Regulation of Colonic Inflammation and Macrophage Homeostasis of IFN-γ-Primed Canine AMSCs in Experimental Colitis in Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of IFN-γ Priming of Canine AMSCs
2.2. Establishment of DSS-Induced Colitis Mouse Model and Assessment of Colitis Disease Severity
2.3. Histological Analysis of Colon Tissues
2.4. Isolation of Mesenteric Lymph Nodes (MLNs) and Colonic Lamina Propria Cells
2.5. Intracellular Cytokine Staining
2.6. Flow Cytometry Analysis
2.7. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis
2.8. Immunohistochemistry and Immunofluorescence Analysis
2.9. Statistical Analysis
3. Results
3.1. Canine IFN-γ-Primed AMSCs Alleviate DSS-Induced Acute Colitis in Mice
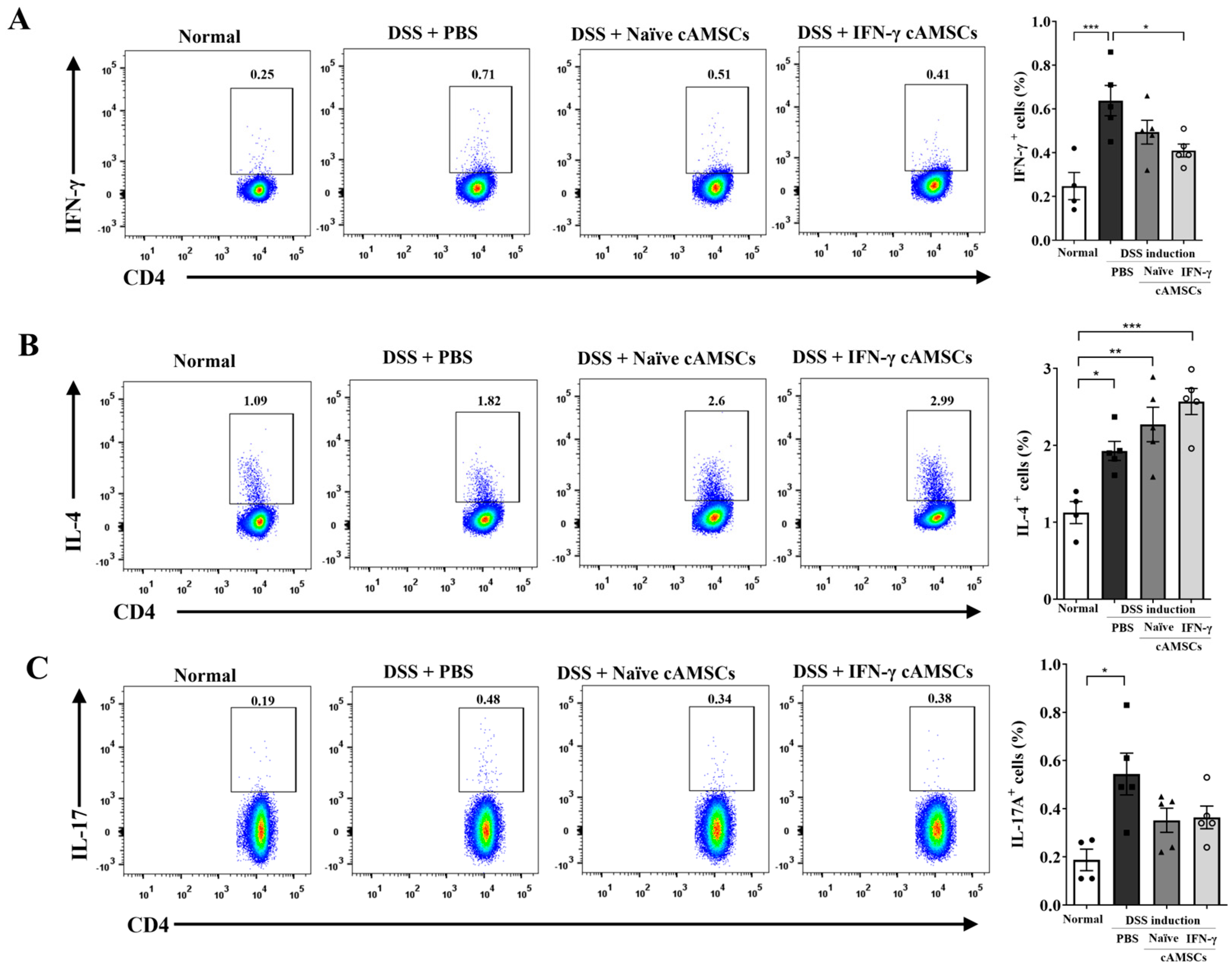
3.2. Suppression of Pathogenic T Cells by Canine IFN-γ-Primed AMSCs
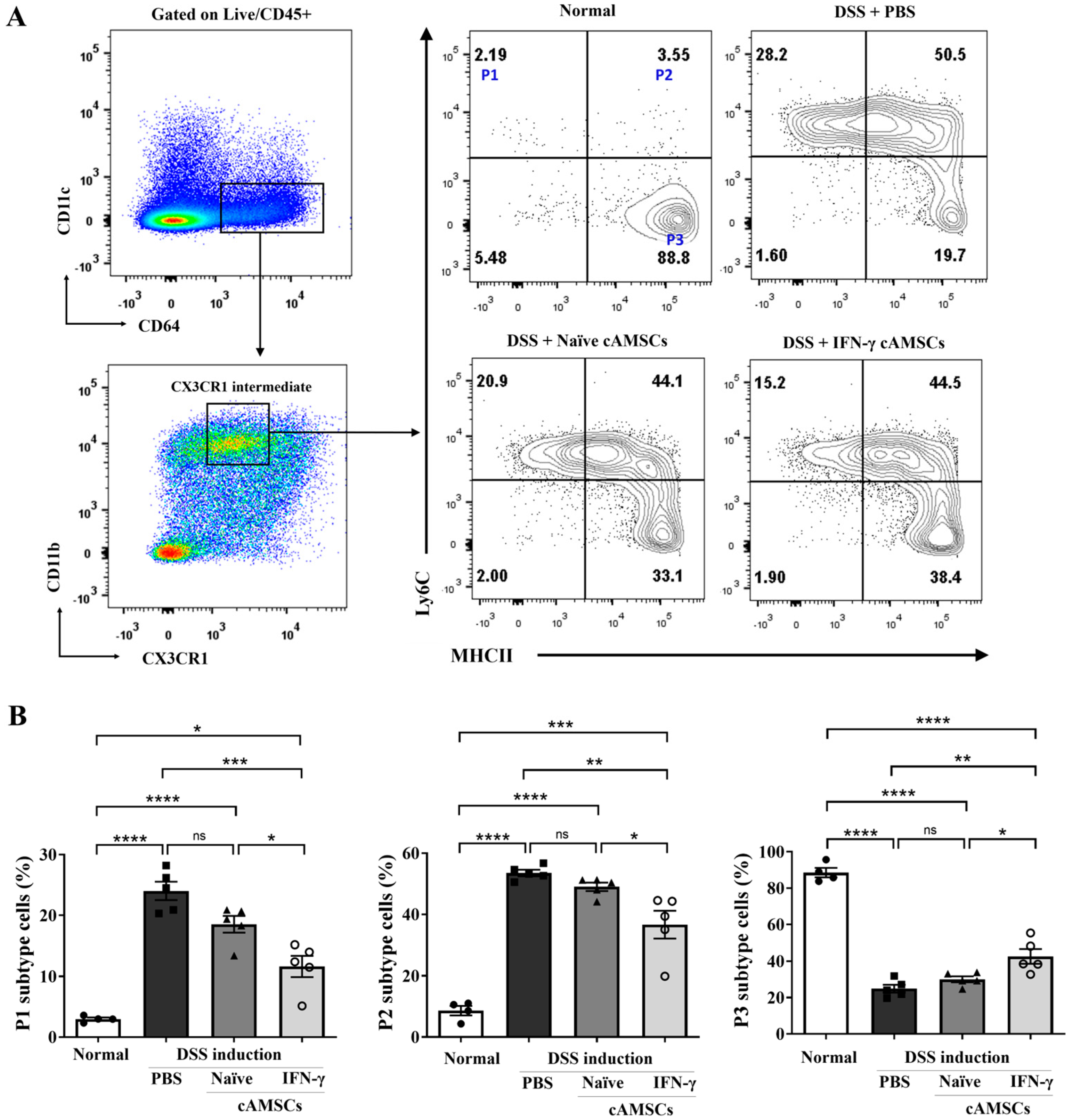
3.3. Reduction in Ly6Chigh Monocyte Accumulation in the Colonic Lamina Propria by Canine IFN-γ-Primed AMSCs
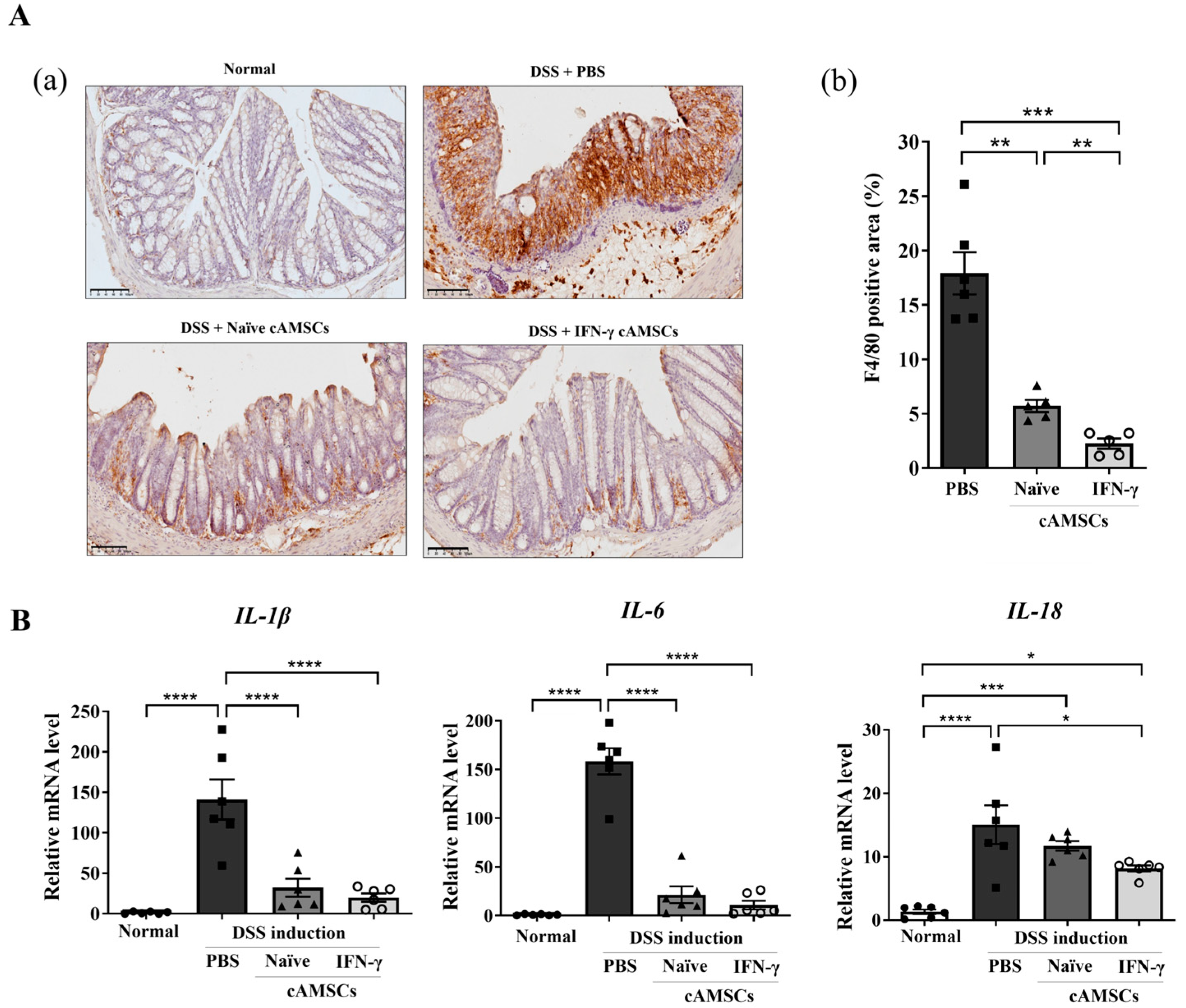
3.4. Reduction in Inflammatory Cytokines and Macrophage Infiltration in the Colon by Canine IFN-γ-Primed AMSCs
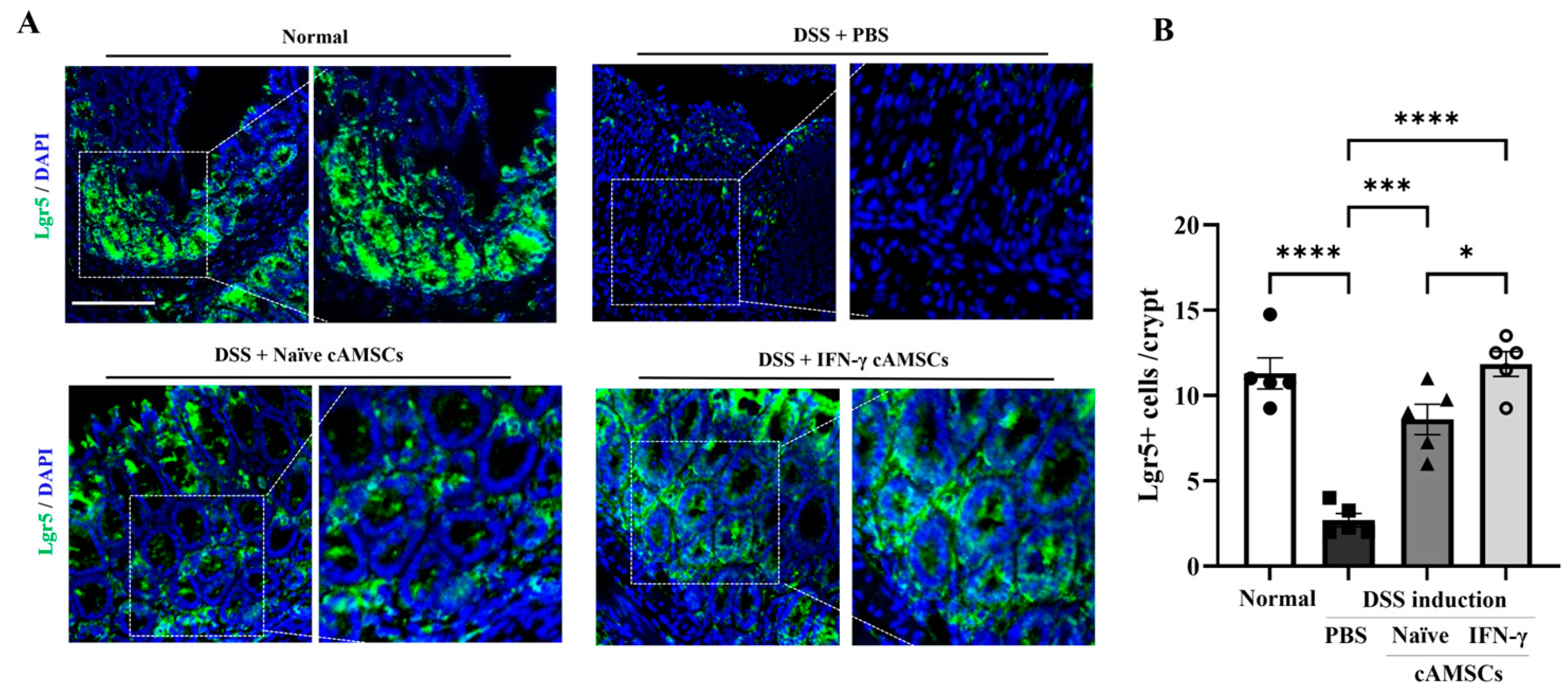
3.5. Induction of Regenerative Ability in Colonic Epithelial Cells by IFN-γ-Primed cAMSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jergens, A.E.; Simpson, K.W. Inflammatory bowel disease in veterinary medicine. Front. Biosci. 2012, 4, 1404–1419. [Google Scholar] [CrossRef]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Clinical and laboratory outcomes. Vet. J. 2015, 206, 385–390. [Google Scholar] [CrossRef]
- Cerquetella, M.; Spaterna, A.; Laus, F.; Tesei, B.; Rossi, G.; Antonelli, E.; Villanacci, V.; Bassotti, G. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J. Gastroenterol. 2010, 16, 1050–1056. [Google Scholar] [CrossRef]
- Langner, C.; Magro, F.; Driessen, A.; Ensari, A.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Borralho Nunes, P.; Cathomas, G.; Fries, W.; et al. The histopathological approach to inflammatory bowel disease: A practice guide. Virchows Arch. 2014, 464, 511–527. [Google Scholar] [CrossRef]
- Dias, I.E.; Dias, I.R.; Franchi-Mendes, T.; Viegas, C.A.; Carvalho, P.P. A Comprehensive Exploration of Therapeutic Strategies in Inflammatory Bowel Diseases: Insights from Human and Animal Studies. Biomedicines 2024, 12, 735. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy-Current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef]
- Dandrieux, J.R.S.; Mansfield, C.S. Chronic Enteropathy in Canines: Prevalence, Impact and Management Strategies. Vet. Med. 2019, 10, 203–214. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Hegarty, L.M.; Jones, G.R.; Bain, C.C. Macrophages in intestinal homeostasis and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 538–553. [Google Scholar] [CrossRef]
- Yip, J.L.K.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Liu, H.; Li, S.; Wang, Q. The Role of Tissue-Resident Macrophages in the Development and Treatment of Inflammatory Bowel Disease. Front. Cell. Dev. Biol. 2022, 10, 896591. [Google Scholar] [CrossRef]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef]
- Dandrieux, J.R.; Martinez Lopez, L.M.; Stent, A.; Jergens, A.; Allenspach, K.; Nowell, C.J.; Firestone, S.M.; Kimpton, W.; Mansfield, C.S. Changes in duodenal CD163-positive cells in dogs with chronic enteropathy after successful treatment. Innate Immun. 2018, 24, 400–410. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Lu, D.; Jiao, X.; Jiang, W.; Yang, L.; Gong, Q.; Wang, X.; Wei, M.; Gong, S. Mesenchymal stem cells influence monocyte/macrophage phenotype: Regulatory mode and potential clinical applications. Biomed. Pharmacother. 2023, 165, 115042. [Google Scholar] [CrossRef]
- Lu, D.; Xu, Y.; Liu, Q.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 681171. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; González-Jubete, A.; González, L.O.; Vizoso, F.J. Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges. Int. J. Mol. Sci. 2022, 23, 8905. [Google Scholar] [CrossRef]
- Shi, M.Y.; Liu, L.; Yang, F.Y. Strategies to improve the effect of mesenchymal stem cell therapy on inflammatory bowel disease. World J. Stem Cells 2022, 14, 684–699. [Google Scholar] [CrossRef]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e120. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Dunn, C.M.; Kameishi, S.; Cho, Y.K.; Song, S.U.; Grainger, D.W.; Okano, T. Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function. Cells 2022, 11, 3738. [Google Scholar] [CrossRef]
- Jauković, A.; Kukolj, T.; Obradović, H.; Okić-Đorđević, I.; Mojsilović, S.; Bugarski, D. Inflammatory niche: Mesenchymal stromal cell priming by soluble mediators. World J. Stem Cells 2020, 12, 922–937. [Google Scholar] [CrossRef]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R.; Vasandan, A.B. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef]
- English, K.; Barry, F.P.; Field-Corbett, C.P.; Mahon, B.P. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wang, Y.; Jin, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Shen, B.; Yin, S.; Liu, W.; Cui, L.; et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008, 18, 846–857. [Google Scholar] [CrossRef]
- Ahmadifard, R.; Jafarzadeh, A.; Mahmoodi, M.; Nemati, M.; Rahmani, M.; Khorramdelazad, H.; Ayoobi, F. Interferon-γ-Treated Mesenchymal Stem Cells Modulate the T Cell-Related Chemokines and Chemokine Receptors in an Animal Model of Experimental Autoimmune Encephalomyelitis. Drug Res. 2023, 73, 213–223. [Google Scholar] [CrossRef]
- Kurawaki, S.; Nakashima, A.; Ishiuchi, N.; Kanai, R.; Maeda, S.; Sasaki, K.; Masaki, T. Mesenchymal stem cells pretreated with interferon-gamma attenuate renal fibrosis by enhancing regulatory T cell induction. Sci. Rep. 2024, 14, 10251. [Google Scholar] [CrossRef]
- Park, A.; Park, H.; Yoon, J.; Kang, D.; Kang, M.H.; Park, Y.Y.; Suh, N.; Yu, J. Priming with Toll-like receptor 3 agonist or interferon-gamma enhances the therapeutic effects of human mesenchymal stem cells in a murine model of atopic dermatitis. Stem Cell Res. Ther. 2019, 10, 66. [Google Scholar] [CrossRef]
- Zigmond, E.; Varol, C.; Farache, J.; Elmaliah, E.; Satpathy, A.T.; Friedlander, G.; Mack, M.; Shpigel, N.; Boneca, I.G.; Murphy, K.M.; et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 2012, 37, 1076–1090. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Nakashima, A.; Doi, S.; Kimura, T.; Yoshida, K.; Maeda, S.; Ishiuchi, N.; Yamada, Y.; Ike, T.; Doi, T.; et al. Interferon-γ enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Sci. Rep. 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, T.; Han, C.; Wang, P.; Liu, X.; Zheng, C.; Bi, J.; Zhou, X. IFN-γ-Primed hUCMSCs Significantly Reduced Inflammation via the Foxp3/ROR-γt/STAT3 Signaling Pathway in an Animal Model of Multiple Sclerosis. Front. Immunol. 2022, 13, 835345. [Google Scholar] [CrossRef]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.; de Jonge-Muller, E.S.; Roelofs, H.; et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, J.; Liu, Z.; Hou, P.; Cao, L.; Zhang, Y.; Liu, R.; Li, Y.; Shang, Q.; Chen, Y.; et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem Cell Res. Ther. 2021, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, X.; Su, D.; Ren, Y.; Cheng, F.; Yao, Y.; Shi, G.; Ji, Y.; Chen, S.; Shi, P.; et al. Therapeutic efficacy of human adipose mesenchymal stem cells in Crohn’s colon fibrosis is improved by IFN-γ and kynurenic acid priming through indoleamine 2,3-dioxygenase-1 signaling. Stem Cell Res. Ther. 2022, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Li, Q.; Bhang, D.H.; Song, W.J.; Youn, H.Y. TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef]
- Song, W.J.; Li, Q.; Ryu, M.O.; Nam, A.; An, J.H.; Jung, Y.C.; Ahn, J.O.; Youn, H.Y. Canine adipose tissue-derived mesenchymal stem cells pre-treated with TNF-alpha enhance immunomodulatory effects in inflammatory bowel disease in mice. Res. Vet. Sci. 2019, 125, 176–184. [Google Scholar] [CrossRef] [PubMed]
- De Calisto, J.; Villablanca, E.J.; Mora, J.R. FcγRI (CD64): An identity card for intestinal macrophages. Eur. J. Immunol. 2012, 42, 3136–3140. [Google Scholar] [CrossRef]
- Li, Y.H.; Shen, S.; Shao, T.; Jin, M.T.; Fan, D.D.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Mesenchymal stem cells attenuate liver fibrosis by targeting Ly6C(hi/lo) macrophages through activating the cytokine-paracrine and apoptotic pathways. Cell Death Discov. 2021, 7, 239. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yan, L.; Wang, C.Z.; Wang, W.H.; Shi, H.; Su, B.B.; Zeng, Q.H.; Du, H.T.; Wan, J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J. Gastroenterol. 2013, 19, 4702–4717. [Google Scholar] [CrossRef]
- Alves, V.B.F.; de Sousa, B.C.; Fonseca, M.T.C.; Ogata, H.; Caliári-Oliveira, C.; Yaochite, J.N.U.; Rodrigues Júnior, V.; Chica, J.E.L.; da Silva, J.S.; Malmegrim, K.C.R.; et al. A single administration of human adipose tissue-derived mesenchymal stromal cells (MSC) induces durable and sustained long-term regulation of inflammatory response in experimental colitis. Clin. Exp. Immunol. 2019, 196, 139–154. [Google Scholar] [CrossRef]
- Guo, G.; Tan, Z.; Liu, Y.; Shi, F.; She, J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res. Ther. 2022, 13, 138. [Google Scholar] [CrossRef]
- Jovanovic, K.; Siebeck, M.; Gropp, R. The route to pathologies in chronic inflammatory diseases characterized by T helper type 2 immune cells. Clin. Exp. Immunol. 2014, 178, 201–211. [Google Scholar] [CrossRef]
- Yu, Z.L.; Gao, R.Y.; Lv, C.; Geng, X.L.; Ren, Y.J.; Zhang, J.; Ren, J.Y.; Wang, H.; Ai, F.B.; Wang, Z.Y.; et al. Notoginsenoside R1 promotes Lgr5(+) stem cell and epithelium renovation in colitis mice via activating Wnt/β-Catenin signaling. Acta Pharmacol. Sin. 2024, 45, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Sequence | Product Size (bp) | Anneal. Tm. (°C) | Accession |
|---|---|---|---|---|
| Il-1β | F: ATGACCTGTTCTTTGAAGTTGACG R: CCTGAAGCTCTTGTTGATGTGC | 128 | 60 | BC011437.1 |
| Il-6 | F: GGCCTTCCCTACTTCACAAG R: ATTTCCACGATTTCCCAGAG | 126 | 60 | NM_001314054.1 |
| Il-18 | F: GACTCTTGCGTCAACTTCAAGG R: TTTGTCAACGAAGAGAACTTGG | 159 | 60 | NM_008360.2 |
| Tbp | F: AGTGAAGAACAATCCAGACTAG R: TATAGGGAACTTCACATCACA | 129 | 60 | NM_013684.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, C.-H.; Lee, S.-Y.; Son, Y.-B.; Lee, W.-J.; Choe, Y.-H.; Lee, H.-J.; Oh, S.-J.; Kim, T.-S.; Hong, C.-Y.; Lee, S.-L.; et al. Regulation of Colonic Inflammation and Macrophage Homeostasis of IFN-γ-Primed Canine AMSCs in Experimental Colitis in Mice. Animals 2024, 14, 3283. https://doi.org/10.3390/ani14223283
Jo C-H, Lee S-Y, Son Y-B, Lee W-J, Choe Y-H, Lee H-J, Oh S-J, Kim T-S, Hong C-Y, Lee S-L, et al. Regulation of Colonic Inflammation and Macrophage Homeostasis of IFN-γ-Primed Canine AMSCs in Experimental Colitis in Mice. Animals. 2024; 14(22):3283. https://doi.org/10.3390/ani14223283
Chicago/Turabian StyleJo, Chan-Hee, Sang-Yun Lee, Young-Bum Son, Won-Jae Lee, Yong-Ho Choe, Hyeon-Jeong Lee, Seong-Ju Oh, Tae-Seok Kim, Chae-Yeon Hong, Sung-Lim Lee, and et al. 2024. "Regulation of Colonic Inflammation and Macrophage Homeostasis of IFN-γ-Primed Canine AMSCs in Experimental Colitis in Mice" Animals 14, no. 22: 3283. https://doi.org/10.3390/ani14223283
APA StyleJo, C.-H., Lee, S.-Y., Son, Y.-B., Lee, W.-J., Choe, Y.-H., Lee, H.-J., Oh, S.-J., Kim, T.-S., Hong, C.-Y., Lee, S.-L., & Rho, G.-J. (2024). Regulation of Colonic Inflammation and Macrophage Homeostasis of IFN-γ-Primed Canine AMSCs in Experimental Colitis in Mice. Animals, 14(22), 3283. https://doi.org/10.3390/ani14223283





