Research Progress on the Impact of Human Chorionic Gonadotropin on Reproductive Performance in Sows
Simple Summary
Abstract
1. Introduction
2. Structure and Function of hCG
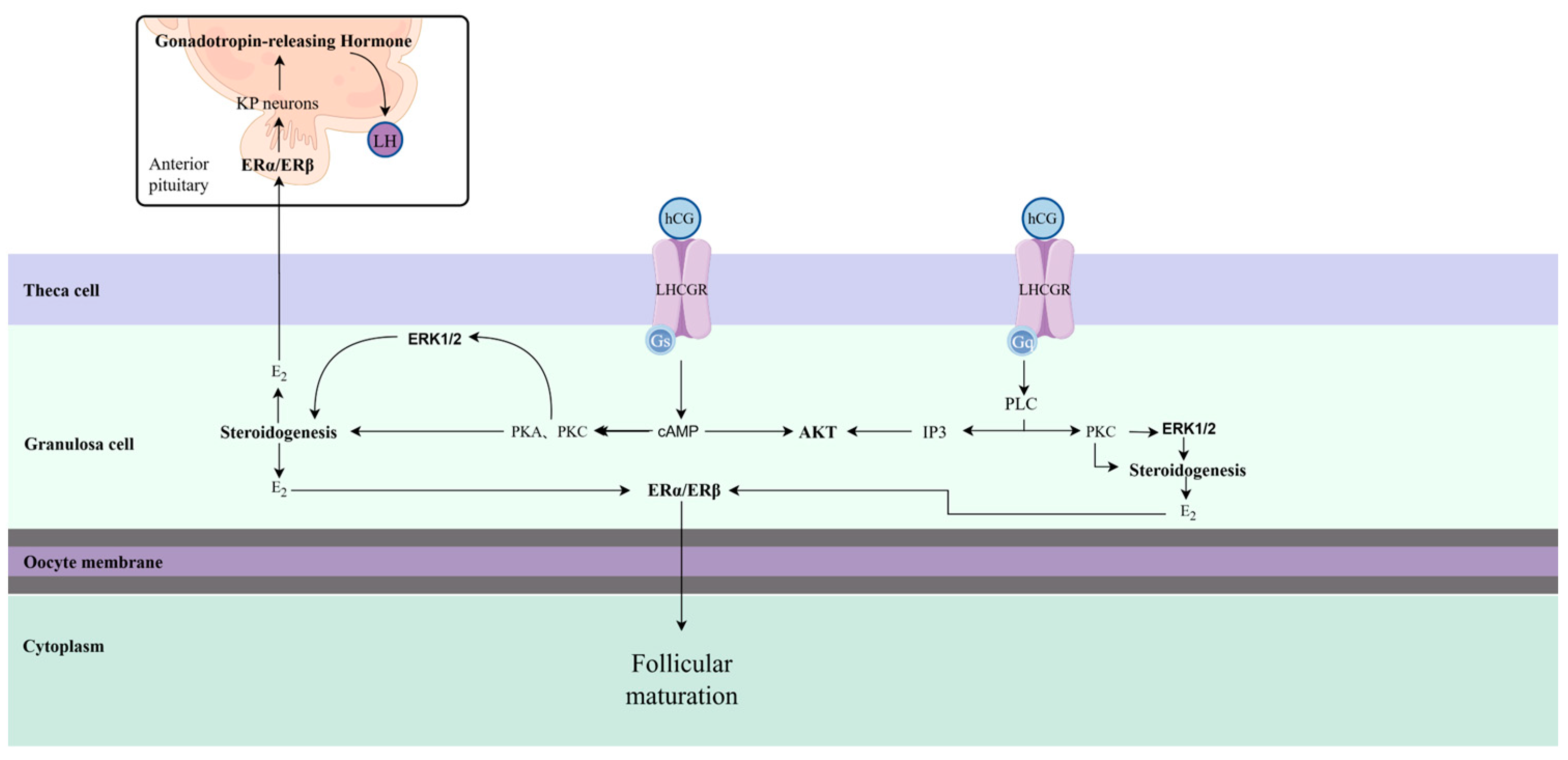
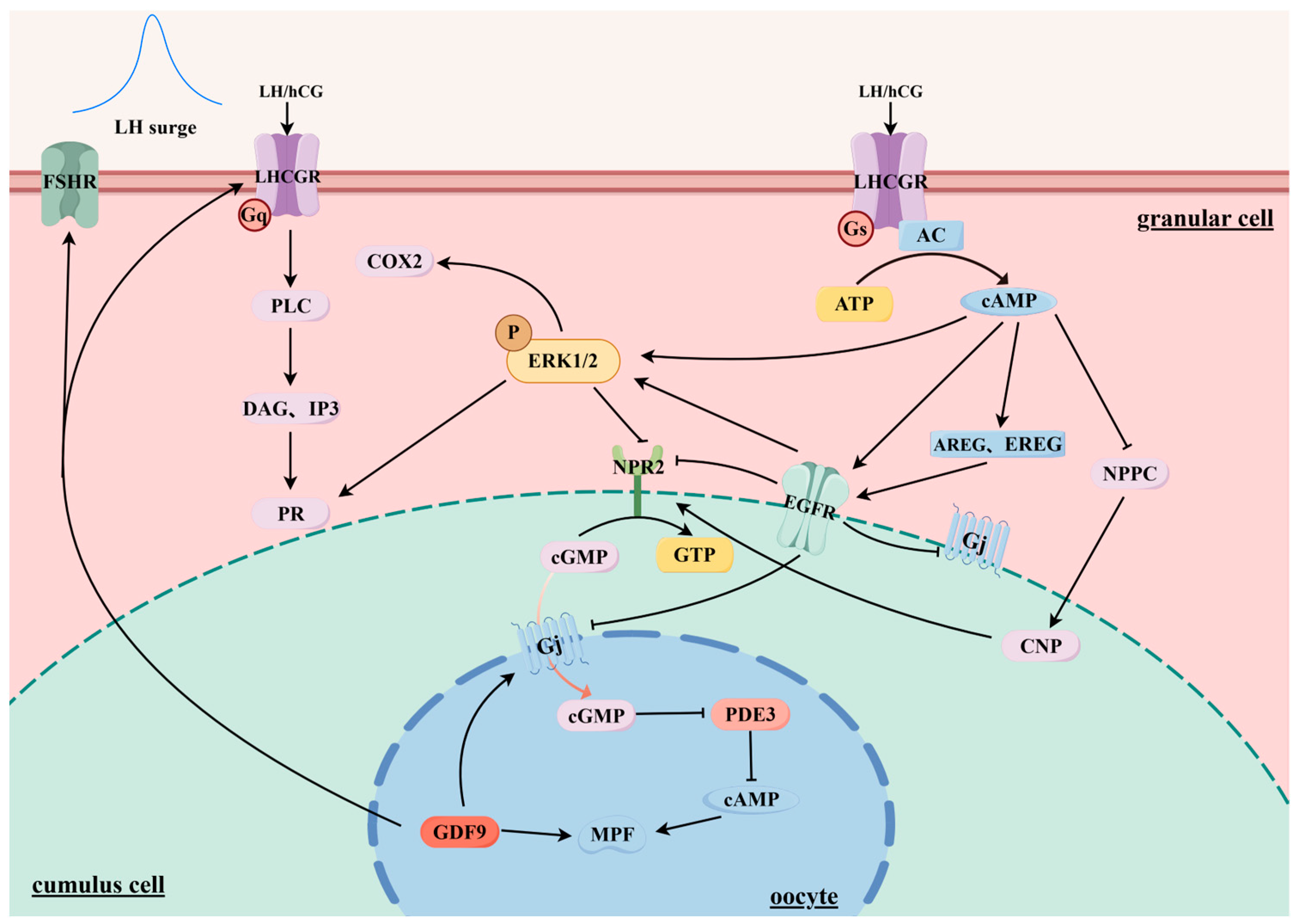
3. hCG Affects Follicle Maturation and Ovulation
4. hCG Supports Luteal Function
5. The Maintenance of Pregnancy by hCG
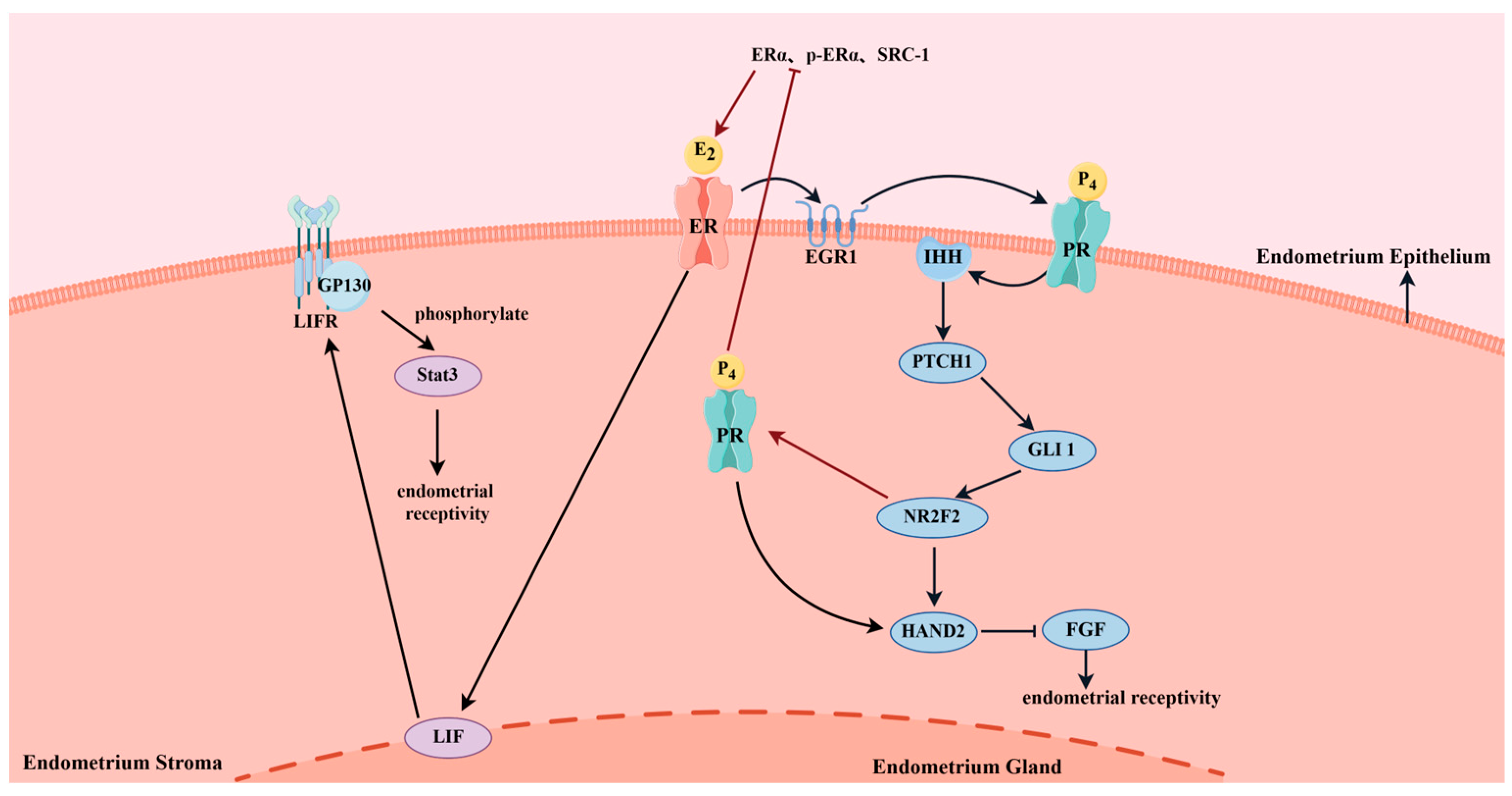
5.1. hCG Affects Endometrial Receptivity
5.2. hCG-Mediated Immunomodulation Contributes to Embryo Implantation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quirino, M.W.; Schultz, C.; Franz, M.; Lucia, T., Jr.; Martelli, A.; Goncalves, P.B.D.; Ulguim, R.D.R.; Gasperin, B.G.; Bianchi, I. Use of chorionic gonadotropins during lactation to optimize postpartum sow reproductive performance: A review. Anim. Reprod. 2024, 21, e20230118. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.D.; Martinuk, S.D. Equine Chorionic Gonadotropin. Endocr. Rev. 1991, 12, 27–44. [Google Scholar] [CrossRef]
- Gifre, L.; Aris, A.; Bach, A.; Garcia-Fruitos, E. Trends in recombinant protein use in animal production. Microb. Cell Fact. 2017, 16, 40. [Google Scholar] [CrossRef]
- Herrera Alvarez, R.; Nogueira Natal, F.L.; Almeida, B.E.; Oliveira, J.E.; Ferreira Melo, A.J.; Carvalho Ribela, M.T.; Bartolini, P. Biological Activity of Different Batches of Equine Chorionic Gonadotropin as Determined by Reversed-phase High-performance Liquid Chromatography and in vivo Assay. J. Adv. Med. Pharm. Sci. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Saunders, H.; Schertz, J.C.; Hecker, C.; Lang, B.; Arriagada, P. The recombinant human chorionic gonadotropin prefilled pen: Results of patient and nurse human factors usability testing. Expert Opin. Drug Deliv. 2012, 9, 893–900. [Google Scholar] [CrossRef]
- Cole, L.A. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod. Biol. Endocrinol. 2009, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. hCG: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Likszo, P.; Gromadzka-Hliwa, K.; Klos, J.; Kaczmarek, M.M.; Ziecik, A.J. Attainment of Sexual Maturity and Gonadotropin Priming in Gilts Determine Follicular Development, Endocrine Milieu and Response to Ovulatory Triggers. Int. J. Mol. Sci. 2022, 23, 9190. [Google Scholar] [CrossRef]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Mol. Cell. Endocrinol. 2014, 383, 203–213. [Google Scholar] [CrossRef] [PubMed]
- d’Hauterive, S.P.; Close, R.; Gridelet, V.; Mawet, M.; Nisolle, M.; Geenen, V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. Int. J. Mol. Sci. 2022, 23, 1380. [Google Scholar] [CrossRef]
- Gromoll, J.; Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG Action on the Same Receptor Results in Quantitatively and Qualitatively Different Intracellular Signalling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef]
- Bates, G.W.; Bowling, M. Physiology of the female reproductive axis. Periodontol. 2000 2013, 61, 89–102. [Google Scholar] [CrossRef]
- Ascheim, S.; Zondek, B. Hypophysenvorderlappen hormone und ovarial hormone im Harn von Schwangeren. Wien. Klin. Wochenschr. 1927, 6, 13–21. [Google Scholar] [CrossRef]
- Jones, A.; Gey, G.; Gey, M. Hormone production by placental cells maintained in continuous culture. Bull. Johns Hopkins Hosp. 1943, 72, 26–38. [Google Scholar]
- Duran, H.E. Controlled Ovarian Stimulation Protocols for IVF: From First IVF Baby in the United States and Beyond. Diminished Ovarian Reserve and Assisted Reproductive Technologies: Current Research and Clinical Management; Bukulmez, O., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 105–117. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S.A. Hyperglycosylated human chorionic gonadotropin and human chorionic gonadotropin free beta-subunit: Tumor markers and tumor promoters. J. Reprod. Med. 2008, 53, 499–512. [Google Scholar] [PubMed]
- Wang, J. Recombinant Human Chorionic Gonadotropin (hCG) Glycosylation Characteristics Analysis of Identification and Quality Control. Master’s Thesis, South China University of Technology, Guangzhou, China, 2020. [Google Scholar]
- Stenman, U.H.; Tiitinen, A.; Alfthan, H.; Valmu, L. The classification, functions and clinical use of different isoforms of HCG. Hum. Reprod. Update 2006, 12, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Gabay, R.; Rozen, S.; Samokovlisky, A.; Amor, Y.; Rosenfeld, R.; Kohen, F.; Amsterdam, A.; Berger, P.; Ben-Menahem, D. The role of the 3′ region of mammalian gonadotropin β subunit gene in the luteinizing hormone to chorionic gonadotropin evolution. Mol. Cell. Endocrinol. 2014, 382, 781–790. [Google Scholar] [CrossRef]
- Ogino, M.H.; Tadi, P. Physiology, Chorionic Gonadotropin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fournier, T.; Guibourdenche, J.; Evain-Brion, D. Review: hCGs: Different sources of production, different glycoforms and functions. Placenta 2015, 36, S60–S65. [Google Scholar] [CrossRef]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, D.; Lee, Y.-G.; Won, J.; Hong, S.-H.; Kim, J.H.; Kang, Y.-J. Effects of intrauterine human chorionic gonadotropin administration on endometrial receptivity and embryo implantation. Life Sci. 2022, 311, 121–154. [Google Scholar] [CrossRef]
- Tang, S.; Han, U.T.; Na, R.; Ma, Y.; Zhao, J.; Wu, H.-Q. Effect of human chorionic gonadotropin on ovulation induction in female donkeys. Anim. Husb. Feed Sci. 2021, 42, 59–65. [Google Scholar]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Weedon-Fekjar, M.S.; Tasken, K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta 2012, 33, S87–S91. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.L.; Ryu, K.-S.; Ji, I.; Ji, T.H. The Luteinizing Hormone/Chorionic Gonadotropin Receptor Has Distinct Transmembrane Conductors for cAMP and Inositol Phosphate Signals. J. Biol. Chem. 1996, 271, 19283–19287. [Google Scholar] [CrossRef] [PubMed]
- Donadeu, F.X.; Esteves, C.L.; Doyle, L.K.; Walker, C.A.; Schauer, S.N.; Diaz, C.A. Phospholipase Cβ3 Mediates LH-Induced Granulosa Cell Differentiation. Endocrinology 2011, 152, 2857–2869. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G. Gonadotropic control of ovarian follicular growth and development. Mol. Cell. Endocrinol. 2001, 179, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Theofanakis, C.; Drakakis, P.; Besharat, A.; Loutradis, D. Human Chorionic Gonadotropin: The Pregnancy Hormone and More. Int. J. Mol. Sci. 2017, 18, 1059. [Google Scholar] [CrossRef]
- Wang, M.; Huang, R.; Liang, X.; Mao, Y.; Shi, W.; Li, Q. Recombinant LH supplementation improves cumulative live birth rates in the GnRH antagonist protocol: A multicenter retrospective study using a propensity score-matching analysis. Reprod. Biol. Endocrinol. 2022, 20, 114. [Google Scholar] [CrossRef]
- Lee, E.B.; Chakravarthi, V.P.; Wolfe, M.W.; Rumi, M.A.K. ERβ Regulation of Gonadotropin Responses during Folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef]
- Benyo, D.F.; Ravindranath, N.; Bassett, S.; Hutchison, J.; Zeleznik, A.J. Cellular aspects of corpus luteum function in the primate. Hum. Reprod. 1993, 8, 102–106. [Google Scholar] [CrossRef]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Fagbohun, C.F.; Downs, S.M. Metabolic Coupling and Ligand-Stimulated Meiotic Maturation in the Mouse Oocyte-Cumulus Cell Complex. Biol. Reprod. 1991, 45, 851–859. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Shimada, M.; Wayne, C.M.; Ochsner, S.A.; White, L.; Richards, J.S. Gene Expression Profiles of Cumulus Cell Oocyte Complexes during Ovulation Reveal Cumulus Cells Express Neuronal and Immune-Related Genes: Does this Expand Their Role in the Ovulation Process? Mol. Endocrinol. 2006, 20, 1300–1321. [Google Scholar] [CrossRef]
- Chan, C.C.; Ng, E.H.; Tang, O.S.; Yeung, W.S.; Lau, E.Y.; Ho, P.C. A prospective, randomized, double-blind study to compare two doses of recombinant human chorionic gonadotropin in inducing final oocyte maturity and the hormonal profile during the luteal phase. J. Clin. Endocrinol. Metab. 2005, 90, 3933–3938. [Google Scholar] [CrossRef]
- Chang, P.; Kenley, S.; Burns, T.; Denton, G.; Currie, K.; DeVane, G.; O’Dea, L. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: Results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization-embryo transfer. Fertil. Steril. 2001, 76, 67–74. [Google Scholar] [CrossRef]
- European Recombinant Human Chorionic Gonadotrophin Study Group. Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment--recombinant HCG versus urinary HCG. Hum. Reprod. 2000, 15, 1446–1451. [Google Scholar] [CrossRef]
- Youssef, M.A.; Abou-Setta, A.M.; Lam, W.S. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst. Rev. 2016, 4, Cd003719. [Google Scholar] [CrossRef]
- International Recombinant Human Chorionic Gonadotropin Study Group. Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle-stimulating hormone: A comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertil. Steril. 2001, 75, 1111–1118. [Google Scholar] [CrossRef]
- Farrag, A.; Costantini, A.; Manna, C.; Grimaldi, G. Recombinant HCG for triggering ovulation increases the rate of mature oocytes in women treated for ICSI. J. Assist. Reprod. Genet. 2008, 25, 461–466. [Google Scholar] [CrossRef]
- Cunha, T.O.; Martins, J.P.N. Graduate Student Literature Review: Effects of human chorionic gonadotropin on follicular and luteal dynamics and fertility in cattle. J. Dairy Sci. 2022, 105, 8401–8410. [Google Scholar] [CrossRef]
- Bao, B.; Garverick, H.A.; Smith, G.W.; Smith, M.F.; Salfen, B.E.; Youngquist, R.S. Changes in Messenger Ribonucleic Acid Encoding Luteinizing Hormone Receptor, Cytochrome P450-Side Chain Cleavage, and Aromatase are Associated with Recruitment and Selection of Bovine Ovarian Follicles. Biol. Reprod. 1997, 56, 1158–1168. [Google Scholar] [CrossRef]
- Xu, Z.; Garverick, H.A.; Smith, G.W.; Smith, M.F.; Hamilton, S.A.; Youngquist, R.S. Expression of Follicle-Stimulating Hormone and Luteinizing Hormone Receptor Messenger Ribonucleic Acids in Bovine Follicles during the First Follicular Wave. Biol. Reprod. 1995, 53, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Bodensteiner, K.J.; Wiltbank, M.C.; Bergfelt, D.R.; Ginther, O.J. Alterations in follicular estradiol and gonadotropin receptors during development of bovine antral follicles. Theriogenology 1996, 45, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Koenigsfeld, A.T.; Cantley, T.C.; Boyd, C.K.; Kobayashi, Y.; Lucy, M.C. Growth and the initiation of steroidogenesis in porcine follicles are associated with unique patterns of gene expression for individual componentsof the ovarian insulin-like growth factor system. Biol. Reprod. 2000, 63, 942–952. [Google Scholar] [CrossRef]
- LaVoie, H.A. Transcriptional control of genes mediating ovarian follicular growth, differentiation, and steroidogenesis in pigs. Mol. Reprod. Dev. 2017, 84, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Filicori, M.; Cognigni, G.E.; Taraborrelli, S.; Spettoli, D.; Ciampaglia, W.; de Fatis, C.T.; Pocognoli, P. Luteinizing hormone activity supplementation enhances follicle-stimulating hormone efficacy and improves ovulation induction outcome. J. Clin. Endocrinol. Metab. 1999, 84, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Filicori, M.; Cognigni, G.E.; Tabarelli, C.; Pocognoli, P.; Taraborrelli, S.; Spettoli, D.; Ciampaglia, W. Stimulation and growth of antral ovarian follicles by selective LH activity administration in women. J. Clin. Endocrinol. Metab. 2002, 87, 1156–1161. [Google Scholar] [CrossRef]
- Filicori, M.; Cognigni, G.E.; Samara, A.; Melappioni, S.; Perri, T.; Cantelli, B.; Parmegiani, L.; Pelusi, G.; DeAloysio, D. The use of LH activity to drive folliculogenesis: Exploring uncharted territories in ovulation induction. Hum. Reprod. Update 2002, 8, 543–557. [Google Scholar] [CrossRef]
- Filicori, M.; Cognigni, G.E.; Gamberini, E.; Parmegiani, L.; Troilo, E.; Roset, B. Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil. Steril. 2005, 84, 394–401. [Google Scholar] [CrossRef]
- Lee, A.; Miller, K.A.; Elkind-Hirsch, K.E.; Scott, R.T. The “Un-Coast”—Use of low-dose hCG alone to complete ovarian folliculogenesis in a high responder. Fertil. Steril. 2004, 82, S120. [Google Scholar] [CrossRef]
- Lopes, T.P.; Padilla, L.; Bolarin, A.; Rodriguez-Martinez, H.; Roca, J. Weaned Sows with Small Ovarian Follicles Respond Poorly to the GnRH Agonist Buserelin. Animals 2020, 10, 1979. [Google Scholar] [CrossRef]
- Lopes, T.P.; Padilla, L.; Bolarin, A.; Rodriguez-Martinez, H.; Roca, J. Ovarian Follicle Growth during Lactation Determines the Reproductive Performance of Weaned Sows. Animals 2020, 10, 1012. [Google Scholar] [CrossRef]
- Sahin, S.; Ozay, A.; Ergin, E.; Turkgeldi, L.; Kurum, E.; Ozornek, H. The risk of ectopic pregnancy following GnRH agonist triggering compared with hCG triggering in GnRH antagonist IVF cycles. Arch. Gynecol. Obstet. 2015, 291, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Beck-Fruchter, R.; Golan, J.; Lavee, M.; Geslevich, Y.; Shalev, E. Ectopic pregnancy risk factors for ART patients undergoing the GnRH antagonist protocol: A retrospective study. Reprod. Biol. Endocrinol. 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhai, Y.; Qu, D. Management of ectopic pregnancy after in vitro fertilization/intracytoplasmic sperm injection and embryo transfer: A case series and mini-review. Asian Biomed. (Res. Rev. News) 2024, 18, 18–23. [Google Scholar] [CrossRef]
- Jiao, X.; Chu, Z.; Li, M.; Wang, J.; Ren, Z.; Wang, L.; Lu, C.; Li, X.; Ren, F.; Wu, X. GnRH-mediated suppression of S100A4 expression inhibits endometrial epithelial cell proliferation in sheep via GNAI2/MAPK signaling. Front. Vet. Sci. 2024, 11, 1445291. [Google Scholar] [CrossRef]
- Taponen, J. Ovarian Function in Dairy Cattle After Gonadotropin-Releasing Hormone Treatments During Perioestrus. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 29 August 2003. [Google Scholar]
- Degenstein, K.L.; O’Donoghue, R.; Patterson, J.L.; Beltranena, E.; Ambrose, D.J.; Foxcroft, G.R.; Dyck, M.K. Synchronization of ovulation in cyclic gilts with porcine luteinizing hormone (pLH) and its effects on reproductive function. Theriogenology 2008, 70, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Driancourt, M.A.; Cox, P.; Rubion, S.; Harnois-Milon, G.; Kemp, B.; Soede, N.M. Induction of an LH surge and ovulation by buserelin (as Receptal) allows breeding of weaned sows with a single fixed-time insemination. Theriogenology 2013, 80, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Martinat-Botte, F.; Venturi, E.; Guillouet, P.; Driancourt, M.A.; Terqui, M. Induction and synchronization of ovulations of nulliparous and multiparous sows with an injection of gonadotropin-releasing hormone agonist (Receptal). Theriogenology 2010, 73, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Li, Y.; Tian, J.; Li, J.; Weng, S.; Zhang, X. Research progress on timed insemination technology in pigs. Chin. J. Anim. Sci. 2019, 55, 28–33. [Google Scholar]
- Magon, N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian J. Endocrinol. Metab. 2011, 15, 261–267. [Google Scholar] [CrossRef]
- Nissen, A.K.; Lehn-Jensen, H.; Hyttel, P.; Greve, T. Follicular development and ovulation in sows: Effect of hCG and GnRH treatment. Acta Vet. Scand. 1995, 36, 123–133. [Google Scholar] [CrossRef]
- Pearodwong, P.; Tretipskul, C.; Soede, N.M.; Tummaruk, P. Factors affecting estrus and ovulation time in weaned sows with induced ovulation by GnRH administration in different seasons. J. Vet. Med. Sci. 2019, 81, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, Y.; Yu, B.; Bai, J.; Xu, X.; Qin, H.; Song, Y.; Liu, Y.; Li, J. Effect of triptorelin on timed insemination in primiparous sows. Chin. J. Anim. Sci. 2022, 58, 146–150. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, Y.J.; Jang, M.; Bae, S.G.; Yun, S.H.; Lee, M.R.; Seo, Y.R.; Cho, J.K.; Kim, S.J.; Lee, W.J. Heat Stress during Summer Attenuates Expression of the Hypothalamic Kisspeptin, an Upstream Regulator of the Hypothalamic-Pituitary-Gonadal Axis, in Domestic Sows. Animals 2022, 12, 2967. [Google Scholar] [CrossRef]
- De Rensis, F.; Kirkwood, R.N. Control of estrus and ovulation: Fertility to timed insemination of gilts and sows. Theriogenology 2016, 86, 1460–1466. [Google Scholar] [CrossRef]
- Youssef, M.A.; Van der Veen, F.; Al-Inany, H.G.; Mochtar, M.H.; Griesinger, G.; Nagi Mohesen, M.; Aboulfoutouh, I.; van Wely, M. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst. Rev. 2014, 2014, CD008046. [Google Scholar] [CrossRef] [PubMed]
- Humaidan, P.; Bredkjar, H.E.; Bungum, L.; Bungum, M.; Grondahl, M.L.; Westergaard, L.; Andersen, C.Y. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: A prospective randomized study. Hum. Reprod. 2005, 20, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Kol, S.; Humaidan, P. GnRH agonist triggering: Recent developments. Reprod. BioMed. Online 2013, 26, 226–230. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Holyoake, P.K.; Evans, G.; Grupen, C.G. Seasonal variation in the ovarian function of sows. Reprod. Fertil. Dev. 2012, 24, 822–834. [Google Scholar] [CrossRef]
- Martinez, M.F.; Adams, G.P.; Bergfelt, D.R.; Kastelic, J.P.; Mapletoft, R.J. Effect of LH or GnRH on the dominant follicle of the first follicular wave in beef heifers. Anim. Reprod. Sci. 1999, 57, 23–33. [Google Scholar] [CrossRef]
- Uddin, A.; Petrovski, K.R.; Song, Y.; Garg, S.; Kirkwood, R.N. Application of Exogenous GnRH in Food Animal Production. Animals 2023, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Jourquin, J.; Kauffold, J.; Sarrazin, S.; Dewulf, J.; Maes, D. Effect of a GnRH analogue (peforelin) on the litter performance of gilts and sows. Porc. Health Manag. 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Seyfang, J.; Langendijk, P.; Chen, T.Y.; Bouwman, E.; Kirkwood, R.N. Human chorionic gonadotrophin in early gestation induces growth of estrogenic ovarian follicles and improves primiparous sow fertility during summer. Anim. Reprod. Sci. 2016, 172, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bolamba, D.; Matton, P.; Sirard, M.; Estrada, R.; Dufour, J. Ovarian morphological conditions and the effect of injection of human chorionic gonadotropin on ovulation rates in prepuberal gilts with two morphologically different ovarian types. J. Anim. Sci. 1991, 69, 3774–3779. [Google Scholar] [CrossRef]
- De Rensis, F.; Valentini, R.; Gorrieri, F.; Bottarelli, E.; Lopez-Gatius, F. Inducing ovulation with hCG improves the fertility of dairy cows during the warm season. Theriogenology 2008, 69, 1077–1082. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Klos, J.; Gromadzka-Hliwa, K.; Dietrich, M.A.; Slowinska, M.; Likszo, P.; Knapczyk-Stwora, K.; Gajewski, Z.; Kaczmarek, M.M. Endocrine and molecular milieus of ovarian follicles are diversely affected by human chorionic gonadotropin and gonadotropin-releasing hormone in prepubertal and mature gilts. Sci. Rep. 2021, 11, 13465. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, Y.; Jansson, L.; Sato, Y.; Deguchi, M.; Kawamura, K.; Hsueh, A.J. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 2015, 29, 2423–2430. [Google Scholar] [CrossRef]
- Sasson, R.; Rimon, E.; Dantes, A.; Cohen, T.; Shinder, V.; Land-Bracha, A.; Amsterdam, A. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol. Human Reprod. 2004, 10, 299–311. [Google Scholar] [CrossRef]
- Kulus, J.; Kranc, W.; Kulus, M.; Dzięgiel, P.; Bukowska, D.; Mozdziak, P.; Kempisty, B.; Antosik, P. Expression of genes regulating cell division in porcine follicular granulosa cells. Cell Div. 2023, 18, 12. [Google Scholar] [CrossRef]
- Kemp, B.; Soede, N.M. Consequences of variation in interval from insemination to ovulation on fertilization in pigs. J. Reprod. Fertil. Suppl. 1997, 52, 79–89. [Google Scholar] [CrossRef]
- Soede, N.M.; Kemp, B. In synchronized pigs, the duration of ovulation is not affected by insemination and is not a determinant for early embryonic diversity. Theriogenology 1993, 39, 1043–1053. [Google Scholar] [CrossRef]
- Cheng, L.; Xiao, Y.; Yang, T.; Lian, C.; Du, C.; Teng, Z. Gonadotropin-releasing hormone analogue was used to update the timed insemination program in sows. Swine Ind. Sci. 2024, 41, 18–24. [Google Scholar]
- Zhu, X.; Zhang, S. Innovative applications of swine batch production in our country. Swine Ind. Sci. 2024, 41, 18–21. [Google Scholar]
- Falceto, M.V.; Suarez-Usbeck, A.; Tejedor, M.T.; Ausejo, R.; Garrido, A.M.; Mitjana, O. GnRH agonists: Updating fixed-time artificial insemination protocols in sows. Reprod. Domest. Anim. 2023, 58, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Brussow, K.P.; Schneider, F.; Kanitz, W.; Ratky, J.; Kauffold, J.; Wahner, M. Studies on fixed-time ovulation induction in the pig. Soc. Reprod. Fertil. Suppl. 2009, 66, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Physiology and Endocrinology Symposium: Factors influencing follicle development in gilts and sows and management strategies used to regulate growth for control of estrus and ovulation. J. Anim. Sci. 2019, 97, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Recent advancements in the hormonal stimulation of ovulation in swine. Vet. Med. 2015, 6, 309–320. [Google Scholar] [CrossRef]
- Polge, C.; Day, B.N.; Groves, T.W. Synchronisation of ovulation and artificial insemination in pigs. Vet. Rec. 1968, 83, 136–142. [Google Scholar] [CrossRef]
- Cassar, G.; Kirkwood, R.N.; Poljak, Z.; Friendship, R. Effect of estrogen formulation and site of deposition on fertility of artificially inseminated sows treated with human chorionic gonadotrophin to induce ovulation. J. Swine Health 2004, 12, 285–287. [Google Scholar] [CrossRef]
- Christenson, R.; Teague, H. Synchronization of ovulation and artificial insemination of sows after lactation. J. Anim. Sci. 1975, 41, 560–563. [Google Scholar] [CrossRef]
- Kirkwood, R.N.; Kauffold, J. Advances in Breeding Management and Use of Ovulation Induction for Fixed-time AI. Reprod. Domest. Anim. 2015, 50, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Belstra, B.A.; Willenburg, K.L.; Gomez-Lopez, D.H.; Knox, R.V.; Stewart, K.R. Effects of the number of sperm and site of uterine semen deposition on conception rate and the number of embryos in weaned sows receiving a single fixed-time insemination. J. Anim. Sci. 2020, 98, skaa260. [Google Scholar] [CrossRef]
- Das, P.K.; Mukherjee, J.; Banerjee, D. Female Reproductive Physiology. In Textbook of Veterinary Physiology; Das, P.K., Sejian, V., Mukherjee, J., Banerjee, D., Eds.; Springer Nature: Singapore, 2023; pp. 513–568. [Google Scholar] [CrossRef]
- Devoto, L.; Kohen, P.; Munoz, A.; Strauss, J.F., 3rd. Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reprod. Biomed. Online 2009, 18 (Suppl. S2), S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Przygrodzka, E.; Kaczmarek, M.M. Corpus Luteum Regression and Early Pregnancy Maintenance in Pigs. In The Life Cycle of the Corpus Luteum; Meidan, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 227–248. [Google Scholar] [CrossRef]
- Bolzan, E.; Andronowska, A.; Bodek, G.; Morawska-Pucinska, E.; Krawczynski, K.; Dabrowski, A.; Ziecik, A.J. The novel effect of hCG administration on luteal function maintenance during the estrous cycle/pregnancy and early embryo development in the pig. Pol. J. Vet. Sci. 2013, 16, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.B.; Bender, R.W.; Souza, A.H.; Ayres, H.; Araujo, R.R.; Guenther, J.N.; Sartori, R.; Wiltbank, M.C. Effect of treatment with human chorionic gonadotropin on day 5 after timed artificial insemination on fertility of lactating dairy cows. J. Dairy Sci. 2013, 96, 2873–2882. [Google Scholar] [CrossRef]
- Vergani, G.B.; da Fonseca, J.F.; Trevizan, J.T.; do Amaral Pereira, V.S.; Garcia, A.R.; Esteves, S.N.; Brandao, F.Z.; Souza-Fabjan, J.M.G.; Oliveira, M.E.F. Luteotropic effects of human chorionic gonadotropin administered 7.5 days after synchronous estrous induction in Morada Nova ewes. Anim. Reprod. Sci. 2020, 223, 106644. [Google Scholar] [CrossRef] [PubMed]
- Am-in, N.; Techakumphu, M.; Kirkwood, R.N. Effect of altering the ratio of exogenous gonadotropins on reproductive performance of primiparous sows during the seasonal infertility period. Can. J. Anim. Sci. 2019, 99, 202–205. [Google Scholar] [CrossRef]
- Pope, W.F. Uterine asynchrony: A cause of embryonic loss. Biol. Reprod. 1988, 39, 999–1003. [Google Scholar] [CrossRef]
- Ellicott, A.R.; Dziuk, P.J. Minimum daily dose of progesterone and plasma concentration for maintenance of pregnancy in ovariectomized gilts. Biol. Reprod. 1973, 9, 300–304. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Przygrodzka, E.; Jalali, B.M.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, R57–R67. [Google Scholar] [CrossRef]
- Bildik, G.; Akin, N.; Esmaeilian, Y.; Hela, F.; Yakin, K.; Onder, T.; Urman, B.; Oktem, O. hCG Improves Luteal Function and Promotes Progesterone Output through the Activation of JNK Pathway in the Luteal Granulosa Cells of the Stimulated IVF Cycles. Biol. Reprod. 2020, 102, 1270–1280. [Google Scholar] [CrossRef]
- Estill, C.T.; Britt, J.H.; Gadsby, J.E. Repeated administration of prostaglandin F2 alpha during the early luteal phase causes premature luteolysis in the pig. Biol. Reprod. 1993, 49, 181–185. [Google Scholar] [CrossRef]
- Grzesiak, M.; Knapczyk-Stwora, K.; Slomczynska, M. The impact of flutamide on prostaglandin F(2α) synthase and prostaglandin F(2α) receptor expression, and prostaglandin F(2α) concentration in the porcine corpus luteum of pregnancy. Domest. Anim. Endocrinol. 2017, 59, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Estill, C.T.; Britt, J.H.; Gadsby, J.E. Does increased PGF2 alpha receptor concentration mediate PGF2 alpha-induced luteolysis during early diestrus in the pig? Prostaglandins 1995, 49, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.H.; Goncalves, J.D.; Arrais, A.M.; Batista, R.I.T.P.; Souza-Fabjan, J.M.G.; Bastos, R.; Siqueira, L.G.B.; Oliveira, M.E.F.; Fonseca, J.F. Single dose of 300 IU hCG in the early luteal phase in superovulated ewes: Effects on corpora lutea, progesterone profile, and embryo recovery. Anim. Reprod. Sci. 2022, 247, 107101. [Google Scholar] [CrossRef] [PubMed]
- Farin, C.E.; Moeller, C.L.; Mayan, H.; Gamboni, F.; Sawyer, H.R.; Niswender, G.D. Effect of Luteinizing Hormone and Human Chorionic Gonadotropin on Cell Populations in the Ovine Corpus Luteum. Biol. Reprod. 1988, 38, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wiesak, T. Effect of pregnancy, injection of oestradiol benzoate or hCG on steroid concentration and release by pig luteal cells. J. Reprod. Fertil. 1989, 86, 247–254. [Google Scholar] [CrossRef]
- Rizos, D.; Scully, S.; Kelly, A.K.; Ealy, A.D.; Moros, R.; Duffy, P.; Al Naib, A.; Forde, N.; Lonergan, P. Effects of human chorionic gonadotrophin administration on Day 5 after oestrus on corpus luteum characteristics, circulating progesterone and conceptus elongation in cattle. Reprod. Fertil. Dev. 2012, 24, 472–481. [Google Scholar] [CrossRef]
- Cunha, T.O.; Statz, L.R.; Domingues, R.R.; Andrade, J.P.N.; Wiltbank, M.C.; Martins, J.P.N. Accessory corpus luteum induced by human chorionic gonadotropin on day 7 or days 7 and 13 of the estrous cycle affected follicular and luteal dynamics and luteolysis in lactating Holstein cows. J. Dairy Sci. 2022, 105, 2631–2650. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Bolt, D.J. Changes in Plasma Estrogen, Luteinizing Hormone, Follicle-Stimulating Hormone and 13, 14-Dihydro-15-Keto-Prostaglandin F2α during Blockade of Luteolysis in Pigs after human Chorionic Gonadotropin Treatment. J. Anim. Sci. 1983, 57, 993–1000. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Rexroad, C.E., Jr. Endometrial Prostaglandin F Release in Vitro and Plasma 13, 14-Dihydro-15-Keto-Prostaglandin F2α in Pigs with Luteolysis Blocked by Pregnancy, Estradiol Benzoate or Human Chorionic Gonadotropin. J. Anim. Sci. 1981, 52, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Howard, H.J.; Scott, R.G.; Britt, J.H. Associations among progesterone, estradiol-17β, oxytocin and prostaglandin in cattle treated with hCG during diestrus to extend corpus luteum function. Prostaglandins 1990, 40, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; Chapdelaine, P.; Madore, E.; Sirois, J.; Fortier, M.A. Prostaglandin Biosynthesis, Transport, and Signaling in Corpus Luteum: A Basis for Autoregulation of Luteal Function. Endocrinology 2004, 145, 2551–2560. [Google Scholar] [CrossRef]
- Langendijk, P. Latest Advances in Sow Nutrition during Early Gestation. Animals 2021, 11, 1720. [Google Scholar] [CrossRef]
- De Rensis, F.; Ziecik, A.J.; Kirkwood, R.N. Seasonal infertility in gilts and sows: Aetiology, clinical implications and treatments. Theriogenology 2017, 96, 111–117. [Google Scholar] [CrossRef]
- de Ruijter-Villani, M.; Stout, T. The Role of Conceptus–maternal Signalling in the Acquisition of Uterine Receptivity to Implantation in Mammals. Reprod. Domest. Anim. 2015, 50, 7–14. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early human trophoblast development: From morphology to function. Cell. Mol. Life Sci. 2022, 79, 345. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Endo, S.; Nagai, L.A.E.; Kobayashi, E.H.; Oike, A.; Kobayashi, N.; Kitamura, A.; Hori, T.; Nashimoto, Y.; Nakato, R.; et al. Modeling embryo-endometrial interface recapitulating human embryo implantation. Sci. Adv. 2024, 10, eadi4819. [Google Scholar] [CrossRef]
- Martinez, E.A.; Martinez, C.A.; Cambra, J.M.; Maside, C.; Lucas, X.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Rodriguez-Martinez, H.; Gil, M.A.; et al. Achievements and future perspectives of embryo transfer technology in pigs. Reprod. Domest. Anim. 2019, 54 (Suppl. S4), 4–13. [Google Scholar] [CrossRef]
- Valadao, L.; da Silva, H.M.; da Silva, F.M. Bovine embryonic development to implantation. In Embryology-Theory and Practice; IntechOpen: London, UK, 2018. [Google Scholar]
- Bulletti, C.; Flamigni, C.; Giacomucci, E. Reproductive failure due to spontaneous abortion and recurrent miscarriage. Hum. Reprod. Update 1996, 2, 118–136. [Google Scholar] [CrossRef]
- Gao, X.; Louwers, Y.V.; Laven, J.S.E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The endometrial microbiota and early pregnancy loss. Hum. Reprod. 2024, 39, 638–646. [Google Scholar] [CrossRef]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Bazer, F.; Burghardt, R.; Spencer, T.; Wu, G.; Bayless, K. Conceptus-uterus interactions in pigs: Endometrial gene expression in response to estrogens and interferons from conceptuses. Soc. Reprod. Fertil. Suppl. 2009, 66, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ka, H.; Seo, H.; Choi, Y.; Yoo, I.; Han, J. Endometrial response to conceptus-derived estrogen and interleukin-1β at the time of implantation in pigs. J. Anim. Sci. Biotechnol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Baird, D.D.; Weinberg, C.R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999, 340, 1796–1799. [Google Scholar] [CrossRef]
- Jiang, N.X.; Li, X.L. The Complicated Effects of Extracellular Vesicles and Their Cargos on Embryo Implantation. Front. Endocrinol. 2021, 12, 681266. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, V. Electron microscopy of the initial stages of placentation in the pig. Anat. Embryol. 1985, 172, 281–293. [Google Scholar] [CrossRef]
- Forde, N.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. ‘Conceptualizing’ the Endometrium: Identification of Conceptus-Derived Proteins During Early Pregnancy in Cattle. Biol. Reprod. 2015, 92, 156. [Google Scholar] [CrossRef]
- Guillomot, M. Cellular interactions during implantation in domestic ruminants. J. Reprod. Fertil. Suppl. 1995, 49, 39–51. [Google Scholar] [CrossRef]
- Kubota, K. Molecular approaches to mammalian uterine receptivity for conceptus implantation. J. Reprod. Dev. 2024, 70, 207–212. [Google Scholar] [CrossRef]
- Spencer, T.E.; Forde, N.; Lonergan, P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5941–5950. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.A.; Bazer, F.W.; Burghardt, R.C. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol. Reprod. 1996, 55, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, Y.S.; Yoon, J.A.; Lyu, S.W.; Shin, H.; Lim, H.J.; Hong, S.H.; Lee, D.R.; Song, H. Egr1 is rapidly and transiently induced by estrogen and bisphenol A via activation of nuclear estrogen receptor-dependent ERK1/2 pathway in the uterus. Reprod. Toxicol. 2014, 50, 60–67. [Google Scholar] [CrossRef]
- Liang, X.H.; Deng, W.B.; Li, M.; Zhao, Z.A.; Wang, T.S.; Feng, X.H.; Cao, Y.J.; Duan, E.K.; Yang, Z.M. Egr1 protein acts downstream of estrogen-leukemia inhibitory factor (LIF)-STAT3 pathway and plays a role during implantation through targeting Wnt4. J. Biol. Chem. 2014, 289, 23534–23545. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- Hudson, K.R.; Vernallis, A.B.; Heath, J.K. Characterization of the receptor binding sites of human leukemia inhibitory factor and creation of antagonists. J. Biol. Chem. 1996, 271, 11971–11978. [Google Scholar] [CrossRef]
- Sun, X.; Bartos, A.; Whitsett, J.A.; Dey, S.K. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Mol. Endocrinol. 2013, 27, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Celichowski, P.; Nawrocki, M.J.; Dyszkiewicz-Konwinska, M.; Jankowski, M.; Budna, J.; Bryja, A.; Kranc, W.; Borys, S.; Knap, S.; Ciesiólka, S.; et al. “Positive regulation of RNA metabolic process” ontology group highly regulated in porcine oocytes matured in vitro: A microarray approach. Biomed. Res. Int. 2018, 2018, 2863068. [Google Scholar] [CrossRef]
- Brazert, M.; Kranc, W.; Nawrocki, M.J.; Sujka-Kordowska, P.; Konwerska, A.; Jankowski, M.; Kocherova, I.; Celichowski, P.; Jeseta, M.; Ozegowska, K.; et al. New markers for regulation of transcription and macromolecule metabolic process in porcine oocytes during in vitro maturation. Mol. Med. Rep. 2020, 21, 1537–1551. [Google Scholar] [CrossRef]
- Takamoto, N.; Zhao, B.; Tsai, S.Y.; DeMayo, F.J. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol. Endocrinol. 2002, 16, 2338–2348. [Google Scholar] [CrossRef]
- Kubota, K.; Yamauchi, N.; Yamagami, K.; Nishimura, S.; Gobaru, T.; Yamanaka, K.; Wood, C.; Soh, T.; Takahashi, M.; Hattori, M.A. Steroidal regulation of Ihh and Gli1 expression in the rat uterus. Cell Tissue Res. 2010, 340, 389–395. [Google Scholar] [CrossRef]
- Katayama, S.; Ashizawa, K.; Gohma, H.; Fukuhara, T.; Narumi, K.; Tsuzuki, Y.; Tatemoto, H.; Nakada, T.; Nagai, K. The expression of Hedgehog genes (Ihh, Dhh) and Hedgehog target genes (Ptc1, Gli1, Coup-TfII) is affected by estrogenic stimuli in the uterus of immature female rats. Toxicol. Appl. Pharmacol. 2006, 217, 375–383. [Google Scholar] [CrossRef]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef]
- Kurihara, I.; Lee, D.K.; Petit, F.G.; Jeong, J.; Lee, K.; Lydon, J.P.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007, 3, e102. [Google Scholar] [CrossRef]
- Dias Da Silva, I.; Wuidar, V.; Zielonka, M.; Pequeux, C. Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview. Cells 2024, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.A.; Southey, B.R.; Everts, R.E.; Marjani, S.L.; Tian, C.X.; Lewin, H.A.; Rodriguez-Zas, S.L. Transferase activity function and system development process are critical in cattle embryo development. Funct. Integr. Genom. 2011, 11, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, A.; Chen, W.; Wei, J.; Fu, J.; Wang, A. Differential Gene Expression in Uterine Endometrium During Implantation in Pigs. Biol. Reprod. 2015, 92, 52. [Google Scholar] [CrossRef] [PubMed]
- Suluburic, A.; Milanovic, S.; Vranjes-Duric, S.; Jovanovic, I.B.; Barna, T.; Stojic, M.; Fratric, N.; Szenci, O.; Gvozdic, D. Progesterone concentration, pregnancy and calving rate in Simmental dairy cows after oestrus synchronisation and hCG treatment during the early luteal phase. Acta Vet. Hung. 2017, 65, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Przala, J.; Wiesak, T.; Grazul, A. Influence of LH, hCG and PRL on steroid release from granulosa cells of early pregnant and pseudopregnant sows. Endocrinol. Exp. 1985, 19, 304–311. [Google Scholar]
- Rajamahendran, R.; Sianangama, P.C. Effect of human chorionic gonadotrophin on dominant follicles in cows: Formation of accessory corpora lutea, progesterone production and pregnancy rates. J. Reprod. Fertil. 1992, 95, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.H.; Beck, N.F.; Khalid, M. The effects of GnRH analogue (buserelin) or hCG (Chorulon) on Day 12 of pregnancy on ovarian function, plasma hormone concentrations, conceptus growth and placentation in ewes and ewe lambs. Anim. Reprod. Sci. 2007, 102, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Manta, M.W.; da Silva, E.P.; Feltrin, S.R.; Prante, A.L.; Aires, K.V.; de Andrade, L.G.; da Silva, A.P.; Amaral, C.D.S.; Wink, L.M.; Portela, V.M.; et al. Human Chorionic Gonadotrophin (hCG) induces changes in IFN-pathway and Interferon-Stimulated Genes (ISGs) on the bovine endometrium at Day 18 of pregnancy. Anim. Reprod. 2024, 21, e20230130. [Google Scholar] [CrossRef]
- Besbaci, M.M.; Abdelli, A.; Belabdi, I.; Benabdelaziz, A.; Khelili, R.; Mebarki, M.; Kaidi, R. Effects of GnRH or hCG on day 11 after artificial insemination in cows luteal activity. J. Hell. Vet. Med. Soc. 2019, 69, 1227–1234. [Google Scholar] [CrossRef]
- Helmer, S.D.; Britt, J.H. Hormone Secretion and Characteristics of Estrous Cycles after Treatment of Heifers with Human Chorionic Gonadotropin or Prostaglandin F2α during Corpus Luteum Formation. J. Anim. Sci. 1987, 64, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.P.; Thatcher, W.W.; Pool, L.; Overton, M.W. Effect of human chorionic gonadotropin on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. J. Anim. Sci. 2001, 79, 2881–2894. [Google Scholar] [CrossRef]
- Nikbakht, K.; Habibizad, J.; Meamar, M. Effect of GnRH and hCG injection on the reproductive performance and serum progesterone concentration of ewes during spring season. Vet. Res. Forum Int. Q. J. 2022, 13, 553–561. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Portaluppi, M.A.; Tenhouse, D.E.; Lloyd, A.; Eborn, D.R.; Kacuba, S.; DeJarnette, J.M. Interventions after artificial insemination: Conception rates, pregnancy survival, and ovarian responses to gonadotropin-releasing hormone, human chorionic gonadotropin, and progesterone. J. Dairy Sci. 2007, 90, 331–340. [Google Scholar] [CrossRef]
- Schmitt, E.J.; Barros, C.M.; Fields, P.A.; Fields, M.J.; Diaz, T.; Kluge, J.M.; Thatcher, W.W. A cellular and endocrine characterization of the original and induced corpus luteum after administration of a gonadotropin-releasing hormone agonist or human chorionic gonadotropin on day five of the estrous cycle. J. Anim. Sci. 1996, 74, 1915–1929. [Google Scholar] [CrossRef]
- Fernandez, J.; Bruno-Galarraga, M.M.; Soto, A.T.; de la Sota, R.L.; Cueto, M.I.; Lacau-Mengido, I.M.; Gibbons, A.E. Effect of GnRH or hCG administration on Day 4 post insemination on reproductive performance in Merino sheep of North Patagonia. Theriogenology 2019, 126, 63–67. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Stanchev, P.D.; Tilton, J.E. Evidence for the Presence of Luteinizing Hormone/Human Chorionic Gonadotropin-Binding Sites in the Porcine Uterus. Endocrinology 1986, 119, 1159–1163. [Google Scholar] [CrossRef]
- Islami, D.; Bischof, P.; Chardonnens, D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol. Hum. Reprod. 2003, 9, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Blitek, A.; Schams, D.; Ziecik, A. Effect of Luteinizing Hormone and Tumour Necrosis Factor-Alpha on VEGF Secretion by Cultured Porcine Endometrial Stromal Cells. Reprod. Domest. Anim. 2010, 45, 481–486. [Google Scholar] [CrossRef]
- Szymanska, M.; Blitek, A. Endometrial and conceptus response to exogenous progesterone treatment in early pregnant gilts following hormonally-induced estrus. Anim. Reprod. Sci. 2016, 174, 56–64. [Google Scholar] [CrossRef]
- Kubota, K.; Miwa, M.; Hayashi, K.G.; Hosoe, M.; Sakatani, M. Steroidal but not embryonic regulation of mucin 1 expression in bovine endometrium. J. Reprod. Dev. 2021, 67, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Brayman, M.; Thathiah, A.; Carson, D.D. MUC1: A multifunctional cell surface component of reproductive tissue epithelia. Reprod. Biol. Endocrinol. 2004, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.A.; Bazer, F.W.; Burghardt, R.C. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vitro. Biol. Reprod. 1997, 56, 409–415. [Google Scholar] [CrossRef]
- de Mezer, M.; Rogalinski, J.; Przewozny, S.; Chojnicki, M.; Niepolski, L.; Sobieska, M.; Przystanska, A. SERPINA3: Stimulator or Inhibitor of Pathological Changes. Biomedicines 2023, 11, 156. [Google Scholar] [CrossRef]
- Sherwin, J.R.A.; Sharkey, A.M.; Cameo, P.; Mavrogianis, P.M.; Catalano, R.D.; Edassery, S.; Fazleabas, A.T. Identification of Novel Genes Regulated by Chorionic Gonadotropin in Baboon Endometrium during the Window of Implantation. Endocrinology 2007, 148, 618–626. [Google Scholar] [CrossRef]
- Zlotkowska, A.; Andronowska, A. Chemokines as the modulators of endometrial epithelial cells remodelling. Sci. Rep. 2019, 9, 12968. [Google Scholar] [CrossRef]
- Freis, A.; Germeyer, A.; Jauckus, J.; Capp, E.; Strowitzki, T.; Zorn, M.; Weber, A.M. Endometrial expression of receptivity markers subject to ovulation induction agents. Arch. Gynecol. Obstet. 2019, 300, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, Y.; Cheong, H.T.; Yang, B.K.; Park, C.K. Effects of 17β-estradiol, Interleukin-1β, and Human Chorionic Gonadotropin on Activity and mRNA Expression of Plasminogen Activators in Porcine Endometrial Cells. Dev. Reprod. 2018, 22, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, B.; Korpelainen, E.; Pepper, M.S.; Mandriota, S.J.; Aase, K.; Kumar, V.; Gunji, Y.; Jeltsch, M.M.; Shibuya, M.; Alitalo, K.; et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 11709–11714. [Google Scholar] [CrossRef]
- Murphy, S.P.; Tayade, C.; Ashkar, A.A.; Hatta, K.; Zhang, J.; Croy, B.A. Interferon gamma in successful pregnancies. Biol. Reprod. 2009, 80, 848–859. [Google Scholar] [CrossRef]
- von Rango, U. Fetal tolerance in human pregnancy—A crucial balance between acceptance and limitation of trophoblast invasion. Immunol. Lett. 2008, 115, 21–32. [Google Scholar] [CrossRef]
- Bidarimath, M.; Khalaj, K.; Wessels, J.M.; Tayade, C. MicroRNAs, immune cells and pregnancy. Cell. Mol. Immunol. 2014, 11, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.; Zenclussen, A.C. How cells of the immune system prepare the endometrium for implantation. Semin. Reprod. Med. 2014, 32, 358–364. [Google Scholar] [CrossRef]
- Li, D.; Zheng, L.; Zhao, D.; Xu, Y.; Wang, Y. The Role of Immune Cells in Recurrent Spontaneous Abortion. Reprod. Sci. 2021, 28, 3303–3315. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, Y.; Qiao, Y.; Tang, Y.; Sui, X.; Yin, P.; Yang, D. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 18434. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Raghupathy, R.; Saito, S.; Szekeres-Bartho, J. Cytokines, Hormones and Cellular Regulatory Mechanisms Favoring Successful Reproduction. Front. Immunol. 2021, 12, 717808. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.R.; Sinedino, L.D.P.; Spencer, T.E. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 2017, 154, F45–F59. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.W.; Malayer, J.R.; Ritchey, J.W.; Geisert, R.D. Characterization of the interleukin-1beta system during porcine trophoblastic elongation and early placental attachment. Biol. Reprod. 2003, 69, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Davies, C.; White, K.; Caron, K.; Golos, T.; Fazleabas, A.; Paria, B.; Mor, G.; Paul, S.; Ye, X.; et al. Interdisciplinary collaborative team for blastocyst implantation research: Inception and perspectives. Am. J. Reprod. Immunol. 2014, 71, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Velez, C.; Clauzure, M.; Williamson, D.; Koncurat, M.A.; Santa-Coloma, T.A.; Barbeito, C. IL-1β, IL-2 and IL-4 concentration during porcine gestation. Theriogenology 2019, 128, 133–139. [Google Scholar] [CrossRef]
- Velez, C.; Williamson, D.; Cánovas, M.L.; Giai, L.R.; Rutland, C.; Pérez, W.; Barbeito, C.G. Changes in Immune Response during Pig Gestation with a Focus on Cytokines. Vet. Sci. 2024, 11, 50. [Google Scholar] [CrossRef]
- Franczak, A.; Zmijewska, A.; Kurowicka, B.; Wojciechowicz, B.; Kotwica, G. Interleukin 1β-induced synthesis and secretion of prostaglandin E2 in the porcine uterus during various periods of pregnancy and the estrous cycle. J. Physiol. Pharmacol. 2010, 61, 733–742. [Google Scholar]
- Guay, F.; Matte, J.J.; Girard, C.L.; Palin, M.F.; Giguere, A.; Laforest, J.P. Effect of folic acid plus glycine supplement on uterine prostaglandin and endometrial granulocyte-macrophage colony-stimulating factor expression during early pregnancy in pigs. Theriogenology 2004, 61, 485–498. [Google Scholar] [CrossRef]
- Hansen, P.J.; Dobbs, K.B.; Denicol, A.C. Programming of the preimplantation embryo by the embryokine colony stimulating factor 2. Anim. Reprod. Sci. 2014, 149, 59–66. [Google Scholar] [CrossRef]
- Schafer, A.; Pauli, G.; Friedmann, W.; Dudenhausen, J.W. Human choriogonadotropin (hCG) and placental lactogen (hPL) inhibit interleukin-2 (IL-2) and increase interleukin-1β (IL-1β), -6 (IL-6) and tumor necrosis factor (TNF-α) expression in monocyte cell cultures. J. Perinat. Med. 1992, 20, 233–240. [Google Scholar] [CrossRef]
- Blitek, A.; Morawska, E.; Ziecik, A.J. Regulation of expression and role of leukemia inhibitory factor and interleukin-6 in the uterus of early pregnant pigs. Theriogenology 2012, 78, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.M.; Krawczynski, K.; Najmula, J.; Reliszko, Z.P.; Sikora, M.; Gajewski, Z. Differential expression of genes linked to the leukemia inhibitor factor signaling pathway during the estrus cycle and early pregnancy in the porcine endometrium. Reprod. Biol. 2014, 14, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Huang, H.; Li, X.; Huang, F.; Adeniran, S.O.; Wang, Z.; Feng, R.; Zhang, G. LRH-A3 and HCG increase pregnancy rate during timed artificial insemination in dairy cows. Anim. Sci. J. 2021, 92, e13549. [Google Scholar] [CrossRef] [PubMed]
- Akoum, A.; Metz, C.N.; Morin, M. Marked Increase in Macrophage Migration Inhibitory Factor Synthesis and Secretion in Human Endometrial Cells in Response to Human Chorionic Gonadotropin Hormone. J. Clin. Endocrinol. Metab. 2005, 90, 2904–2910. [Google Scholar] [CrossRef]
- Calandra, T.; Bernhagen, J.; Metz, C.N.; Spiegel, L.A.; Bacher, M.; Donnelly, T.; Cerami, A.; Bucala, R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995, 377, 68–71. [Google Scholar] [CrossRef]
- Paulesu, L.; Cateni, C.; Romagnoli, R.; Ietta, F.; Dantzer, V. Variation in Macrophage-Migration-Inhibitory-Factor Immunoreactivity During Porcine Gestation. Biol. Reprod. 2005, 72, 949–953. [Google Scholar] [CrossRef]
- Onodera, S.; Suzuki, K.; Matsuno, T.; Kaneda, K.; Takagi, M.; Nishihira, J. Macrophage migration inhibitory factor induces phagocytosis of foreign particles by macrophages in autocrine and paracrine fashion. Immunology 1997, 92, 131–137. [Google Scholar] [CrossRef]
- Enders, A.C.; Blankenship, T.N. Comparative placental structure. Adv. Drug Deliv. Rev. 1999, 38, 3–15. [Google Scholar] [CrossRef]
- Hafez, S. Comparative Placental Anatomy: Divergent Structures Serving a Common Purpose. Prog. Mol. Biol. Transl. Sci. 2017, 145, 1–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhu, X.; Zhu, W.; Li, L.; Wei, H.; Zhang, S. Research Progress on the Impact of Human Chorionic Gonadotropin on Reproductive Performance in Sows. Animals 2024, 14, 3266. https://doi.org/10.3390/ani14223266
Li J, Zhu X, Zhu W, Li L, Wei H, Zhang S. Research Progress on the Impact of Human Chorionic Gonadotropin on Reproductive Performance in Sows. Animals. 2024; 14(22):3266. https://doi.org/10.3390/ani14223266
Chicago/Turabian StyleLi, Jiahao, Xuedan Zhu, Wenjun Zhu, Li Li, Hengxi Wei, and Shouquan Zhang. 2024. "Research Progress on the Impact of Human Chorionic Gonadotropin on Reproductive Performance in Sows" Animals 14, no. 22: 3266. https://doi.org/10.3390/ani14223266
APA StyleLi, J., Zhu, X., Zhu, W., Li, L., Wei, H., & Zhang, S. (2024). Research Progress on the Impact of Human Chorionic Gonadotropin on Reproductive Performance in Sows. Animals, 14(22), 3266. https://doi.org/10.3390/ani14223266




