A Qualitative Model Demonstrating the Adaptation of Amphibians to Semi-Arid and Arid Habitats: Comparing the Green Toad Bufotes sitibundus (Pallas, 1771) and Pelophylax bedriagae (Camerano, 1882)
Simple Summary
Abstract
1. Introduction
2. Green Toad and Water Frog Classification
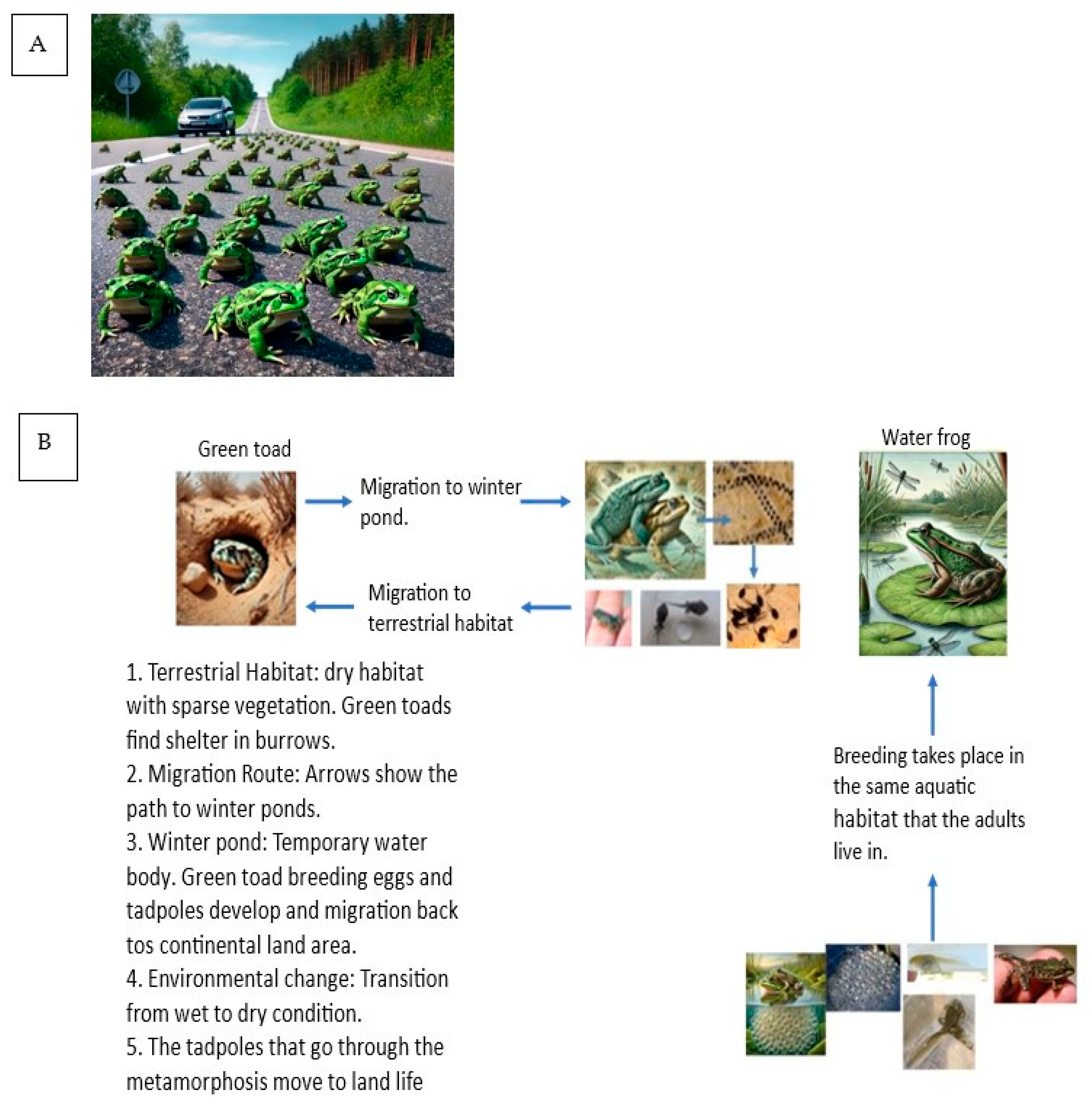
3. Habitat Variations Between Green Toads and Water Frogs in Israel
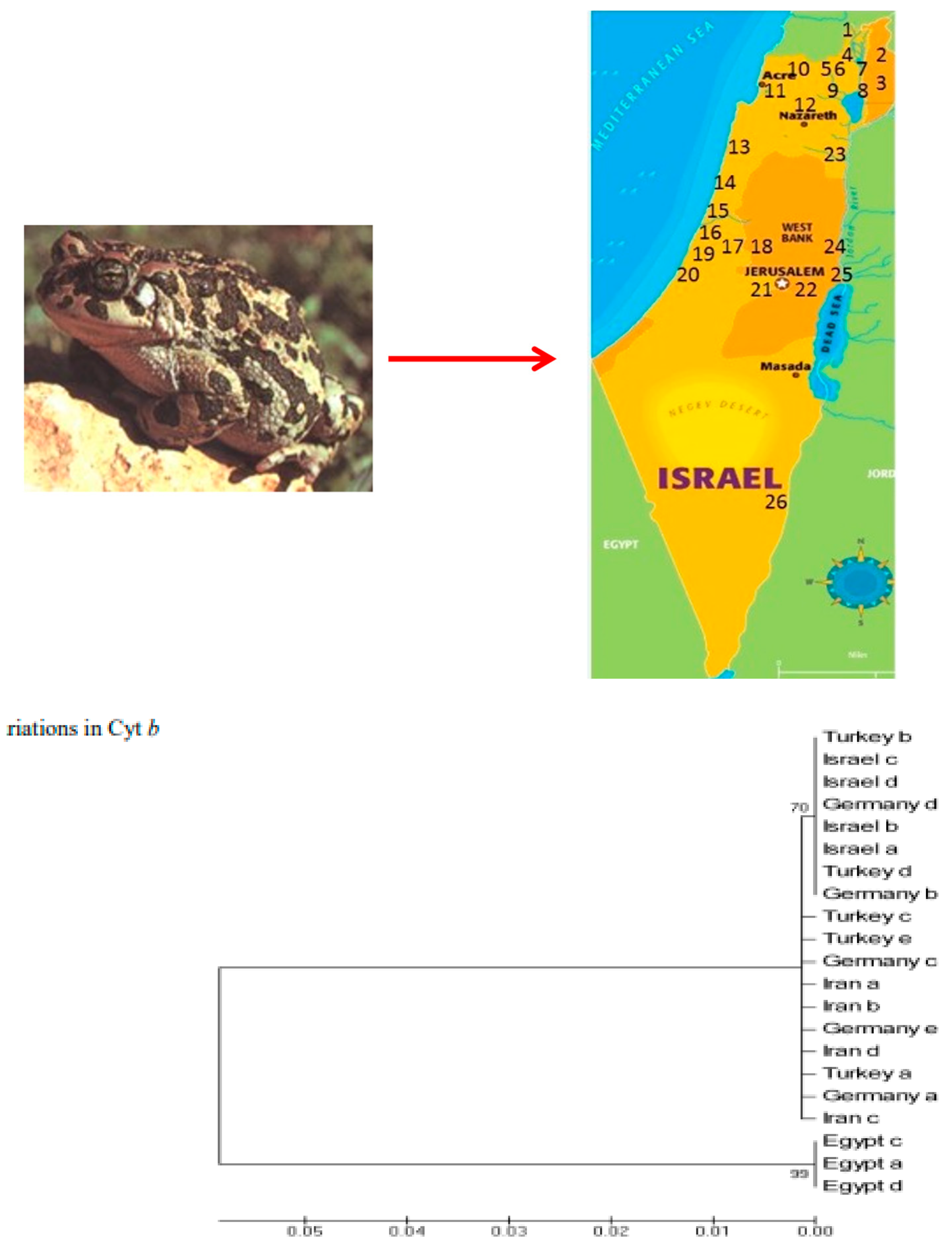
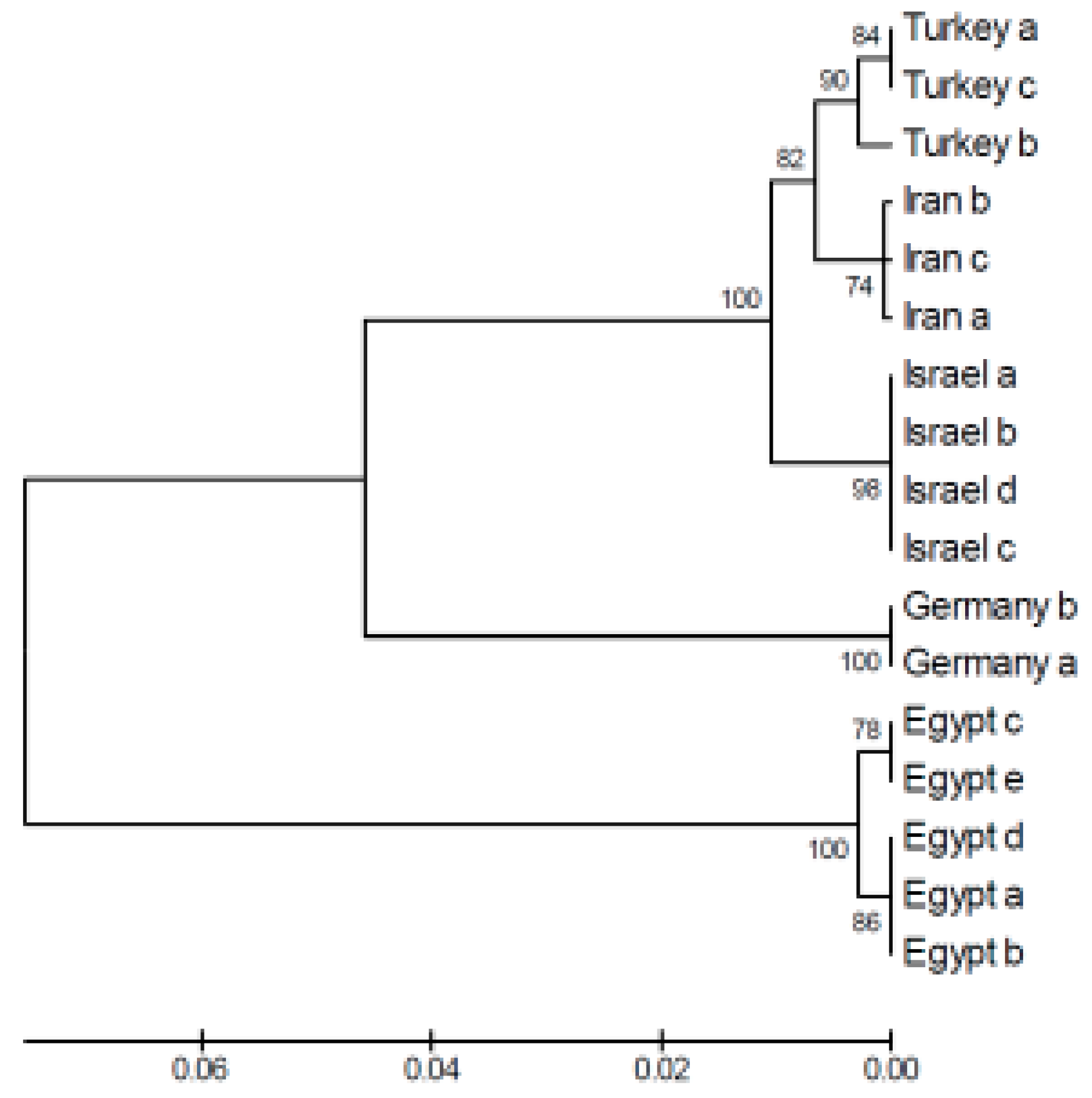
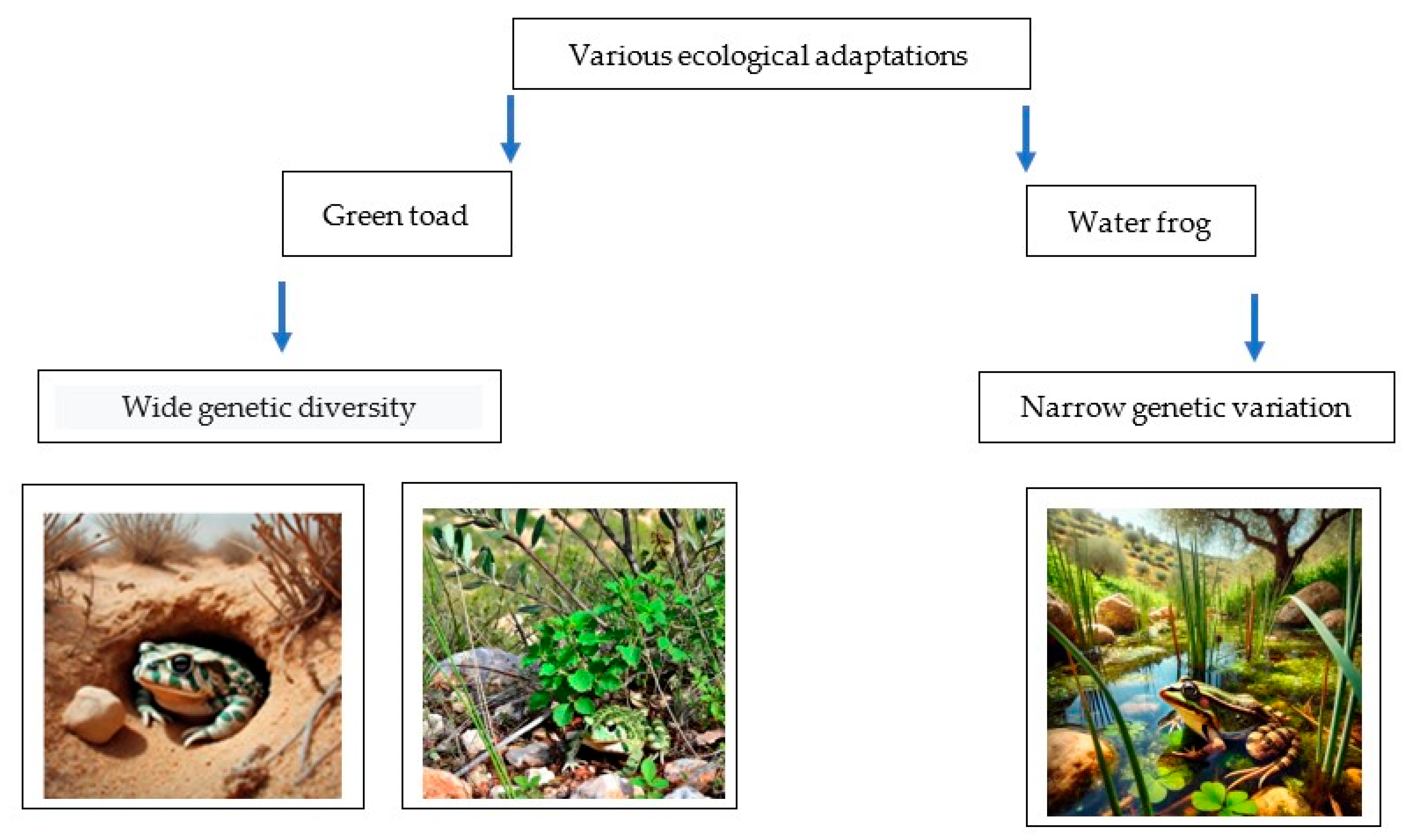
4. Comparison of Genetic Variation Between Green Toads and Water Frogs in Israel
5. Reproduction
5.1. Breeding Season
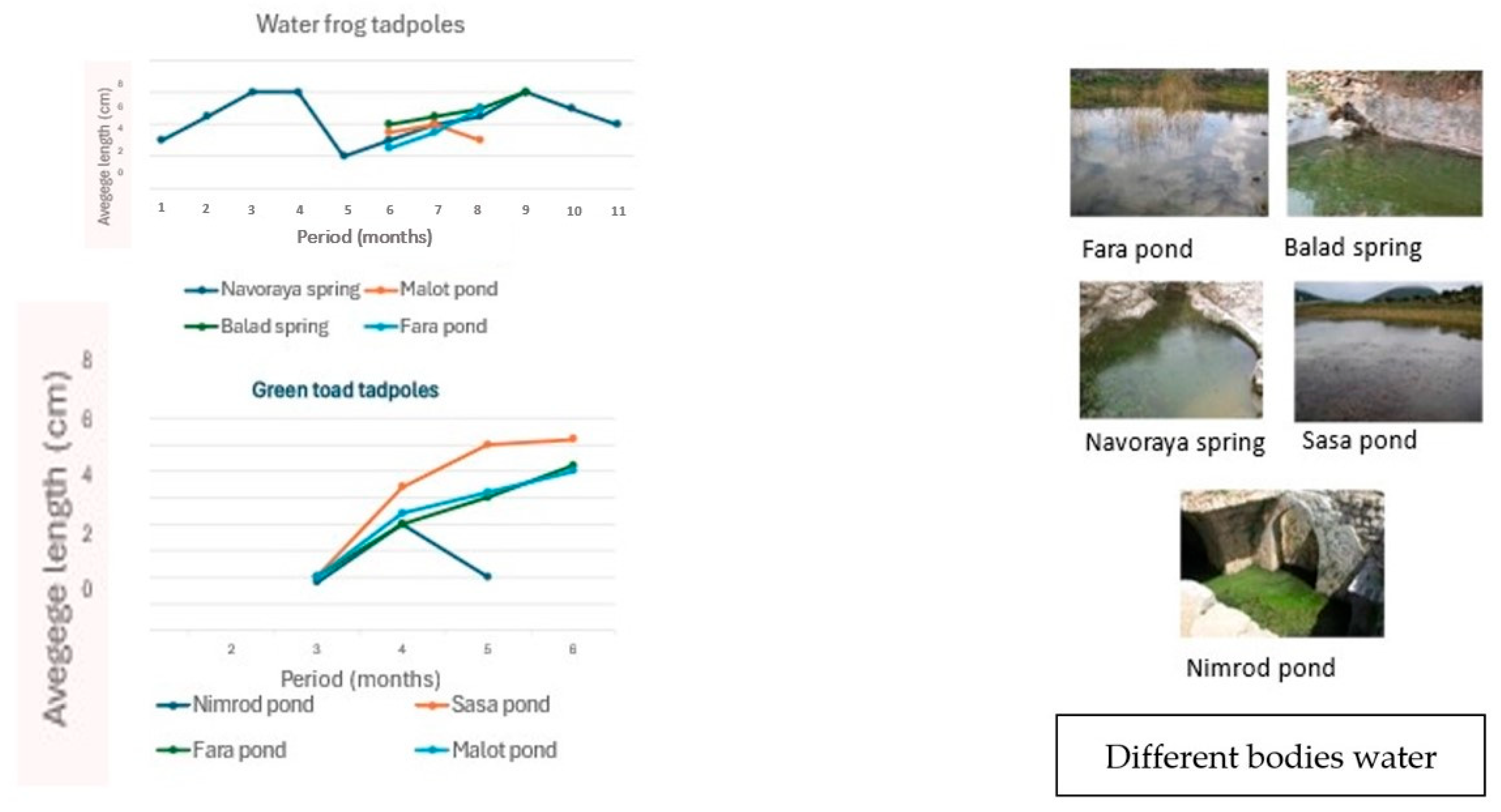
5.2. Breeding Sites
5.3. Mating Behavior
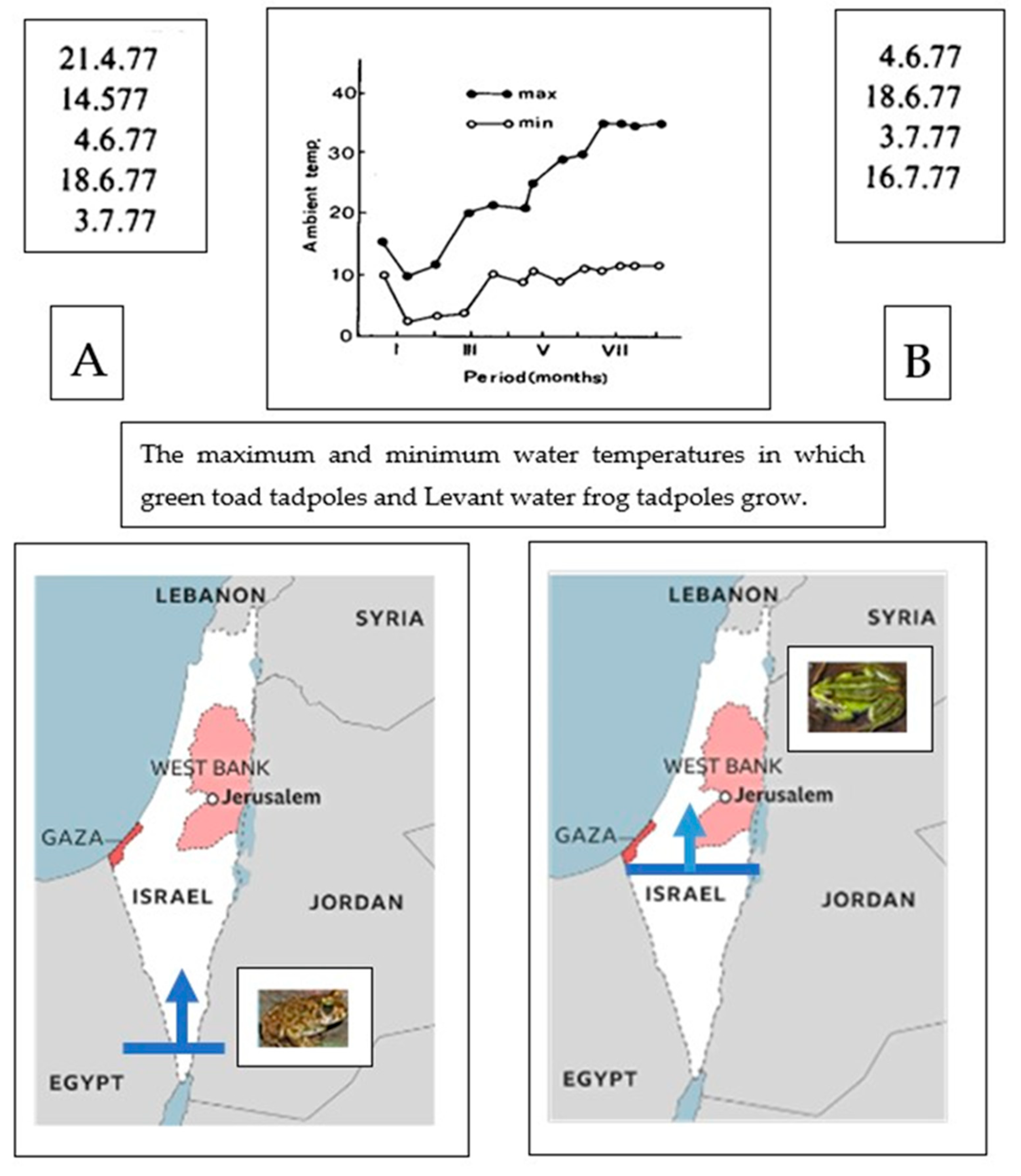
6. Tadpole Growth and Metamorphosis in Water Frogs and Green Toads
7. Different Physiological Adaptations to Terrestrial Life of Green Toads and Water Frogs
7.1. Green Toad
7.2. Water Frog
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degani, G. Biological Adaptations of Anuran Species Across Diverse Habitats, Spanning Mediterranean to Desert Climates; Scientific Research Publishing: Wuhan, China, 2024; pp. 1–92. [Google Scholar]
- Laurila, A. Breeding habitat selection and larval performance of two anurans in freshwater rockpools. Ecography 1998, 21, 484–494. [Google Scholar] [CrossRef]
- Degani, G.; Goldberg, T.; Gazith, A.; Elorom, E.; Nevo, E. The DNA Variation of Pseudepidalea viridis (Syn. Bufo viridis) from Various Habitats. Zool. Stud. 2013, 52, 18. [Google Scholar]
- Lymberakis, P.; Poulakakis, N.; Manthalou, G.; Tsigenopoulos, C.S.; Magoulas, A.; Mylonas, M. Mitochondrial phylogeography of Rana (Pelophylax) ridibunda and the Rana (Pelophylax) bedriagae species complex in the eastern Mediterranean region. Mol. Phylogenetics Evol. 2007, 44, 1153–1165. [Google Scholar] [CrossRef]
- Plötner, J.; Ohst, T.; Baier, F.; Schreiber, R.; Köhler, F. Mitochondrial and nuclear markers support a paraphyletic origin of the Levantine water frog Pelophylax bedriagae. Zootaxa 2009, 2067, 1–21. [Google Scholar]
- Dufresnes, C.; Litvinchuk, S.N. Diversity, distribution and molecular species delimitation in frogs and toads from the Eastern Palaearctic. Zool. J. Linn. Soc. 2022, 195, 695–760. [Google Scholar] [CrossRef]
- Dufresnes, C.; Mazepa, G.; Jablonski, D.; Oliveira, R.C.; Wenseleers, T.; Shabanov, D.A.; Auer, M.; Ernst, R.; Koch, C.; Ramírez-Chaves, H.E.; et al. Fifteen shades of green: The evolution of Bufotes toads re-visited. Mol. Phylogenetics Evol. 2019, 141, 106615. [Google Scholar] [CrossRef] [PubMed]
- Mendelssohn, H.; Steinitz, H. Contribution to the ecological zoogeography of the amphibians in Palestine. Istanb. Univ. Fen Fak. Mecm. 1944, 9, 289–298. [Google Scholar]
- Degani, G. Amphibian tadpole interaction in a winter pond. Hydrobiologia 1982, 96, 3–8. [Google Scholar] [CrossRef]
- Degani, G. Growth and behavior of six species of amphibian larvae in a winter pond in Israel. Hydrobiologia 1986, 140, 5–10. [Google Scholar] [CrossRef]
- Degani, G.; Goldberg, T. Effect of human activity in creating a death trap for Salamandra infraimmaculata seeking breeding places during colonization of new breeding sites. Am. Open Anim. Sci. J. 2015, 2, 1–11. [Google Scholar]
- Degani, G.; Goldberg, T.; Yom-Din, S. The ecology and variation in DNA of Rana bedreagae from various breeding site in North Israel. Res. Open. J. Anim. Sci. 2013, 1, 1–14. [Google Scholar]
- Degani, G.; Kaplan, D. Distribution of amphibian larvae in Israeli habitats with changeable water availability. Hydrobiologia 1999, 405, 49–56. [Google Scholar] [CrossRef]
- Degani, G.; Silanikove, N.; Shkolnik, A. Adaptation of Green Toad (Bufo viridis) to terrestrial life by urea accumulation. Comp. Biochem. Physiol. 1984, 77, 585–587. [Google Scholar] [CrossRef]
- Goldberg, T.; Nevo, E.; Degani, G. Amphibian Larval in Various Water Bodies in the Semi-arid Zone. Zool. Stud. 2012, 51, 345–361. [Google Scholar]
- Litvinchuk, S.N.; Skorinov, D.V.; Ivanov, A.Y.; Ermakov, O.A. Detection of glacial refugia and post-glacial colonization routes of morphologically cryptic marsh frog species (Anura: Ranidae: Pelophylax) using environmental niche modeling. Diversity 2024, 16, 94. [Google Scholar] [CrossRef]
- Askenderov, A.D.; Mazanaeva, L.F.; Mikhaylov, R.A.; Fayzulin, A.I. Spawning water bodies and their role in conservation of rare amphibian species in the foothills of the Republic of Dagestan (Russia). Nat. Conserv. Res. 2018, 3, 83–97. [Google Scholar] [CrossRef]
- Nessi, A.; Cioccarelli, S.; Tremolada, P.; Gariano, P.; Grandinetti, M.; Balestrieri, A.; Manenti, R. Environmental Factors Affecting Amphibian Communities in River Basins of the Southern Apennines. Diversity 2023, 15, 625. [Google Scholar] [CrossRef]
- Gvoždík, V.; Moravec, J.; Klütsch, C.; Kotlík, P. Phylogeography of the green toad complex (Bufo viridis subgroup) with insights on their dispersal routes in the Western Palearctic. Mol. Phylogenetics Evol. 2010, 55, 1108–1120. [Google Scholar]
- Geffen, E.; Yom-Tov, Y. Are the populations of the green toad (Bufo viridis) in Israel sexually dimorphic in size. Isr. J. Zool. 2000, 46, 221–228. [Google Scholar]
- Goldberg, T.; Degani, G.; Gazith, A.; Elron, E.; Nevo, E. Sequence variation in the mitochondrial DNA of Pseudepidalea viridis (Syn. Bufo viridis) in Israel. Bull. UASVM Anim. Sci. Biotechnol. 2011, 68, 51–57. [Google Scholar]
- Plötner, J.; Baier, F.; Demirsoy, A.; Uzzell, T. Genetic diversity and distribution of the water frogs in the eastern Mediterranean (Anura: Ranidae: Pelophylax). Zool. Scr. 2001, 30, 351–362. [Google Scholar]
- Degani, G. Ecological and Genetic Variation of the Distribution of Various Species of Amphibians at the Southern Border of their Distribution. Int. J. Plant Anim. Environ. Sci. 2019, 9, 21–41. [Google Scholar]
- Tóth, G.; Bán, Z.; Vörös, J.; Puky, M.; Végvári, Z.; Lesku, B.; Korsós, Z.; Varga, Z.; Horváth, R.; Farkas, J. Movements of green toads (Bufo viridis) in a Hungarian agricultural landscape. Herpetol. J. 2013, 23, 15–24. [Google Scholar]
- Daversa, D.; Erin Muths, E.; Jaime Bosch, J. Terrestrial Movement Patterns of the Common Toad (Bufo bufo) in Central Spain Reveal Habitat of Conservation Importance. J. Herpetol. 2012, 46, 658–664. [Google Scholar] [CrossRef]
- Goldberg, T.; Nevo, E.; Degani, G. Breeding site selection according to suitability for amphibian larval growth under various ecological conditions in the semi-arid zone of northern Israel. Ecol. Mediterr. 2009, 35, 65–74. [Google Scholar] [CrossRef]
- Sutherland, D.R. Water conservation strategies in amphibians. J. Herpetol. 1992, 26, 457–462. [Google Scholar]
- Degani, G. Urea tolerance and osmoregulation in Bufo viridis and Rana ridibunda. Comp. Biochem. Physiol. A 1985, 82, 833–836. [Google Scholar] [CrossRef]
- Degani, G. Osmoregulation in red blood cells of Bufo viridis. Comp. Biochem. Physiol. A 1985, 81, 451–453. [Google Scholar] [CrossRef]
- Smith, G.R.; Green, D.M. Habitat preferences of river frogs. Can. J. Zool. 1984, 62, 1401–1407. [Google Scholar]
- Griffiths, R.A. The effects of habitat alteration on amphibian populations. Herpetol. J. 1996, 6, 1–8. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degani, G. A Qualitative Model Demonstrating the Adaptation of Amphibians to Semi-Arid and Arid Habitats: Comparing the Green Toad Bufotes sitibundus (Pallas, 1771) and Pelophylax bedriagae (Camerano, 1882). Animals 2024, 14, 3351. https://doi.org/10.3390/ani14233351
Degani G. A Qualitative Model Demonstrating the Adaptation of Amphibians to Semi-Arid and Arid Habitats: Comparing the Green Toad Bufotes sitibundus (Pallas, 1771) and Pelophylax bedriagae (Camerano, 1882). Animals. 2024; 14(23):3351. https://doi.org/10.3390/ani14233351
Chicago/Turabian StyleDegani, Gad. 2024. "A Qualitative Model Demonstrating the Adaptation of Amphibians to Semi-Arid and Arid Habitats: Comparing the Green Toad Bufotes sitibundus (Pallas, 1771) and Pelophylax bedriagae (Camerano, 1882)" Animals 14, no. 23: 3351. https://doi.org/10.3390/ani14233351
APA StyleDegani, G. (2024). A Qualitative Model Demonstrating the Adaptation of Amphibians to Semi-Arid and Arid Habitats: Comparing the Green Toad Bufotes sitibundus (Pallas, 1771) and Pelophylax bedriagae (Camerano, 1882). Animals, 14(23), 3351. https://doi.org/10.3390/ani14233351



