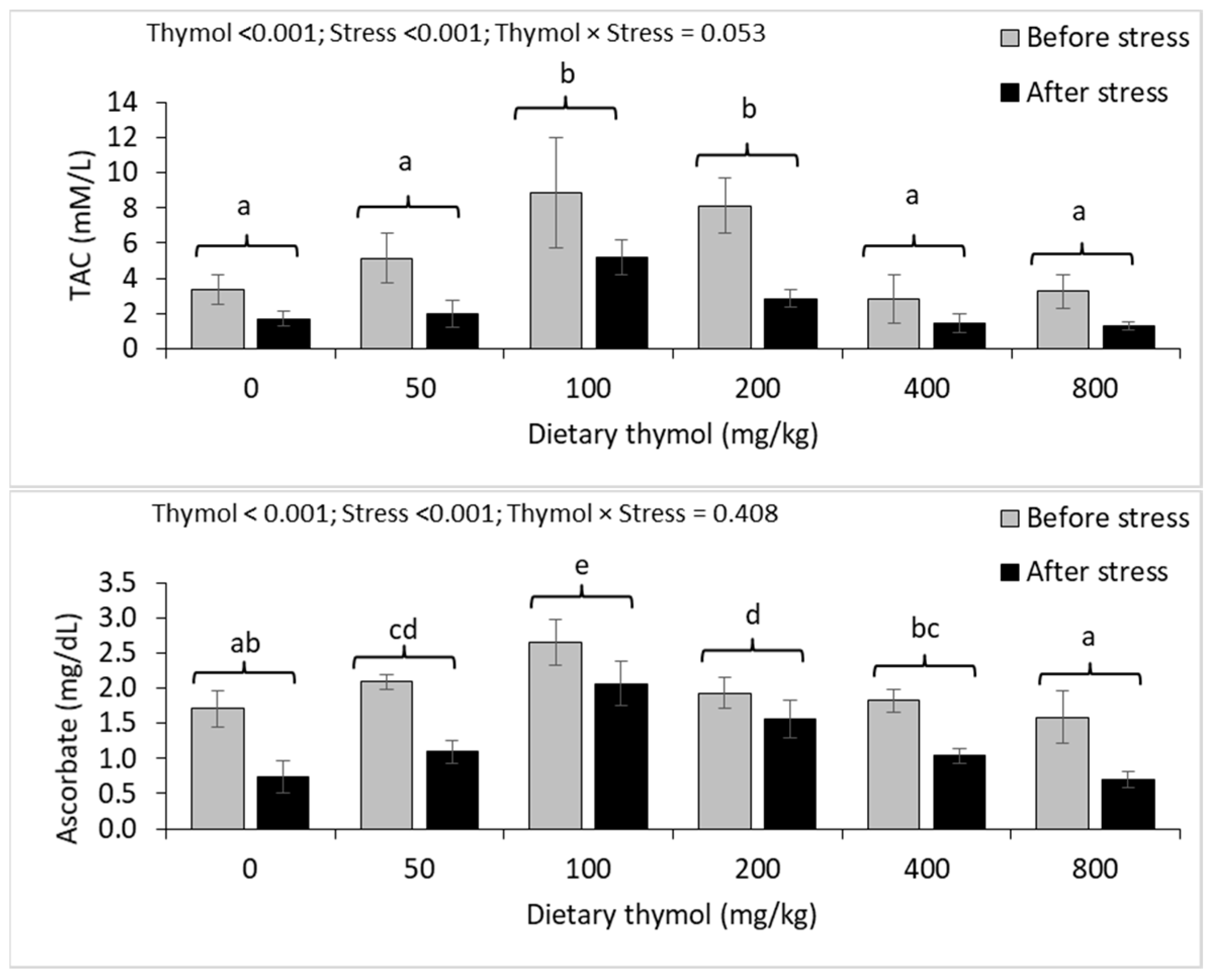
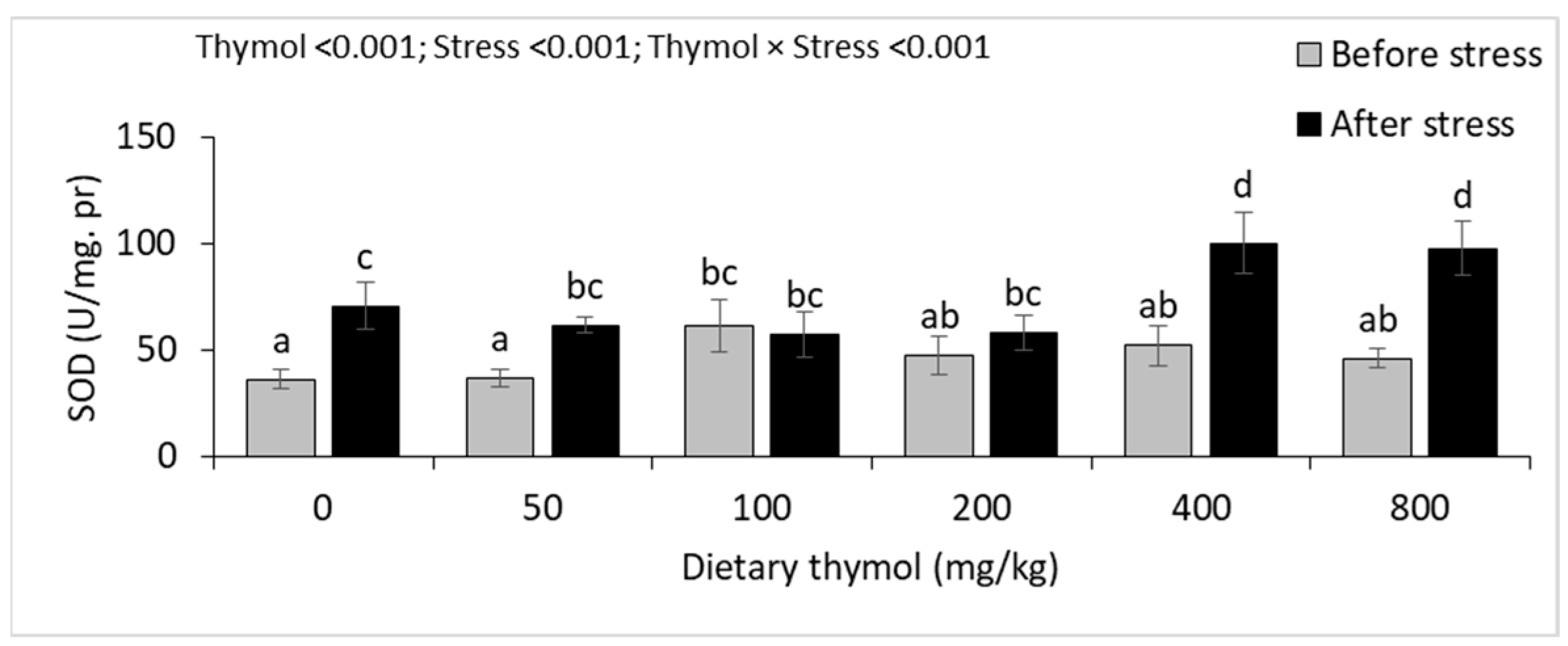
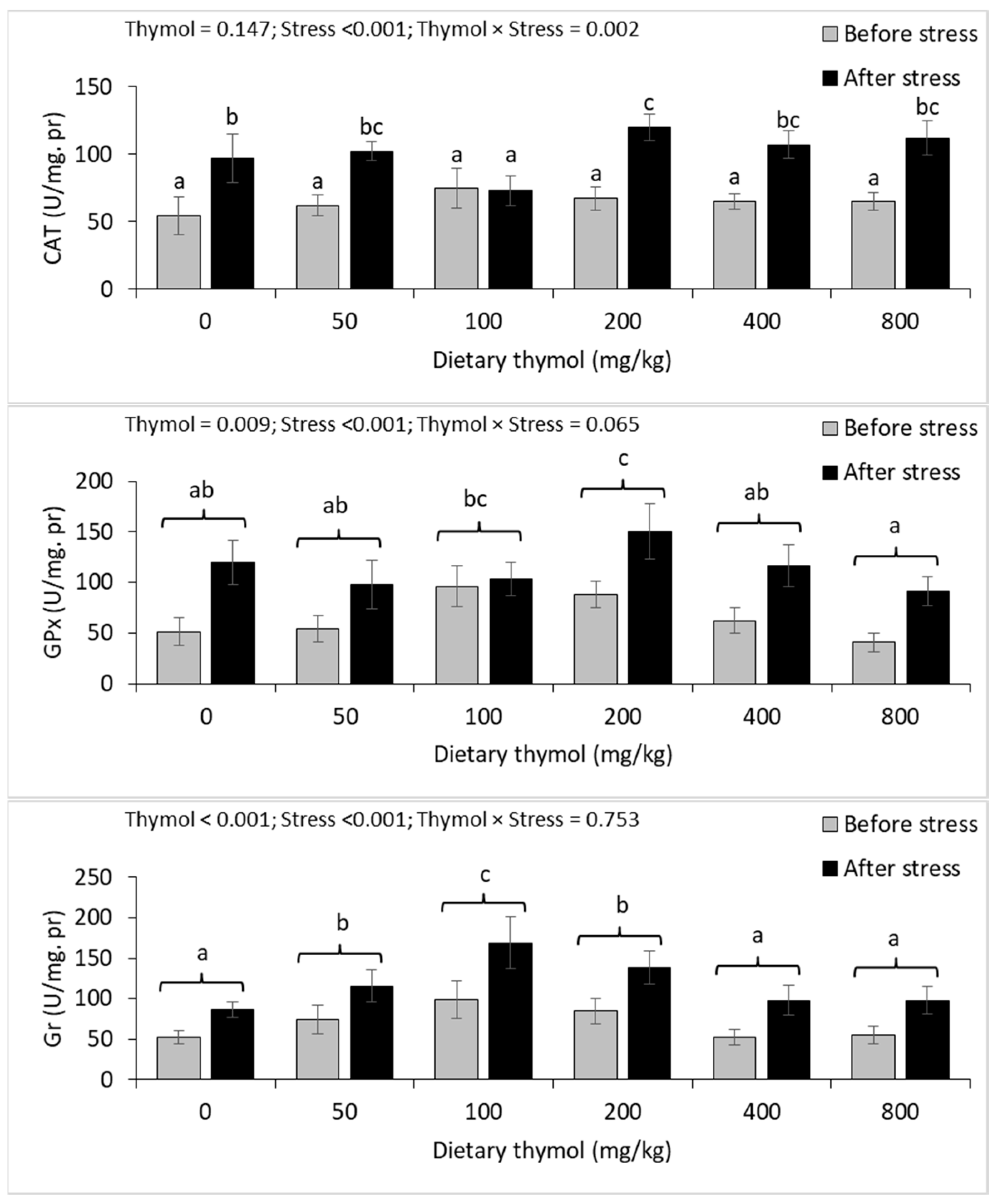
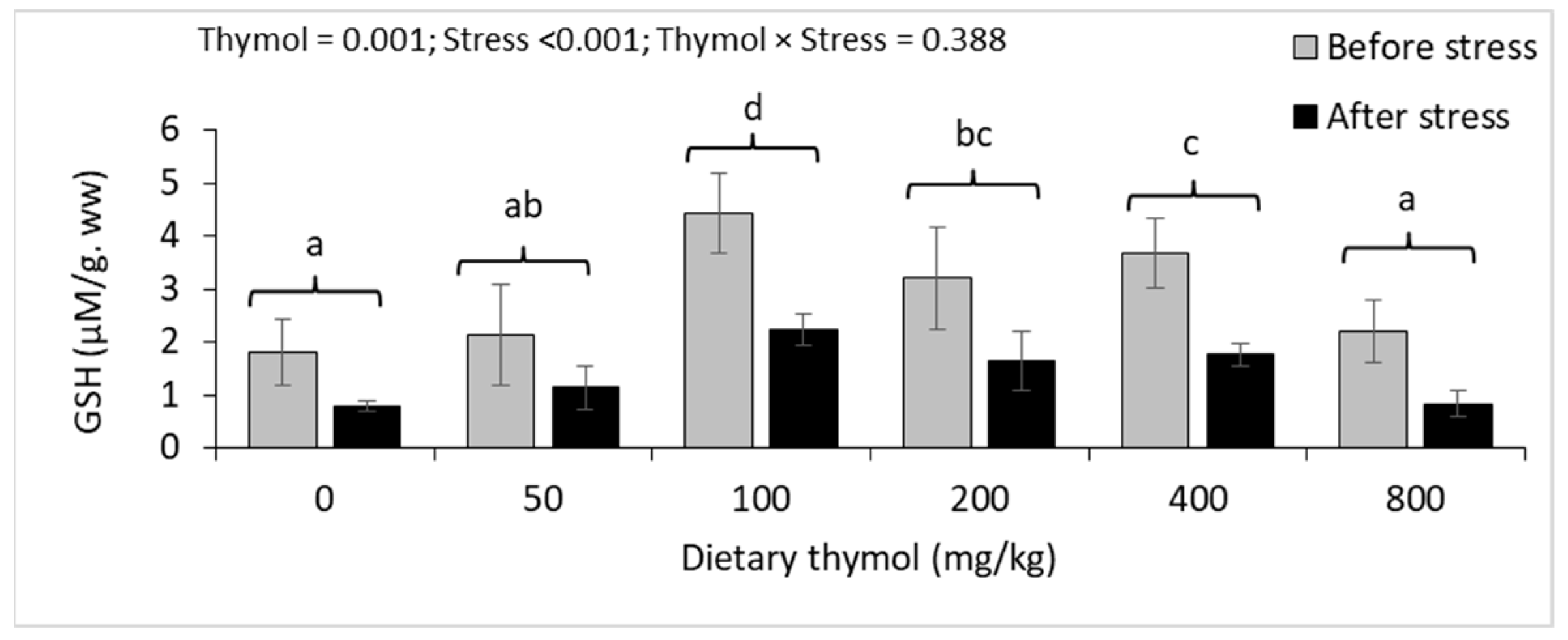
3.4. Erythrocyte Antioxidant Parameters
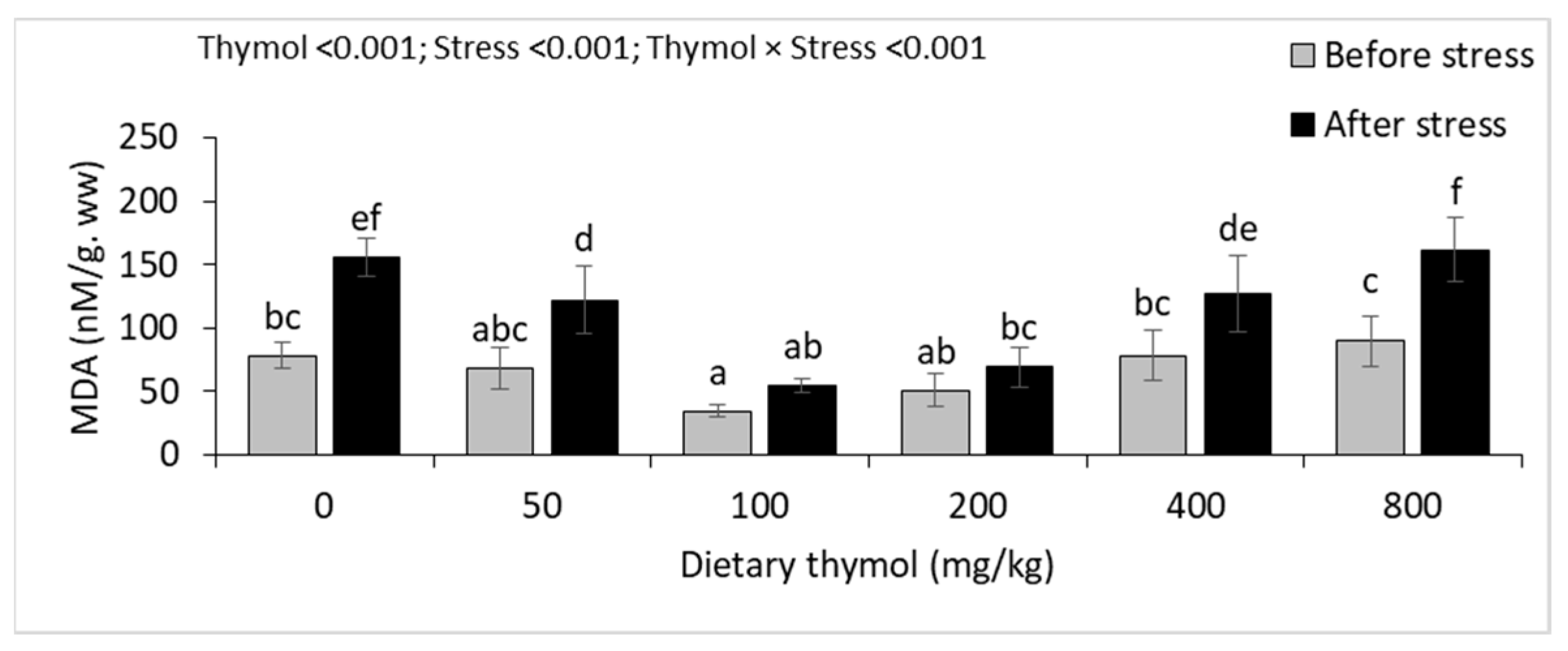
3.5. Hepatic Antioxidant Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; Food and Agriculture Organization: Roma, Italy, 2022. [Google Scholar]
- Galappaththi, E.K.; Ichien, S.T.; Hyman, A.A.; Aubrac, C.J.; Ford, J.D. Climate change adaptation in aquaculture. Rev. Aquac. 2020, 12, 2160–2176. [Google Scholar] [CrossRef]
- Yang, C.; Dong, J.; Sun, C.; Li, W.; Tian, Y.; Liu, Z.; Gao, F.; Ye, X. Exposure to heat stress causes downregulation of immune response genes and weakens the disease resistance of Micropterus salmoides. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101011. [Google Scholar] [CrossRef] [PubMed]
- Schleger, I.C.; Pereira, D.M.C.; Resende, A.C.; Romão, S.; Herrerias, T.; Neundorf, A.K.A.; Sloty, A.M.; Guimarães, I.M.; de Souza, M.R.D.P.; Carster, G.P.; et al. Cold and warm waters: Energy metabolism and antioxidant defenses of the freshwater fish Astyanax lacustris (Characiformes: Characidae) under thermal stress. J. Comp. Physiol. B 2022, 192, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Chainy, G.B.N. Effects of temperature on complexes I and II mediated respiration, ROS generation and oxidative stress status in isolated gill mitochondria of the mud crab Scylla serrata. J. Therm. Biol. 2014, 41, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Hoseini, S.M.; Kulikov, E.V.; Seleznev, S.B.; Petrov, A.K.; Babichev, N.V.; Kochneva, M.V.; Davies, S.J. Effects of dietary Hyssop, Hyssopus officinalis, extract on physiological and antioxidant responses of rainbow trout, Oncorhynchus mykiss, juveniles to thermal stress. Front. Vet. Sci. 2022, 9, 1042063. [Google Scholar] [CrossRef]
- Zhu, F. A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture 2020, 526, 735422. [Google Scholar] [CrossRef]
- Magouz, F.I.; Amer, A.A.; Faisal, A.; Sewilam, H.; Aboelenin, S.M.; Dawood, M.A.O. The effects of dietary oregano essential oil on the growth performance, intestinal health, immune, and antioxidative responses of Nile tilapia under acute heat stress. Aquaculture 2022, 548, 737632. [Google Scholar] [CrossRef]
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE 2019, 14, e0219792. [Google Scholar] [CrossRef]
- García Beltrán, J.M.; Esteban, M.Á. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Kong, Y.-D.; Li, M.; Xia, C.-G.; Zhao, J.; Niu, X.-t.; Shan, X.-F.; Wang, G.-Q. The optimum thymol requirement in diets of Channa argus: Effects on growth, antioxidant capability, immune response and disease resistance. Aquac. Nutr. 2021, 27, 712–722. [Google Scholar] [CrossRef]
- Morselli, M.B.; Reis, J.H.; Baldissera, M.D.; Souza, C.F.; Baldisserotto, B.; Petrolli, T.G.; Paiano, D.; Lopes, D.L.A.; Da Silva, A.S. Benefits of thymol supplementation on performance, the hepatic antioxidant system, and energetic metabolism in grass carp. Fish Physiol. Biochem. 2020, 46, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Aanyu, M.; Betancor, M.B.; Monroig, O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; Zheng, C.; Khalil, S.R.; Farag, M.R.; Elsabbagh, H.S.; Siddique, M.S.; Mawed, S.A.; Azzam, M.M.; Di Cerbo, A.; Elkhadrawey, B.A. Thymol-enriched diet alleviates the toxic impacts of zinc oxide nanoparticles on growth performance, blood biochemistry, oxidant/antioxidant status and stress-related genes and histology of liver and gills in Oreochromis niloticus. Aquac. Rep. 2023, 33, 101750. [Google Scholar] [CrossRef]
- Hafsan, H.; Saleh, M.M.; Zabibah, R.S.; Obaid, R.F.; Jabbar, H.S.; Mustafa, Y.F.; Sultan, M.Q.; Gabr, G.A.; Ramírez-Coronel, A.A.; Khodadadi, M.; et al. Dietary thymol improved growth, body composition, digestive enzyme activities, hematology, immunity, antioxidant defense, and resistance to Streptococcus iniae in the rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2022, 2022, 3288139. [Google Scholar] [CrossRef]
- Meshkini, S.; Tafy, A.-A.; Tukmechi, A.; Farhang-Pajuh, F. Effects of chitosan on hematological parameters and stress resistance in rainbow trout (Oncorhynchus mykiss). Vet. Res. Forum 2012, 3, 49–54. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Siwicki, A.; Anderson, D. Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. In Fish Disease Diagnosis and Prevention Methods; Siwicki, A., Anderson, D., Waluga, J., Eds.; Wydawnictwo Instytutu Rybactwa Srodladowego: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Ed.; SOS Publication: Ikej, Nigeria, 1990; pp. 101–103. [Google Scholar]
- Yano, T. Assays of hemolytic complement activity. In Techniques in Fish Immunology; Stolen, J.S., Ed.; SOS Publication: Ikej, Nigeria, 1992; pp. 131–141. [Google Scholar]
- Omaye, S.T.; David Turnbull, J.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1979; pp. 3–11. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Hu, M.-L. Measurement of protein thiol groups and glutathione in plasma. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; pp. 380–385. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Fleischer, S., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 1978; pp. 302–310. [Google Scholar]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- LeBlanc, S.; Höglund, E.; Gilmour, K.M.; Currie, S. Hormonal modulation of the heat shock response: Insights from fish with divergent cortisol stress responses. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2011, 302, R184–R192. [Google Scholar] [CrossRef] [PubMed]
- Khieokhajonkhet, A.; Aeksiri, N.; Ratanasut, K.; Kannika, K.; Suwannalers, P.; Tatsapong, P.; Inyawilert, W.; Kaneko, G. Effects of dietary Hericium erinaceus powder on growth, hematology, disease resistance, and expression of genes related immune response against thermal challenge of Nile tilapia (Oreochromis niloticus). Anim. Feed. Sci. Technol. 2022, 290, 115342. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress altered growth performance and metabolism and induced anaemia and liver disorder in Labeo rohita. Aquac. Res. 2020, 51, 1406–1414. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World. Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Dabrowski, K.; Lee, K.-J.; Guz, L.; Verlhac, V.; Gabaudan, J. Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 2004, 233, 383–392. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. Rsc. Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Ming, J.; Xie, J.; Xu, P.; Ge, X.; Liu, W.; Ye, J. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish Shellfish Immunol. 2012, 32, 651–661. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M.; Abbasi, M.; Kulikov, E.V.; Drukovsky, S.G.; Petrov, A.K.; Krotova, E.A.; Hoseinifar, S.H.; Van Doan, H. Improvement of growth performance, hepatic and erythrocyte antioxidant capacity, innate immunity, and biochemical parameters of Persian sturgeon, Acipenser persicus, by sulfur amino acids’ supplementation. Aquac. Nutr. 2022, 2022, 2025855. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.; Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol. 2015, 5, 216. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef]
- Souza, M.R.D.D.; Zaleski, T.; Machado, C.; Kandalski, P.K.; Forgati, M.; D’BASTIANI, E.; Piechnik, C.A.; Donatti, L. Effect of heat stress on the antioxidant defense system and erythrocyte morphology of Antarctic fishes. An. Acad. Bras. Cienc. 2021, 94, e20190657. [Google Scholar] [CrossRef]
- Pichaud, N.; Ekström, A.; Breton, S.; Sundström, F.; Rowinski, P.; Blier, P.U.; Sandblom, E. Adjustments of cardiac mitochondrial phenotype in a warmer thermal habitat is associated with oxidative stress in European perch, Perca fluviatilis. Sci. Rep. 2020, 10, 17697. [Google Scholar] [CrossRef]

| CTL | 50 TM | 100 TM | 200 TM | 400 TM | 800 TM | |
|---|---|---|---|---|---|---|
| Corn meal | 50 | 50 | 50 | 50 | 50 | 50 |
| Wheat meal | 220 | 220 | 220 | 220 | 220 | 220 |
| Soybean meal | 170 | 170 | 170 | 170 | 170 | 170 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 | 70 |
| Kilka fishmeal 1 | 254 | 254 | 254 | 254 | 254 | 254 |
| Fish process byproduct 2 | 130 | 130 | 130 | 130 | 130 | 130 |
| Poultry byproduct 3 | 9 | 9 | 9 | 9 | 9 | 9 |
| Vitamin premix 4 | 5 | 5 | 5 | 5 | 5 | 5 |
| Mineral premix 4 | 5 | 5 | 5 | 5 | 5 | 5 |
| Methionine | 3 | 3 | 3 | 3 | 3 | 3 |
| Lysine | 3 | 3 | 3 | 3 | 3 | 3 |
| Proximate composition | ||||||
| Moisture | 91.5 | 90.8 | 91.6 | 90.3 | 91.0 | 91.3 |
| Crude protein | 431.7 | 435.2 | 430.6 | 433.6 | 432.0 | 436.9 |
| Crude fat | 154.8 | 152.9 | 150.4 | 153.6 | 155.7 | 158.3 |
| Crude ash | 75.8 | 76.1 | 76.3 | 75.8 | 76.7 | 76.3 |
| Crude fiber | 28.7 | 27.6 | 28.0 | 28.6 | 28.7 | 28.3 |
| Dietary Thymol (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | 800 | Sig. | |
| Initial weight (g) | 5.53 ± 0.15 | 5.60 ± 0.10 | 5.50 ± 0.10 | 5.50 ± 0.10 | 5.53 ± 0.21 | 5.53 ± 0.15 | 0.956 |
| Final weight (g) | 24.7 ± 0.46 | 25.7 ± 0.67 | 26.2 ± 1.32 | 24.2 ± 0.34 | 25.5 ± 1.33 | 24.3 ± 1.27 | 0.152 |
| Weight gain (%) | 346 ± 20.1 | 359 ± 18.8 | 376 ± 16.9 | 341 ± 13.6 | 360 ± 14.9 | 338 ± 10.9 | 0.105 |
| SGR (%/d) | 2.67 ± 0.08 | 2.72 ± 0.07 | 2.78 ± 0.06 | 2.65 ± 0.05 | 2.73 ± 0.06 | 2.63 ± 0.04 | 0.109 |
| Feed intake (g/tank) | 432 ± 8.88 | 451 ± 15.8 | 466 ± 24.4 | 425 ± 8.55 | 438 ± 25.3 | 421 ± 21.5 | 0.088 |
| Feed efficiency (%) | 88.6 ± 1.02 | 89.1 ± 0.64 | 88.7 ± 1.57 | 88.2 ± 1.46 | 91.0 ± 1.62 | 89.0 ± 1.35 | 0.221 |
| Survival (%) | 100 | 100 | 100 | 100 | 100 | 100 | - |
| Dietary Thymol (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | 800 | Sig. | |
| Lysozyme (U/mL) | 28.0 ± 3.61 | 28.7 ± 4.04 | 32.7 ± 6.65 | 28.0 ± 2.65 | 26.3 ± 2.52 | 26.3 ± 3.05 | 0.452 |
| ACH50 (%) | 33.7 ± 6.56 | 36.0 ± 7.54 | 41.3 ± 6.11 | 35.7 ± 5.13 | 36.3 ± 5.51 | 37.3 ± 4.72 | 0.742 |
| Total Ig (g/dL) | 6.20 ± 0.95 | 7.60 ± 1.21 | 7.53 ± 1.50 | 6.43 ± 0.84 | 6.93 ± 1.31 | 6.27 ± 0.74 | 0.492 |
| Total protein (g/dL) | 3.40 ± 0.40 | 3.37 ± 0.45 | 3.43 ± 0.38 | 3.37 ± 0.31 | 3.27 ± 0.31 | 3.20 ± 0.26 | 0.964 |
| Albumin (g/dL) | 1.61 ± 0.18 | 1.66 ± 0.18 | 1.65 ± 0.13 | 1.64 ± 0.12 | 1.62 ± 0.10 | 1.60 ± 0.10 | 0.995 |
| Globulin (g/dL) | 1.79 ± 0.25 | 1.71 ± 0.27 | 1.79 ± 0.26 | 1.73 ± 0.18 | 1.64 ± 0.21 | 1.60 ± 0.17 | 0.883 |
| Thermal | Dietary Thymol (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stress | 0 | 50 | 100 | 200 | 400 | 800 | Thymol | Stress | Thymol × Stress | |
| SOD (U/mg. pr) | Before | 2.28 ± 0.95 ab | 2.67 ± 0.71 ab | 5.23 ± 1.00 c | 3.70 ± 0.80 b | 2.79 ± 1.02 ab | 1.97 ± 0.40 a | 0.016 | 0.007 | 0.036 |
| After | 3.39 ± 1.02 A | 4.58 ± 0.89 A* | 3.88 ± 0.46 A | 4.02 ± 0.41 A | 4.11 ± 0.58 A | 4.18 ± 0.71 A* | ||||
| CAT (U/mg. pr) | Before | 365 ± 153 | 405 ± 37.4 | 398 ± 88.5 | 420 ± 45.6 | 400 ± 43.2 | 419 ± 11.5 | 0.905 | <0.001 | 0.952 |
| After | 824 ± 128 | 954 ± 107 | 919 ± 146 | 925 ± 178 | 894 ± 287 | 975 ± 99.4 | ||||
| GSH (mM/mg. pr) | Before | 2.35 ± 0.77 | 2.16 ± 0.44 | 1.85 ± 0.49 | 1.97 ± 0.10 | 1.96 ± 0.42 | 1.99 ± 0.52 | 0.867 | <0.001 | 0.711 |
| After | 1.21 ± 0.31 | 1.07 ± 0.22 | 1.37 ± 0.25 | 1.32 ± 0.28 | 1.13 ± 0.31 | 1.36 ± 0.26 | ||||
| MDA (nM/mg. pr) | Before | 0.10 ± 0.02 a | 0.10 ± 0.01 a | 0.11 ± 0.02 a | 0.10 ± 0.01 a | 0.09 ± 0.01 a | 0.10 ± 0.01 a | 0.013 | <0.001 | <0.001 |
| After | 0.15 ± 0.02 B* | 0.16 ± 0.01 B* | 0.09 ± 0.02 A | 0.07 ± 0.02 A | 0.15 ± 0.01 B* | 0.15 ± 0.01 B* | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi, M.; Hoseini, S.M.; Vatnikov, Y.A.; Karamyan, A.; Kulikov, E.V. Dietary Thymol Supplementation Promotes Antioxidant Responses and Thermal Stress Resistance in Rainbow Trout, Oncorhynchus mykiss. Animals 2024, 14, 2988. https://doi.org/10.3390/ani14202988
Yousefi M, Hoseini SM, Vatnikov YA, Karamyan A, Kulikov EV. Dietary Thymol Supplementation Promotes Antioxidant Responses and Thermal Stress Resistance in Rainbow Trout, Oncorhynchus mykiss. Animals. 2024; 14(20):2988. https://doi.org/10.3390/ani14202988
Chicago/Turabian StyleYousefi, Morteza, Seyyed Morteza Hoseini, Yury Anatolyevich Vatnikov, Arfenya Karamyan, and Evgeny Vladimirovich Kulikov. 2024. "Dietary Thymol Supplementation Promotes Antioxidant Responses and Thermal Stress Resistance in Rainbow Trout, Oncorhynchus mykiss" Animals 14, no. 20: 2988. https://doi.org/10.3390/ani14202988
APA StyleYousefi, M., Hoseini, S. M., Vatnikov, Y. A., Karamyan, A., & Kulikov, E. V. (2024). Dietary Thymol Supplementation Promotes Antioxidant Responses and Thermal Stress Resistance in Rainbow Trout, Oncorhynchus mykiss. Animals, 14(20), 2988. https://doi.org/10.3390/ani14202988






