Comparison of Universal mtDNA Primers in Species Identification of Animals in a Sample with Severely Degraded DNA
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Data Analyses
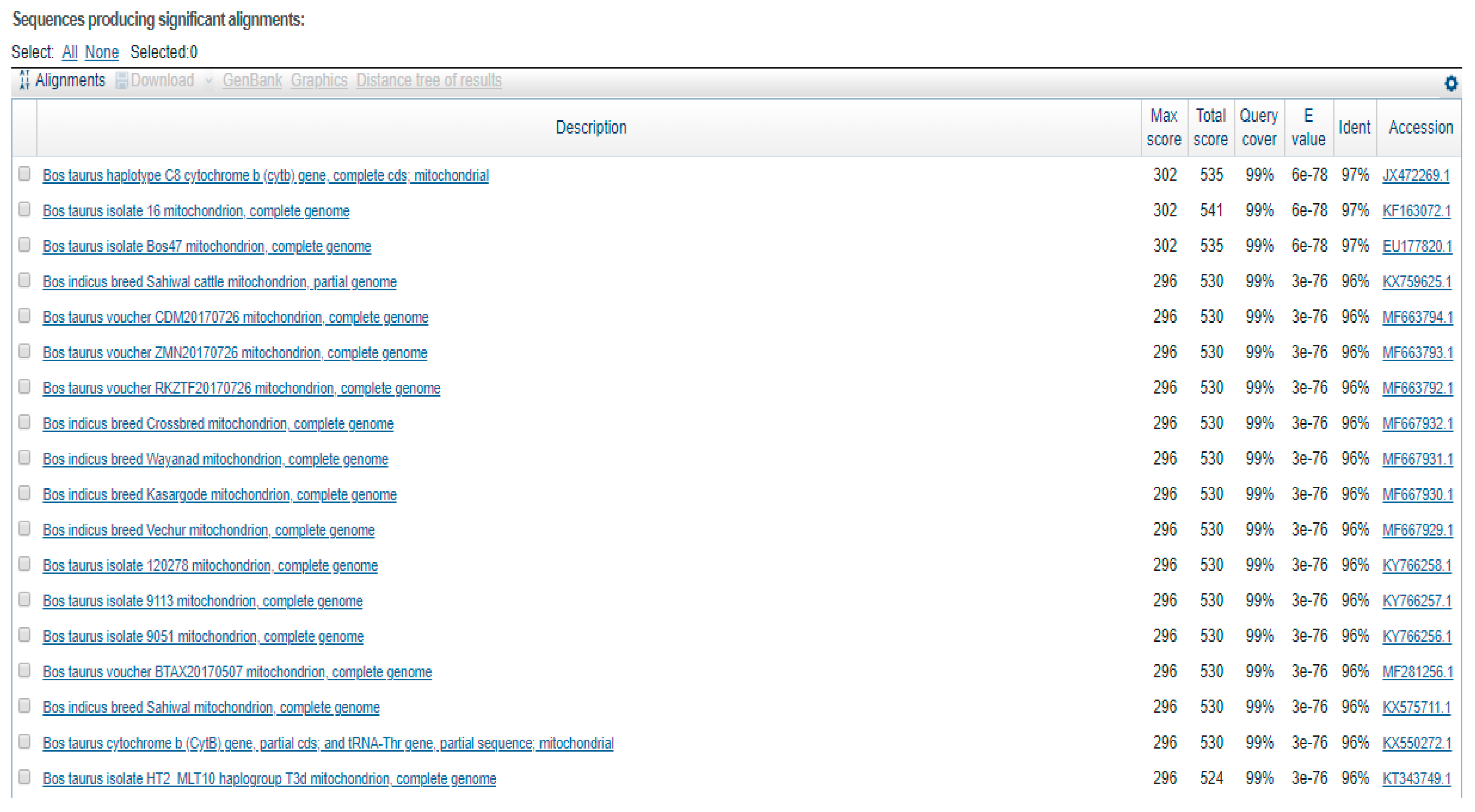
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giles, R.E.; Blanc, H.; Cann, H.M.; Wallace, D.C. Maternal inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 6715–6719. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, S.; Grela, M.; Listos, P.; Gryzinska, M. Molekularne metody identyfikacji gatunkowej wykorzystywane w ekspertyzach sądowych. J. Anim. Sci. Biol. 2019, 37, 31–39. [Google Scholar] [CrossRef]
- Dalvin, S.; Glover, K.A.; Sørvik, A.G.; Seliussen, B.B.; Taggart, J.B. Forensic identification of severely degraded Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) tissues. Investig Genet. 2010, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.D.N.; Stoeckle, M.Y.; Zemlak TSFrancis, C.M. Identification of Birds through DNA Barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar]
- Anderson, S.; Bruijn, M.H.; Coulson, A.R.; Eperon, I.C.; Sanger, F.; Young, I.G. Complete sequence of bovine mitochondrial DNA, conserved features of the mammalian mitochondrial genome. J. Mol. Biol. 1982, 156, 683–684. [Google Scholar] [CrossRef]
- Xu, X.; Aranson, U. The complete mitochondrial DNA sequence of the horse Equus caballus: Extensive heteroplasmy of the control region. Gene 1994, 48, 357–362. [Google Scholar]
- Hiendleder, S.; Lewalski, H.; Wassmuth, R.; Janke, A. The complete mitochondrial DNA sequence of the domestic sheep (Ovis aries) and comparison with the other major ovine haplotype. J. Mol. Evol. 1998, 47, 441–448. [Google Scholar] [CrossRef]
- Ursing, B.M.; Arnason, U. The complete mitochondrial DNA sequence of the pig (Sus scrofa). J. Mol. Evol. 1998, 47, 302–306. [Google Scholar] [CrossRef]
- Luikart, G.; Gielly, L.; Excoffier, L.; Vigne, J.D.; Bouvet, J.; Taberlet, P. Multiple maternal origins and weak phylogeographic structure in domestic goats. Proc. Natl. Acad. Sci. USA 2001, 98, 5927–5932. [Google Scholar] [CrossRef]
- Cooper, A.; Lalueza-Fox, C.; Anderson, A.; Rambaut, A.; Austin, J.; Ward, R. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature 2001, 409, 704–706. [Google Scholar] [CrossRef]
- Haddrath, O.; Baker, A.J. Complete mitochondrial DNA geonome sequences of extinct birds: Ratite phylogenetics and the vicariance biogeography hypothesis. Proc. Biol. Sci. 2001, 268, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Hofreiter, M.; Paijmans, J.L.; Goodchild, H.; Camilla, F.; Speller, C.F.; Barlow, A.; Fortes, G.G.; Thomas, A.; Ludwig, A.; Collins, M.J. The future of ancient DNA: Technical advances and conceptual shifts. Bioessays 2014, 36, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Skuza, L.; Demska, K.; Adamczyk, A. Barkoding jako nowoczesne narzędzie biologii molekularnej. Postępy Biol. Komórki 2015, 42, 621–632. [Google Scholar]
- Ajmal Ali, M.; Gyulai, G.; Hidvégi, N.; Kerti, B.; Al Hemaid, F.M.; Pandey, A.K.; Lee, J. The changing epitome of species identification—DNA barcoding. Saudi J. Biol. Sci. 2014, 21, 204–231. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Zawadzka, E.; Szewczuk, D.; Gryzińska, M.; Jakubczak, A. Molecular markers used in forensic genetics. Med. Sci. Law 2018, 58, 201–209. [Google Scholar] [CrossRef]
- Kelle, J.; Carmon, J.; Pucherelli, S.; Hosler, D. Identification of Unknown Organisms by DNA Barcoding: A Molecular Method for Species Classification. Tech. Memo. 2014, 86, 14–15. [Google Scholar]
- Kitano, T.; Umetsu, K.; Tian, W.; Osawa, M. Two universal primer setsfor species identification among vertebrates. Int. J. Legal Med. 2007, 121, 423–427. [Google Scholar] [CrossRef]
- Wultsch, C.; Waits, L.P.; Kelly, M.J. Noninvasive individual and species identification of jaguars (Panthera onca), pumas (Puma concolor) and ocelots (Leopardus pardalis) in Belize, Central America using cross-species microsatellites and faecal DNA. Mol. Ecol. Resour. 2014, 14, 1171–1182. [Google Scholar] [CrossRef]
- Dell, B.A.; Masembe, C.; Gerhold, R.; Willcox, A.; Okafor, C.; Souza, M. Species misidentification in local markets: Discrepancies between reporting and molecular identification of bushmeat species in northern Uganda. One Health 2021, 13, 100251. [Google Scholar] [CrossRef]
- Boukhdoud, L.; Saliba, C.; Kahale, R.; Bou Dagher Kharrat, M. Tracking mammals in a Lebanese protected area using environmental DNA-based approach. Environ. DNA 2021, 3, 792–799. [Google Scholar] [CrossRef]
- Angulo, A.S.; Fajardo, F.E.; Salom-Pérez, R.; Carazo-Salazar, J.; Taylor, F.; Pilé, E.; Quesada-Alvarado, F.; Blanco-Peña, K. Identification of anthropogenic impact on natural habitats by antimicrobial resistance quantification in two neotropical wild cats and their geospatial analysis. J. Wildl. Dis. 2023, 59, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wu, X.; Yang, R.; Li, X.; Yang, B. DNA-based species identification for faecal samples: An application on the mammalian survey in Mountain Huangshan Scenic Spot. Afr. J. Biotechnol. 2011, 10, 12134–12141. [Google Scholar]
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1992L0043:20070101:en:PDF (accessed on 8 November 2024).
- Convention on International Trade in Endangered Species of Wild Fauna and Flora (Signed at Washington, D.C., on 3 March 1973. Available online: https://cites.org/sites/default/files/eng/disc/CITES-Convention-EN.pdf (accessed on 8 November 2024).
- Lopez-Ocejaa, A.; Gamarraa, D.; Borraganb, S.; Jiménez-Morenoc, S.; de Pancorboa, M.M. New cyt b gene universal primer set for forensic analysis. Forensic Sci. Int.-Genet. 2016, 23, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.; Markert, J.A.; Kilpatrick, C.W. Phylogeography and molecular systematics of the Peromyscus aztecus species group (Rodentia: Muridae) inferred using parsimony and likelihood. Syst. Biol. 1997, 46, 426–440. [Google Scholar] [CrossRef]
- Wade, N.L. Molecular Systematics of Neotropical Deer Mice of the Peromyscus mexicanus Species Group. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 1999. [Google Scholar]
- Hebert, P.D.; Cywinska, A.; Ball, S.L. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Parson, W.; Pegoraro, K.; Niederstätter, H.; Föger, M.; Steinlechner, M. Species identification by means of the cytochrome b gene. Int. J. Legal Med. 2000, 114, 23–28. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, W.; Zhou, Y.; Liu, Z.; Chen, Y.; Zhao, Z. Identification of mammalian species using the short and highly variable regions of mitochondrial DNA. Mitochondrial DNA 2015, 26, 550–554. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Smith, M.; Janzen, D.H.; Rodriguez, J.J.; Whitfield, J.B.; Hebert, P.D. A minimalist barcode can identify a specimen whose DNA is degraded. Mol. Ecol. Notes 2006, 6, 959–964. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Singer, G.A.; Clare, E.L.; Hebert, P.D. Design and applicability of DNA arrays and DNA barcodes in biodiversity monitoring. BMC Biol. 2007, 5, 24. [Google Scholar] [CrossRef]
- Hsieh, H.M.; Chiang, H.L.; Tsai, L.C.; Lai, S.Y.; Huang, N.E.; Linacre, A.; Lee, J.C. Cytochrome b gene for species identification of the conservation animals. Forensic Sci. Int. 2001, 122, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Tobe, S.S.; Kitchener, A.C.; Linacre, A.M. Reconstructing mammalian phylogenies: A detailed comparison of the cytochrome B and cytochrome oxidase subunit I mitochondrial genes. PLoS ONE 2010, 5, e14156. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ding, F.; Chen, H.; He, M.; Zhu, S.; Ma, X.; Jang, L.; Li, H. DNA Barcoding for the Identification and Authentication of Animal Species in Traditional Medicine. Evid. Based Complement. Altern. Med. 2018, 51, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Canada, J.; Parboosingh, S.; Bridge, P.J. Identification of Archaeological Animal Bone by PCR/DNA. J. Archaeol. Sci. 2002, 29, 77–84. [Google Scholar] [CrossRef]
- Lopez-Ocejaa, A.; Lekubeb, X.; Ruizc, L.; Mujika-Alustizad, J.A.; De Pancorboa, M.M. CYT B L15601 and H15748 primers for genetic identification of cetacean species. Forensic Sci. Int.-Genet. 2019, 7, 771–772. [Google Scholar] [CrossRef]
- Arnaout, Y.; Djelouadji, Z.; Robardet, E.; Cappelle, J.; Cliquet, F.; Touzalin, F.; Jimenez, G.; Suzel Hurstel, S.; Borel, C.; Picard-Meyer, E. Genetic identification of bat species for pathogen surveillance across France. PLoS ONE 2022, 17, e0261344. [Google Scholar] [CrossRef]
- Boukhdoud, L.; Saliba, C.; Parker, L.D.; McInerney, N.R.; Ishac Mouawad, G.; Kharrat, M.; Kahale, R.; Chahine, T.; Maldonado, J.E.; Bou Dagher Kharrat, M. First DNA sequence reference library for mammals and plants of the Eastern Mediterranean Region. Genome 2020, 64, 39–49. [Google Scholar] [CrossRef]
- Dalén, L.; Lagerholm, V.K.; Nylander, J.A.A.; Barton, N.; Bochenski, Z.M.; Tomek, T.; Rudling, D.; Ericson, P.G.P.; Irestedt, M.; Stewart, J.R. Identifying Bird Remains Using Ancient DNA Barcoding. Genes 2017, 8, 169. [Google Scholar] [CrossRef]
- Kohn, M.; Knauer, F.; Stoffella, A.; Schröder, W.; Pääbo, S. Conservation genetics of the European brown bear—A study Rusing excremental PCR of nuclear and mitochondrial sequences. Mol. Ecol. 1995, 4, 95–103. [Google Scholar] [CrossRef]
- Evans, N.T.; Olds, B.P.; Renshaw, M.A.; Turner, C.R.; Li, Y.; Jerde, C.L.; Mahon, A.R.; Pfrender, M.E.; Lamberti, G.A.; Lodge, D.M. Quantification of mesocosm fish and amphibian species diversity via environmental DNA metabarcoding. Mol. Ecol. Resour. 2016, 16, 29–41. [Google Scholar] [CrossRef]
- Schneider, P.M.; Bender, K.; Mayr, W.R.; Parson, W.; Hoste, B.; Decorte, R.; Cordonnier, J.; Vanek, D.; Morling, N.; Karjalainen, M.; et al. STR analysis of artificially degraded DNA-results of a collaborative European exercise. Forensic Sci. Int. 2004, 139, 123–134. [Google Scholar] [CrossRef] [PubMed]


| Locus | Forward | Sequence (5′–3′) | Reverse | Sequence (5′–3′) | Size (bp) | References |
|---|---|---|---|---|---|---|
| Cytochrome b | L15601 | TACGCAATCCTACGATCAATTCC | H15748 | GGTTGTCCTCCAATTCATGTT | 148 | [25] |
| Cytochrome b | L14841 | CCATCCAACATCTCCGCATGATGAAA | H15149 | CCCTCAGAATGATATTTGGCCTCA | 306 | [26] |
| Cytochrome b | L14553 | CTACCATGAGGACAAATATC | MOUSE-TR | TTCCATTTYTGGTTTACAAGACCA | 990 | [27,28] |
| 12S rRNA | L1085 | CCCAAACTGGGATTAGATACCC | H1259 | GTTTGCTGAAGATGGCGGTA | 215 | [17] |
| 16S rRNA | L2513 | GCCTGTTTACCAAAAACATCAC | H2714 | CTCCATAGGGTCTTCTCGTCTT | 244 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figura, A.; Gryzinska, M.; Jakubczak, A. Comparison of Universal mtDNA Primers in Species Identification of Animals in a Sample with Severely Degraded DNA. Animals 2024, 14, 3256. https://doi.org/10.3390/ani14223256
Figura A, Gryzinska M, Jakubczak A. Comparison of Universal mtDNA Primers in Species Identification of Animals in a Sample with Severely Degraded DNA. Animals. 2024; 14(22):3256. https://doi.org/10.3390/ani14223256
Chicago/Turabian StyleFigura, Aleksandra, Magdalena Gryzinska, and Andrzej Jakubczak. 2024. "Comparison of Universal mtDNA Primers in Species Identification of Animals in a Sample with Severely Degraded DNA" Animals 14, no. 22: 3256. https://doi.org/10.3390/ani14223256
APA StyleFigura, A., Gryzinska, M., & Jakubczak, A. (2024). Comparison of Universal mtDNA Primers in Species Identification of Animals in a Sample with Severely Degraded DNA. Animals, 14(22), 3256. https://doi.org/10.3390/ani14223256





