Sustainable Poultry Nutrition Using Citric Acid By-Products from Rice to Boost Growth and Carcass Yield in Thai KKU 1 Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Source of Citric Acid By-Product from Rice (CABR)
2.3. Animals and Experimental Design
2.4. Data Collection
2.4.1. Performance Parameters
2.4.2. Carcass Yield
2.4.3. Meat Characteristic
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Carcass Yield
3.3. Meat Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehdikhany, S.; Zarei, A.; Lotfollahian, H.; Hoseini, S.A. Determination of nutritive value and the effect of citric acid production by-product on broiler performance. Indian J. Anim. Res. 2012, 46, 143–147. [Google Scholar]
- Suriyapha, C.; Suntara, C.; Cherdthong, A. Nutrients quality improvement of agro-industrial citric acid residues by fermentation with yeast waste from bioethanol processing to be used as ruminant feed. Waste Biomass Valor. 2024, 15, 4029–4042. [Google Scholar] [CrossRef]
- Reena, R.; Sindhu, R.; Balakumaran, P.A.; Pandey, A.; Awasthi, M.K.; Binod, P. Insight into citric acid: A versatile organic acid. Fuel 2022, 327, 125181. [Google Scholar] [CrossRef]
- Oryza, S.M.; Wongtangtintharn, S.; Tengjaroenkul, B.; Cherdthong, A.; Tanpong, S.; Bunchalee, P.; Pootthachaya, P.; Reungsang, A.; Polyorach, S. Physico-Chemical characteristics and amino acid content evaluation of citric acid by-product produced by microbial fermentation as a potential use in animal feed. Fermentation 2021, 7, 149. [Google Scholar] [CrossRef]
- Kudzai, C.T.; Ajay, K.; Ambika, P. Citric acid production by Aspergillus niger using different substrates. Malays. J. Microbiol. 2018, 12, 199–204. [Google Scholar] [CrossRef]
- Rehman, Z.; Mirza, M.; Mukhtar, N. Poultry research use of organic acids as potential feed additives in poultry production. J. World Poult. Res. 2016, 6, 105–116. [Google Scholar]
- Islam, M.Z.; Khandaker, Z.H.; Chowdhury, S.D.; Islam, K.M.S. Effect of citric acid and acetic acid on the performance of broilers. J. Bangladesh Agric. Univ. 2008, 6, 315–320. [Google Scholar] [CrossRef]
- Deepa, C.; Jeyanthi, G.P.; Chandrasekaran, D. Effect of phytase and citric acid supplementation on the growth performance, phosphorus, calcium and nitrogen retention on broiler chicks fed with low level of available phosphorus. Asian J. Poult. Sci. 2011, 5, 28–34. [Google Scholar] [CrossRef]
- Islam, K.M.S. Use of citric acid in broiler diets. Worlds Poult. Sci. J. 2012, 68, 104–118. [Google Scholar] [CrossRef]
- Tanpong, S.; Cherdthong, A.; Tengjaroenkul, B.; Tengjaroenkul, U.; Wongtangtintharn, S. Evaluation of physical and chemical properties of citric acid industrial waste. Trop. Anim. Health Prod. 2019, 51, 2167–2174. [Google Scholar] [CrossRef]
- Wattanachant, S. Factors affecting the quality characteristics of Thai indigenous chicken meat. Suranaree J. Sci. Technol. 2008, 15, 1–16. [Google Scholar]
- Jaturasitha, S.; Chaiwang, N.; Kreuzer, M. Thai native chicken meat: An option to meet the demands for specific meat quality by certain groups of consumers; a review. Anim. Prod. Sci. 2016, 57, 1582–1587. [Google Scholar] [CrossRef]
- Sungkhapreecha, P.; Chankitisakul, V.; Duangjinda, M.; Boonkum, W. Combining abilities, heterosis, growth performance, and carcass characteristics in a diallel cross from black-bone chickens and Thai native chickens. Animals 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Molee, A.; Kuadsantia, P.; Kaewnakian, P. Gene effects on body weight, carcass yield, and meat quality of Thai indigenous chicken. J. Poult. Sci. 2018, 55, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tongsiri, S.; Jeyaruban, G.; Hermesch, S.; van der Werf, J.; Li, L.; Chormai, T. Genetic parameters and inbreeding effects for production traits of Thai native chickens. Asian-Australas. J. Anim. Sci. 2019, 32, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Chomchuen, K.; Tuntiyasawasdikul, V.; Chankitisakul, V.; Boonkum, W. Comparative study of phenotypes and genetics related to the growth performance of crossbred Thai indigenous (KKU1 vs. KKU2) chickens under hot and humid conditions. Vet. Sci. 2022, 9, 263. [Google Scholar] [CrossRef]
- Meeprom, S.; Jaratmetakul, P.; Boonkum, W. Examining the effect of consumer experience on co-creation and loyalty for healthy meat consumption. Front. Sustain. Food Syst. 2023, 7, 1123984. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Diarra, S.S.; Sandakabatu, D.; Perera, D.; Tabuaciri, P.; Mohammed, U. Growth performance and carcass yield of broiler chickens fed commercial finisher and cassava copra meal-based diets. J. Appl. Anim. Res. 2015, 43, 352–356. [Google Scholar] [CrossRef]
- Guo, Q.; Richert, B.T.; Burgess, J.R.; Webel, D.M.; Orr, D.E.; Blair, M.; Fitzner, G.E.; Hall, D.D.; Grant, A.L.; Gerrard, D.E. Effects of dietary vitamin E and fat supplementation on pork quality. J. Anim. Sci. 2006, 84, 3089–3099. [Google Scholar] [CrossRef]
- Malila, Y.; Jandamuk, A.; Uopasai, T.; Buasook, T.; Srimarut, Y.; Sanpinit, P.; Phasuk, Y.; Kunhareang, S. Effects of cyclic thermal stress at later age on production performance and meat quality of fast-growing, medium-growing and Thai native chickens. Animals 2021, 11, 3532. [Google Scholar] [CrossRef] [PubMed]
- Küçüközet, A.O.; Uslu, M.K. Cooking loss, tenderness, and sensory evaluation of chicken meat roasted after wrapping with edible films. Food Sci. Technol. Int. 2018, 24, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.I.; Claus, J.R.; Duncan, S.E.; Marriott, N.G.; Solomon, M.B.; Kathman, S.J.; Marini, M.E. Quality and sensory characteristics of selected post-rigor, early deboned broiler breast meat tenderized using hydrodynamic shock waves. Poult. Sci. 2000, 79, 126–136. [Google Scholar] [CrossRef] [PubMed]
- SAS. Statistical Analysis with SAS® University Edition and SAS® Studio, 6th ed.; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Hassan, R.I.; Mosaad, G.M.; Abd Elstar, M. Effect of feeding citric acid on performance of broiler ducks fed different protein levels. J. Adv. Vet. Res. 2016, 6, 18–26. Available online: https://www.advetresearch.com/index.php/AVR/article/view/33 (accessed on 20 May 2023).
- Ricke, S.C. Perspective on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef]
- Behera, D.P.; Sethi, A.P.S.; Pathak, D.; Singh, U.; Wadhwa, M. Effect of Different levels of citrus waste (Kinnow sp.) on duodenal morphology of broiler birds without and with cocktail of enzymes. J. Anim. Res. 2018, 8, 775–782. [Google Scholar] [CrossRef]
- Yadav, S.; Malik, A.; Pathera, A.; Islam, R.U.; Sharma, D. Development of dietary fibre enriched chicken sausages by incorporating corn bran, dried apple pomace and dried tomato pomace. Nutr. Food Sci. 2016, 46, 16–29. [Google Scholar] [CrossRef]
- Kralik, G.; Djurkin, I.; Kralik, Z.; Skrtic, Z.; Radisic, Z. Quality indicators of broiler breast meat in relation to colour. Anim. Sci. Pap. Rep. 2014, 32, 173–178. [Google Scholar]
- Dirinck, P.; De Winne, A.; Casteels, M.; Frigg, M. Studies on vitamin e and meat quality. 1. effect of feeding high vitamin e levels on time-related pork quality. J. Agric. Food Chem. 1996, 44, 65–68. [Google Scholar] [CrossRef]
- Angalet, S.A.; Fry, J.L.; Damron, B.L.; Harms, R.H. Evaluation of waste activated sludge (citrus) as a poultry feed ingredient. II. Quality and flavor of broilers, egg yolk color and egg flavor. Poult. Sci. 1976, 55, 1219–1225. [Google Scholar] [CrossRef]
- Sarikhan, M.; Shahryar, H.A.; Gholizadeh, B. Effects of insoluble fiber on growth performance, carcass traits and ileum morphological parameters on broiler chick males. Int. J. Agric. Bio. 2010, 12, 531–536. [Google Scholar]
- Shahin, K.A.; El Azeem, F.A. Effects of breed, sex and diet and their interactions on fat deposition and partitioning among depots of broiler chickens. Arch. Anim. Breed. 2006, 49, 181–193. [Google Scholar] [CrossRef]
- Le Huu, H.; Khammeng, T. Effect of yeast fermented cassava pulp (FCP) on nutrient digestibility and nitrogen balance of post-weaning pigs. Livest. Res. Rural Dev. 2014, 26, 149. [Google Scholar]
- Kanto, U.; Payombon, S.; Juttupornpong, S. Utilization of citric acid fermentation by-product as pig feed. In Proceedings of the 35th Kasetsart University Annual Conference, Bankok, Thailand, 3–7 February 1997. [Google Scholar]
- Oryza, S.M.; Wongtangtintharn, S.; Tengjaroenkul, B.; Cherdthong, A.; Tanpong, S.; Pootthachaya, P.; Boonkum, W.; Pintaphrom, N. Investigation of citric acid by-products from rice produced by microbial fermentation on growth performance and villi histology of Thai broiler chicken (KKU 1). Vet. Sci. 2021, 8, 284. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O. Effect of acetic acid supplementation on egg quality characteristics of commercial laying hens during hot season. Int. J. Poult. Sci. 2008, 7, 1015–1021. [Google Scholar]
- Mohammed, A.A.; Habib, A.B.; Eltrefi, A.M.E.; Shulukh, E.S.A.; Abubaker, A.A.; Abdelwahid, H.H.; Basheer, E.O.; Hassouna, S.M. Effect of dietary supplementation of increasing levels of organic acid mixture on performance and carcass characteristics of broiler chickens. IOSR J. Agric. Vet. Sci. 2018, 11, 54–58. [Google Scholar] [CrossRef]
- Reda, F.M.; Ismail, I.E.; Attia, A.I.; Fikry, A.M.; Khalifa, E.; Alagawany, M. Use of fumaric acid as a feed additive in quail’s nutrition: Its effect on growth rate, carcass, nutrient digestibility, digestive enzymes, blood metabolites, and intestinal microbiota. Poult. Sci. J. 2021, 100, 101493. [Google Scholar] [CrossRef]
| Ingredients (%) | Starter (1–21 Days) | Grower (22–49 Days) | Finisher (50–56 Days) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | CABR | Control | CABR | Control | CABR | ||||||||||
| 3% | 6% | 9% | 12% | 3% | 6% | 9% | 12% | 3% | 6% | 9% | 12% | ||||
| Corn meal | 50.0 | 48.0 | 46.0 | 44.0 | 42.0 | 55.6 | 53.47 | 51.4 | 49.3 | 47.2 | 59.5 | 57.4 | 55.27 | 53.2 | 51.1 |
| Soybean meal, 44% crude protein | 26.9 | 25.8 | 24.7 | 23.6 | 22.5 | 19.3 | 18.3 | 17.3 | 16.3 | 15.3 | 12.5 | 11.5 | 10.5 | 9.5 | 8.5 |
| Full-fat soybean | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | 19.0 | 19.0 | 19.0 | 19.0 | 19.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| Dicalcium phosphate (P21%) | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Limestone | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 |
| DL-Methionine | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 |
| L-Lysine | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.180 | 0.180 | 0.180 | 0.180 | 0.180 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 |
| Rice bran oil | 1.50 | 1.60 | 1.70 | 1.80 | 1.90 | 2.00 | 2.10 | 2.20 | 2.30 | 2.40 | 2.20 | 2.30 | 2.40 | 2.50 | 2.60 |
| Salt | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| Choline chloride (60%) | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Premix a | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 |
| Citric acid by-product from rice (CABR) b | 0.00 | 3.00 | 6.00 | 9.00 | 12.00 | 0.00 | 3.00 | 6.00 | 9.00 | 12.00 | 0.00 | 3.00 | 6.00 | 9.00 | 12.00 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutritional composition | |||||||||||||||
| Crude protein, % | 22.4 | 22. | 22.3 | 22.2 | 22.1 | 20.1 | 20.0 | 20.0 | 20.0 | 19.9 | 18.3 | 18.3 | 18.2 | 18.2 | 18.17 |
| Crude fiber | 4.40 | 4.62 | 4.84 | 5.06 | 5.28 | 4.16 | 4.38 | 4.61 | 4.83 | 5.05 | 3.98 | 4.42 | 4.42 | 4.65 | 4.87 |
| Calcium | 1.67 | 1.44 | 1.34 | 1.53 | 1.21 | 5.56 | 5.83 | 5.51 | 5.83 | 5.51 | 6.17 | 7.76 | 7.85 | 7.92 | 7.88 |
| Phosphorus | 0.697 | 0.704 | 0.501 | 0.541 | 0.568 | 0.090 | 0.086 | 0.068 | 0.065 | 0.071 | 0.718 | 0.507 | 0.419 | 0.487 | 0.463 |
| ME, kcal/kg | 3013 | 3004 | 2994 | 2985 | 2975 | 3129 | 3118 | 3108 | 3097 | 3087 | 3209 | 3198 | 3188 | 3177 | 3167 |
| Parameter | Treatments | SEM | p-Value | Linier | Quadratic | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 3% CABR | 6% CABR | 9% CABR | 12% CABR | |||||
| Initial weight (g/b) | 32.77 | 32.73 | 32.80 | 32.89 | 32.64 | 0.222 | 0.954 | 0.0000 | 0.0020 |
| Starter (1–21 days) | |||||||||
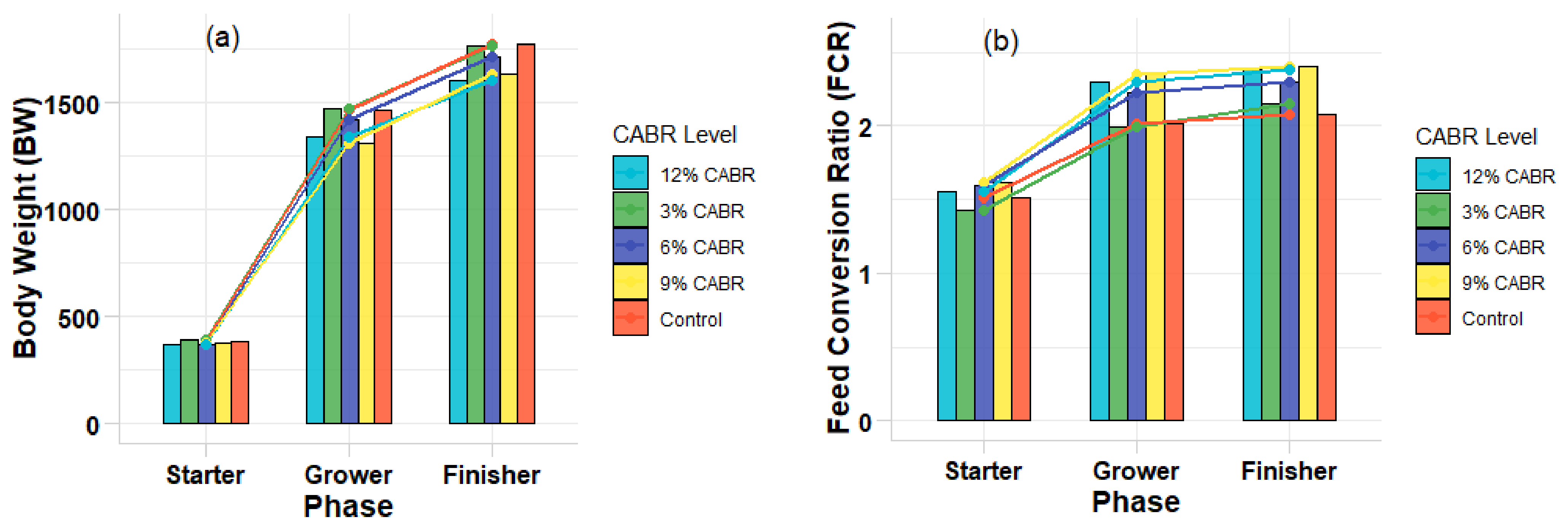
| BW (g/b) | 384 | 392 | 3712 | 377 | 371.33 | 11.15 | 0.647 | 0.0010 | 0.0030 |
| BWG (g/b) | 351 | 360 | 339 | 344 | 338.69 | 11.17 | 0.650 | 0.0000 | 0.0000 |
| FI (g/b) | 526 | 515 | 541 | 544 | 542 | 14.40 | 0.500 | 0.0000 | 0.0000 |
| FCR | 1.51 | 1.43 | 1.60 | 1.59 | 1.62 | 0.0608 | 0.214 | 0.0000 | 0.0025 |
| SR (%) | 100 | 100 | 96.9 | 100 | 100 | 0.807 | 0.0528 | 0.0050 | 0.0060 |
| Grower (22–49 days) | |||||||||
| BW (g/b) | 1461 ab | 1475 a | 1423 abc | 1313 c | 1343 bc | 55.28 | 0.0408 | 0.0020 | 0.0030 |
| BWG (g/b) | 10,781 a | 1083 a | 1051 ab | 936.32 c | 972.12 bc | 44.63 | 0.0172 | 0.0030 | 0.0040 |
| FI (g/b) | 2171 | 2158 | 2091 | 2070 | 2313 | 77.64 | 0.0568 | 0.0010 | 0.0020 |
| FCR | 2.02 b | 1.99 b | 1.99 b | 2.22 a | 2.35 a | 0.0784 | 0.0011 | 0.0020 | 0.0030 |
| SR (%) | 93.8 | 95.3 | 96.9 | 95.3 | 98.4 | 4.92 | 0.903 | NS | NS |
| Finisher (50–56 days) | |||||||||
| BW (g/b) | 1774 a | 1770 a | 1715 ab | 1638 b | 1604 b | 42.96 | 0.4152 | 0.0020 | 0.0030 |
| BWG (g/b) | 312 | 295 | 290 | 325 | 260 | 19.90 | 0.415 | 0.0010 | 0.0020 |
| FI (g/b) | 875 | 866 | 946 | 905 | 885 | 30.90 | 0.245 | 0.0030 | 0.0040 |
| FCR | 2.87 | 2.97 | 3.30 | 2.82 | 3.42 | 0.277 | 0.142 | 0.0020 | 0.0030 |
| SR (%) | 100 | 100 | 100 | 96.8 | 100 | 1.16 | 0.0531 | NS | NS |
| Overall period (1–56 days) | |||||||||
| BWG (g/b) | 1741 a | 1737 a | 1680 ab | 1604 b | 1570 b | 40.8 | 0.031 | 0.0030 | 0.0040 |
| FI (g/b) | 3616 | 3566 | 3597 | 3738 | 3753 | 85.29 | 0.432 | 0.0010 | 0.0020 |
| FCR | 2.08 c | 2.05 c | 2.15 bc | 2.33 ab | 2.39 a | 0.06 | 0.004 | 0.0020 | 0.0030 |
| SR (%) | 93.1 | 95.3 | 93.8 | 92.2 | 98.4 | 3.45 | 0.755 | NS | NS |
| Feed cost (USD/bird) | 0.50 | 0.49 | 0.49 | 0.48 | 0.47 | - | - | - | - |
| Parameter | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 3% CABR | 6% CABR | 9% CABR | 12% CABR | Linear | Quadratic | |||
| Live weight (g) | 1787 | 1694 | 1704 | 1595 | 1568 | 75.1 | 0.128 | NS | NS |
| Dressing percentage (%) | 68.3 a | 67.7 ab | 67.8 a | 67.5 a | 63.8 b | 1.17 | 0.0074 | 0.0010 | 0.0020 |
| External organs | |||||||||
| Breast (%) | 24.0 | 25.3 | 23.6 | 23.8 | 23.2 | 1.50 | 0.741 | NS | NS |
| Thigh (%) | 17.2 | 18.1 | 17.1 | 17.4 | 18.3 | 2.18 | 0.975 | NS | NS |
| Drumstick (%) | 15.6 | 15.5 | 15.8 | 16.0 | 15.7 | 0.665 | 0.822 | NS | NS |
| Wing (%) | 12.9 | 13.0 | 13.9 | 13.7 | 14.0 | 0.520 | 0.571 | NS | NS |
| Internal organs | |||||||||
| Liver (%) | 2.86 | 3.15 | 3.07 | 3.43 | 3.63 | 0.508 | 0.747 | NS | NS |
| Heart (%) | 0.71 | 0.64 | 0.66 | 0.74 | 0.76 | 0.074 | 0.760 | NS | NS |
| Pancreas (%) | 0.36 | 0.36 | 0.33 | 0.34 | 0.39 | 0.040 | 0.605 | NS | NS |
| Gizzard (%) | 3.13 | 3.25 | 3.06 | 3.40 | 3.64 | 0.266 | 0.205 | NS | NS |
| Abdominal fat (%) | 2.21 | 2.55 | 1.56 | 1.78 | 1.66 | 0.497 | 0.641 | NS | NS |
| Parameter | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 3% CABR | 6% CABR | 9% CABR | 12% CABR | Linear | Quadratic | |||
| Breast meat | |||||||||
| Color | |||||||||
| L* | 61.3 | 60.5 | 62.3 | 61.6 | 62.0 | 0.720 | 0.358 | NS | NS |
| a* | 11.0 | 11.9 | 10.4 | 10.0 | 10.3 | 0.890 | 0.487 | NS | NS |
| b* | 13.1 ab | 15.2 a | 11.3 b | 14.2 a | 11.2 b | 1.02 | 0.019 | NS | NS |
| Drip loss (%) | 6.01 | 5.83 | 5.49 | 5.89 | 5.98 | 0.570 | 0.954 | NS | NS |
| Cooking loss (%) | 17.3 | 17.0 | 18.4 | 16.6 | 19.0 | 1.40 | 0.618 | NS | NS |
| Shear force (kg/cm2) | 4.14 | 4.02 | 4.05 | 3.69 | 3.93 | 0.340 | 0.881 | NS | NS |
| Thigh meat | |||||||||
| Color | |||||||||
| L* | 60.6 | 61.4 | 60.3 | 61.3 | 62.5 | 1.02 | 0.430 | NS | NS |
| a* | 11.5 | 11.4 | 12.0 | 11.4 | 11.3 | 0.530 | 0.904 | NS | NS |
| b* | 9.09 | 10.8 | 8.51 | 10.6 | 8.87 | 1.10 | 0.401 | NS | NS |
| Drip loss (%) | 6.80 | 6.74 | 6.64 | 6.84 | 6.90 | 1.06 | 0.997 | NS | NS |
| Cooking loss (%) | 13.9 | 13.6 | 13.4 | 13.0 | 12.2 | 1.44 | 0.823 | NS | NS |
| Shear force (kg/cm2) | 3.81 | 3.56 | 3.28 | 3.99 | 3.73 | 0.430 | 0.318 | NS | NS |
| Parameter | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 3% CABR | 6% CABR | 9% CABR | 12% CABR | Linear | Quadratic | |||
| Breast meat | |||||||||
| Dry matter (%) | 27.5 | 30.0 | 30.0 | 28.1 | 29.3 | 0.582 | 0.056 | 0.038 | 0.070 |
| Gross energy (kcal/kg) | 4405 b | 4745 a | 4398 b | 4522 ab | 4816 a | 113.62 | 0.040 | 0.019 | NS |
| Ether extract (%) | 1.83 b | 3.48 a | 2.71 ab | 2.55 ab | 1.74 b | 0.440 | 0.048 | 0.035 | NS |
| Crude protein (%) | 64.8 | 74.2 | 78.6 | 78.5 | 78.4 | 8.55 | 0.157 | 0.015 | NS |
| Thigh meat | |||||||||
| Dry matter (%) | 27.0 | 23.3 | 28.8 | 26.5 | 24.8 | 1.60 | 0.098 | 0.045 | 0.082 |
| Gross energy (kcal/kg) | 5106 b | 5148 b | 5288 a | 5152 b | 5328 a | 19.3 | 0.003 | 0.000 | 0.015 |
| Ether extract (%) | 12.6 | 12.1 | 12.0 | 13.9 | 12.5 | 1.00 | 0.435 | NS | NS |
| Crude protein (%) | 67.4 | 66.7 | 72.5 | 71.4 | 73.0 | 4.46 | 0.550 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oryza.S, M.; Pootthachaya, P.; Pintaphrom, N.; Tanpong, S.; Unnawong, N.; Cherdthong, A.; Tengjaroenkul, B.; Wongtangtintharn, S. Sustainable Poultry Nutrition Using Citric Acid By-Products from Rice to Boost Growth and Carcass Yield in Thai KKU 1 Broiler Chickens. Animals 2024, 14, 3358. https://doi.org/10.3390/ani14233358
Oryza.S M, Pootthachaya P, Pintaphrom N, Tanpong S, Unnawong N, Cherdthong A, Tengjaroenkul B, Wongtangtintharn S. Sustainable Poultry Nutrition Using Citric Acid By-Products from Rice to Boost Growth and Carcass Yield in Thai KKU 1 Broiler Chickens. Animals. 2024; 14(23):3358. https://doi.org/10.3390/ani14233358
Chicago/Turabian StyleOryza.S, Mutyarsih, Padsakorn Pootthachaya, Nisakon Pintaphrom, Sirisak Tanpong, Narirat Unnawong, Anusorn Cherdthong, Bundit Tengjaroenkul, and Sawitree Wongtangtintharn. 2024. "Sustainable Poultry Nutrition Using Citric Acid By-Products from Rice to Boost Growth and Carcass Yield in Thai KKU 1 Broiler Chickens" Animals 14, no. 23: 3358. https://doi.org/10.3390/ani14233358
APA StyleOryza.S, M., Pootthachaya, P., Pintaphrom, N., Tanpong, S., Unnawong, N., Cherdthong, A., Tengjaroenkul, B., & Wongtangtintharn, S. (2024). Sustainable Poultry Nutrition Using Citric Acid By-Products from Rice to Boost Growth and Carcass Yield in Thai KKU 1 Broiler Chickens. Animals, 14(23), 3358. https://doi.org/10.3390/ani14233358








