Transcriptome Profiling Unveils the Mechanisms of Inflammation, Apoptosis, and Fibrosis in the Liver of Juvenile Largemouth Bass Micropterus salmoides Fed High-Starch Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Feeding Management
2.3. Sampling
2.4. Nutrient Composition Analysis
2.5. Serum Biochemical Analysis
2.6. Histological Examination
2.7. Transcriptome Analysis
2.7.1. cDNA Library Construction, Sequencing, and Transcriptome Assembly
2.7.2. Differential Expression Analysis
2.7.3. Functional Annotation and Enrichment Analysis
2.8. Real-Time PCR Analysis
2.9. Calculations
2.10. Statistical Analysis
3. Results
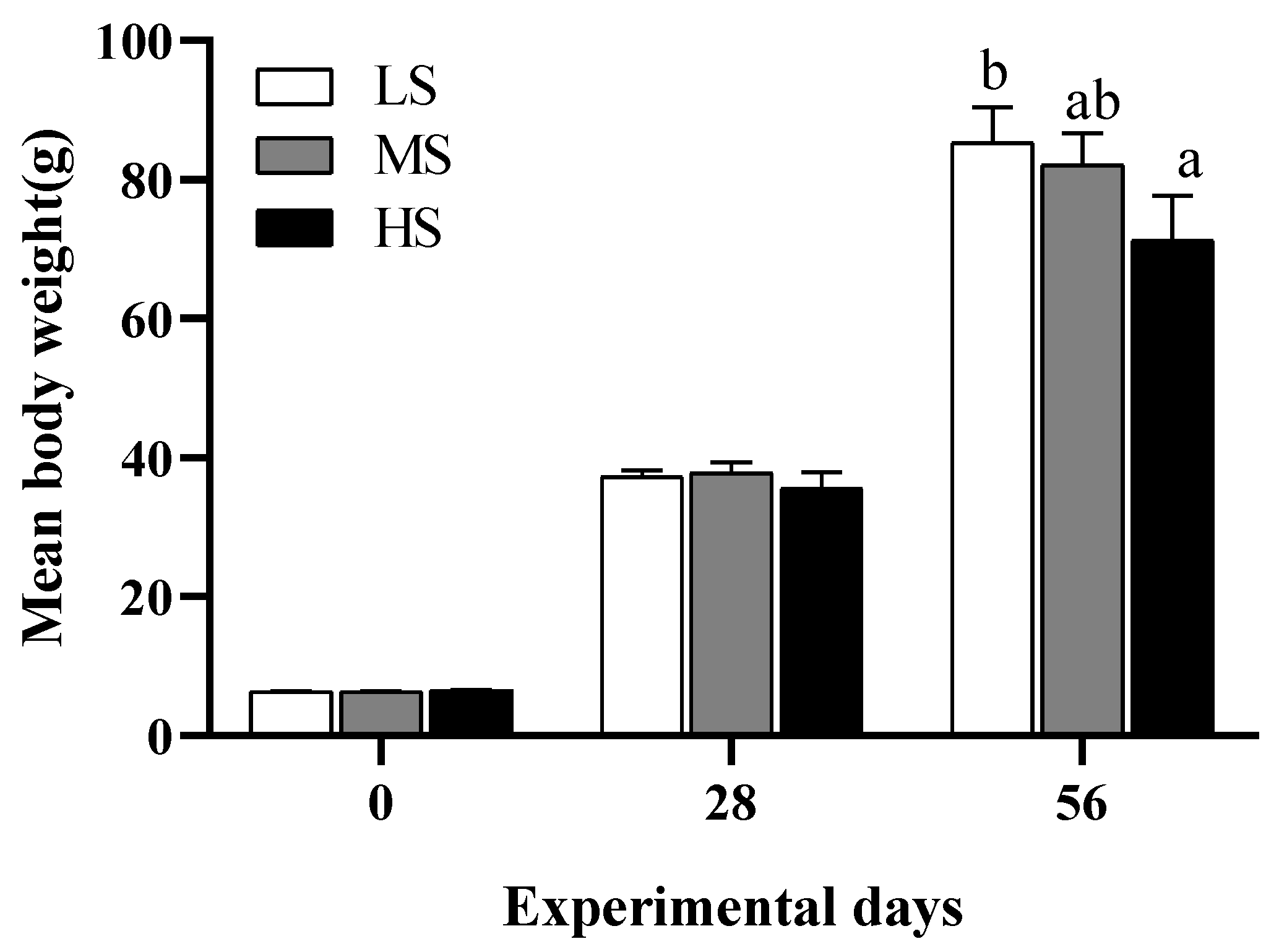
3.1. Growth, Feed Utilization, and Morphological Parameters
3.2. Serum Biochemical Indices
3.3. Nutrient Composition
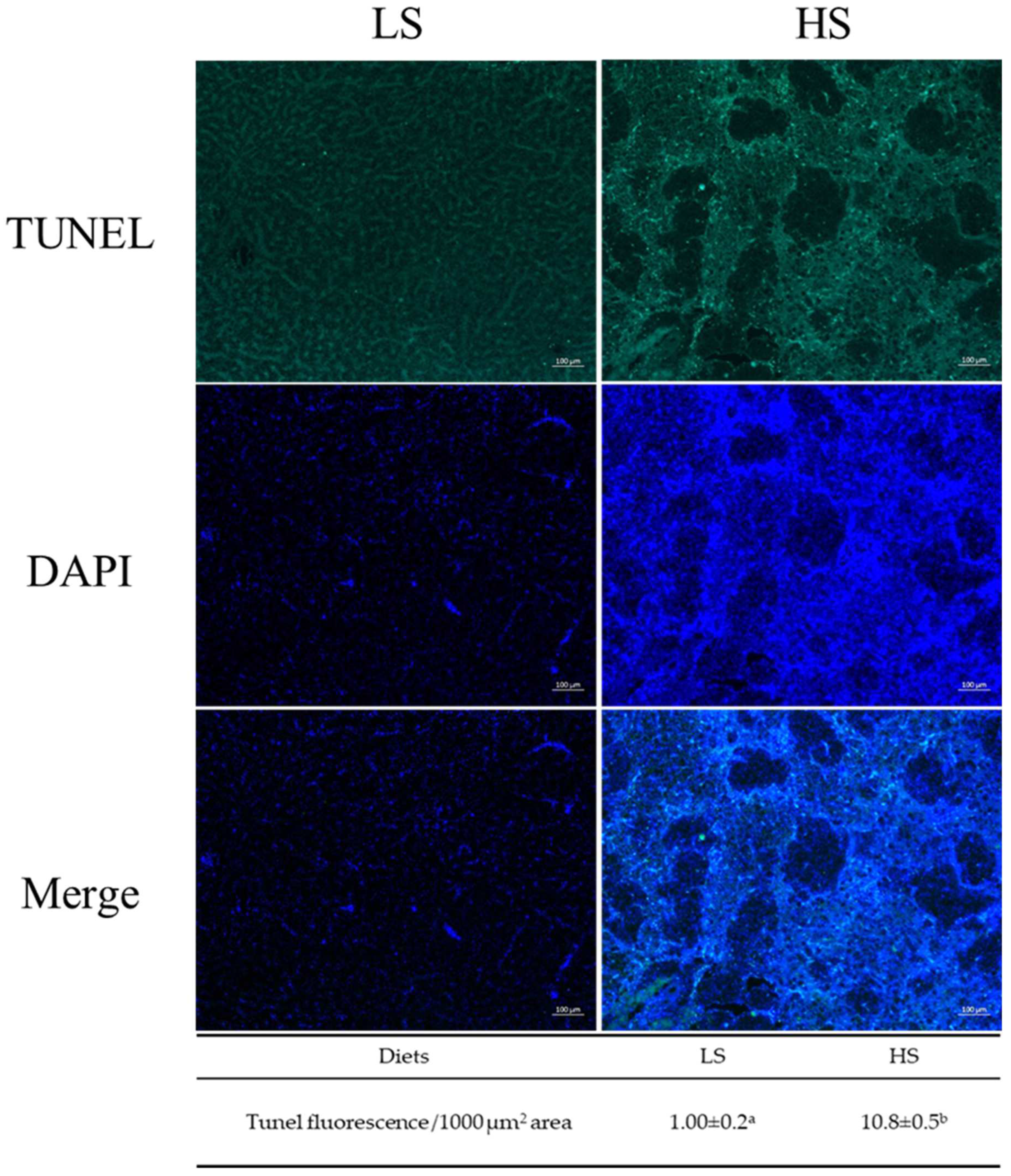
3.4. Histological Observation
3.5. Quality Assessment and Sequence Alignment of Transcriptome
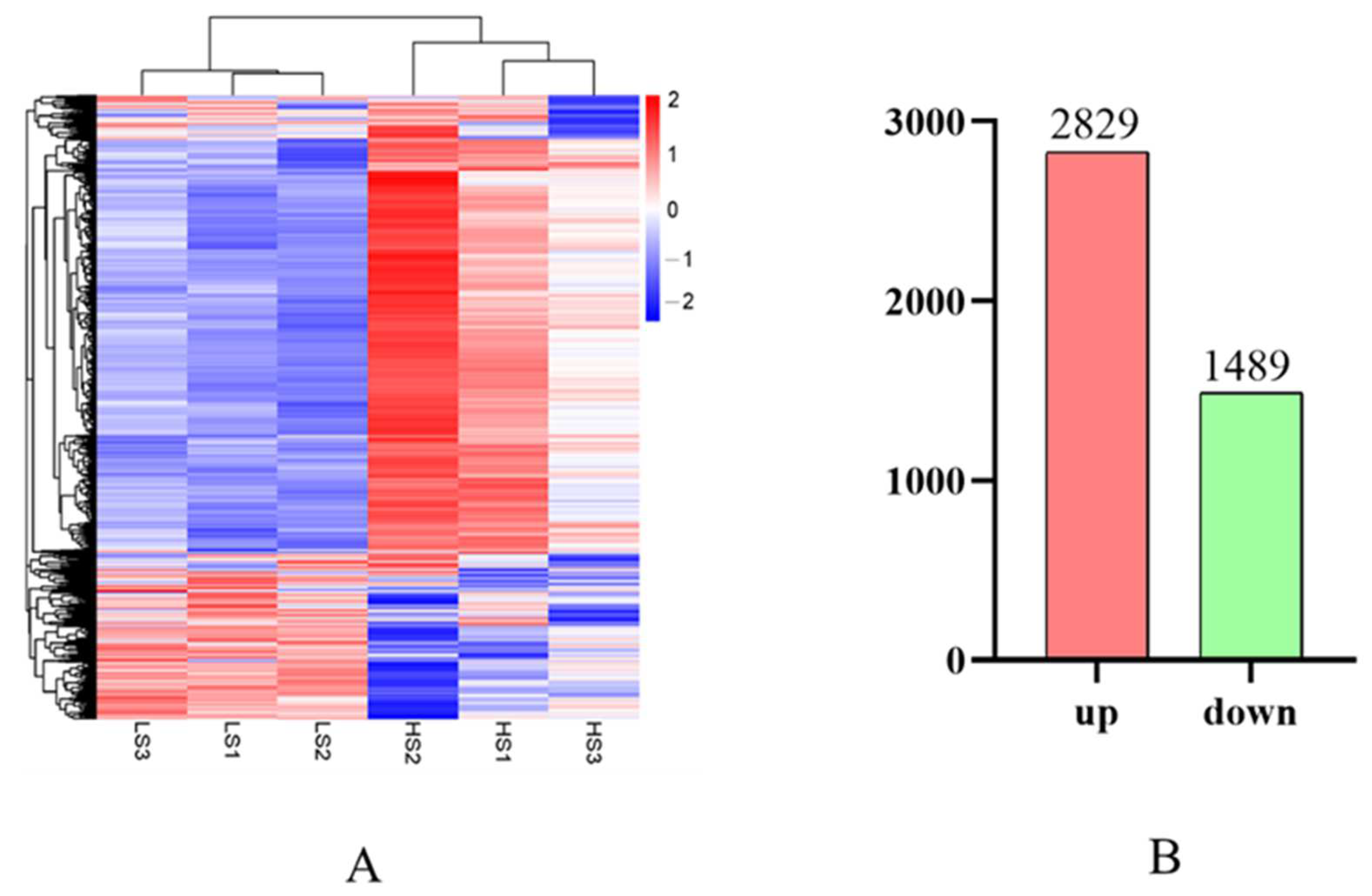
3.6. Differential Expression Analysis
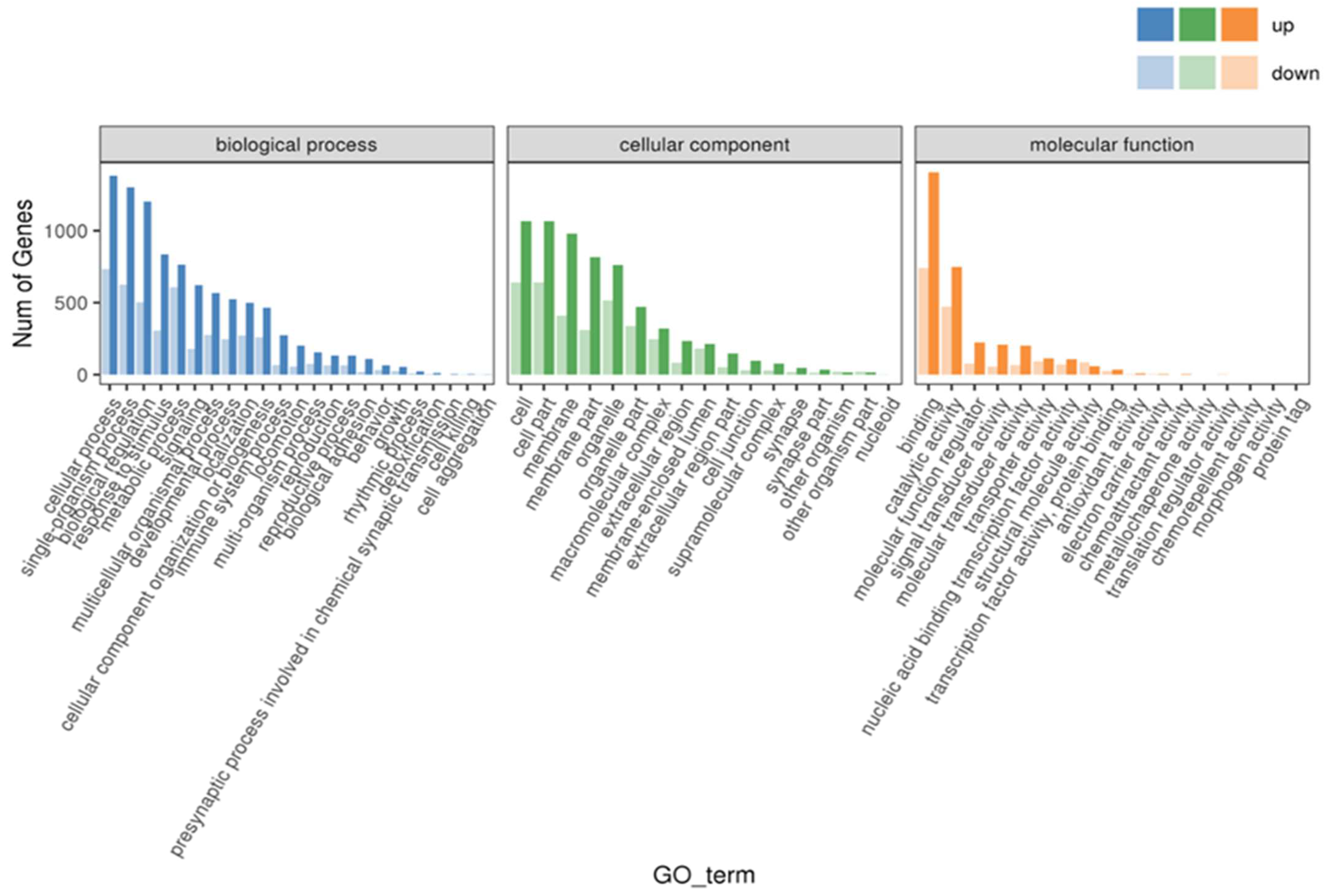
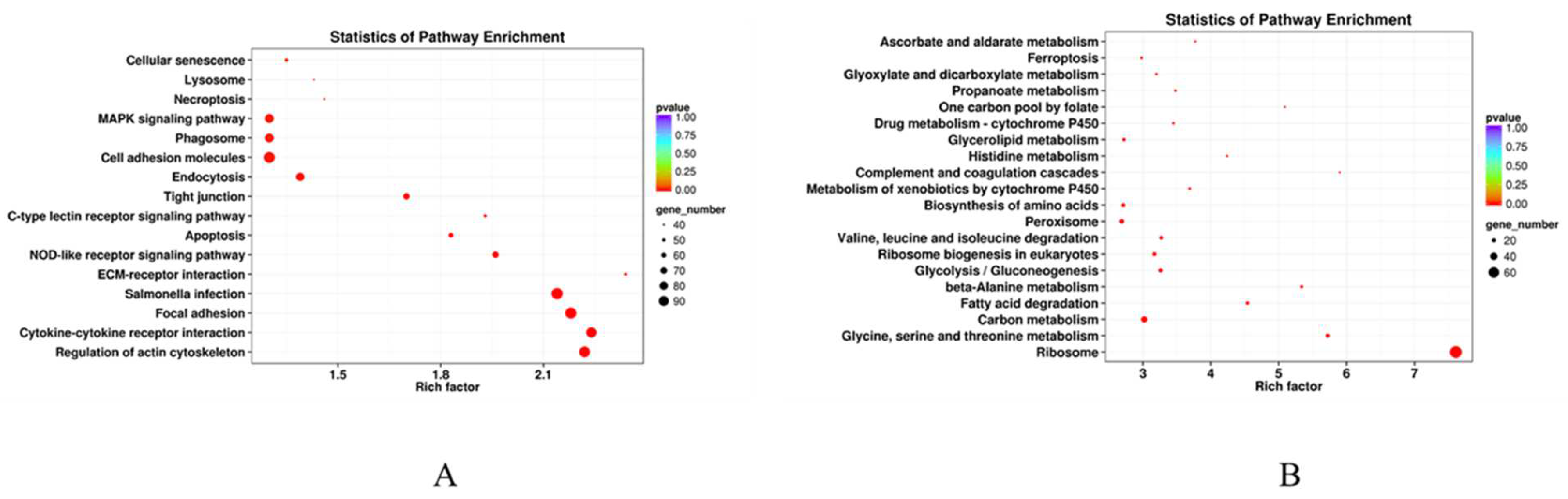
3.7. GO and KEGG Enrichment Analysis
3.8. Real-Time PCR Analysis
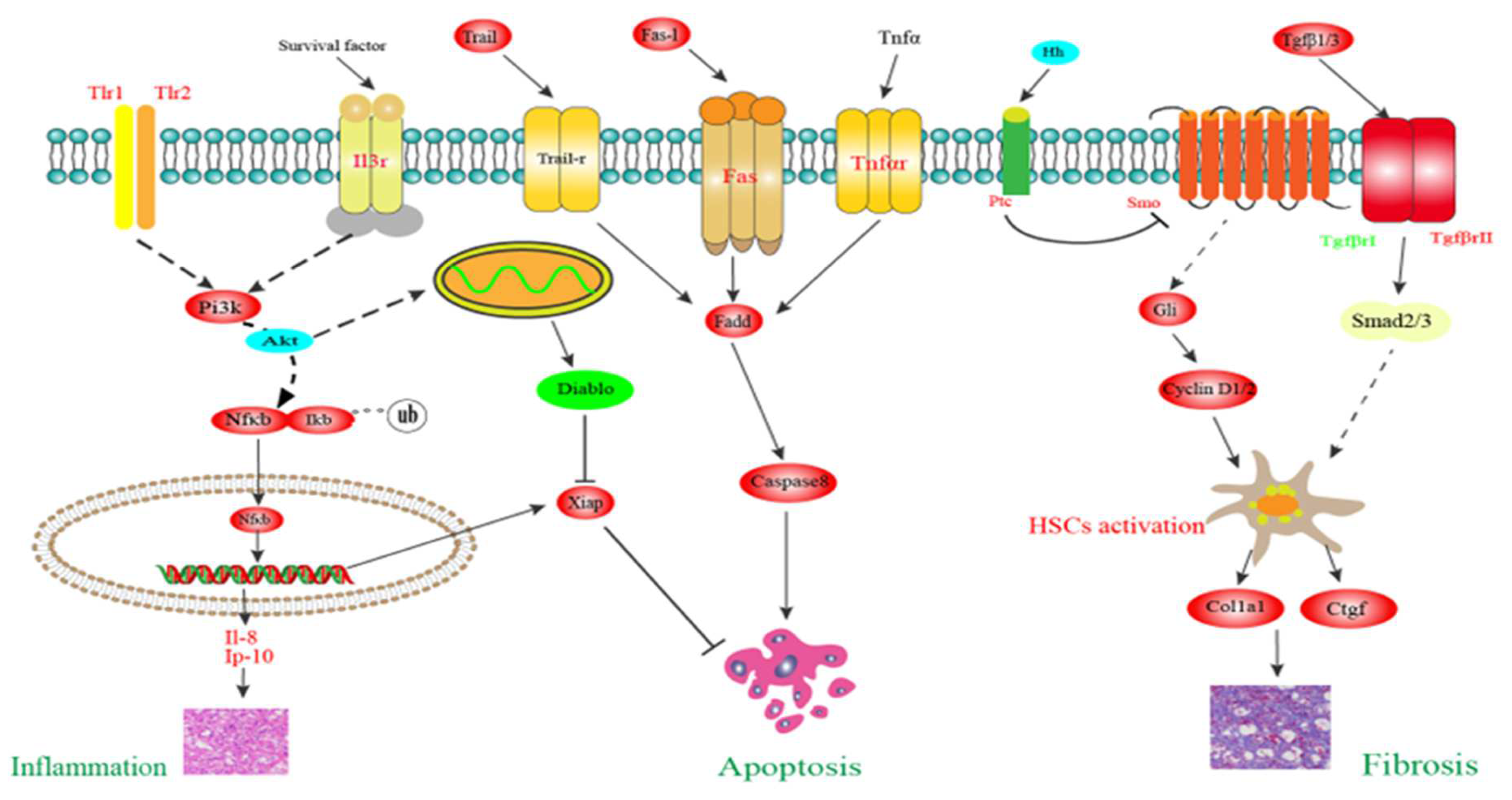
3.9. Putative Molecular Mechanism of Liver Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- BeMiller, J.N. Carbohydrate analysis. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 333–360. [Google Scholar]
- Panchal, S.K.; Poudyal, H.; Arumugam, T.V.; Brown, L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011, 141, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Vetuschi, A.; Gaudio, E.; Tessitore, A.; Capelli, R.; Alesse, E.; Latella, G.; Sferra, R.; Onori, P. Long-term abuse of a high-carbohydrate diet is as harmful as a high-fat diet for development and progression of liver injury in a mouse model of NAFLD/NASH. Nutrition 2020, 75, 110782. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.A. Dietary carbohydrate utilization by fish. Rev. Fish. Sci. 2003, 11, 337–369. [Google Scholar] [CrossRef]
- Honorato, C.A.; Almeida, L.C.; Da Silva Nunes, C.; Carneiro, D.J.; Moraes, G. Effects of processing on physical characteristics of diets with distinct levels of carbohydrates and lipids: The outcomes on the growth of pacu (Piaractus mesopotamicus). Aquacult. Nutr. 2010, 16, 91–99. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tian, L.X.; Yang, H.J.; Chen, P.F.; Yuan, Y.; Liu, Y.J.; Liang, G.Y. Effect of protein and starch level in practical extruded diets on growth, feed utilization, body composition, and hepatic transaminases of juvenile grass carp, Ctenopharyngodon idella. J. World Aquac. Soc. 2012, 43, 187–197. [Google Scholar] [CrossRef]
- Prisingkorn, W.; Prathomya, P.; Jakovlić, I.; Liu, H.; Zhao, Y.H.; Wang, W.M. Transcriptomics, metabolomics and histology indicate that high-carbohydrate diet negatively affects the liver health of blunt snout bream (Megalobrama amblycephala). BMC Genom. 2017, 18, 856. [Google Scholar] [CrossRef]
- Deng, K.Y.; Pan, M.Z.; Liu, J.H.; Yang, M.X.; Gu, Z.X.; Zhang, Y.; Liu, G.X.; Liu, D.; Zhang, W.B.; Mai, K.S. Chronic stress of high dietary carbohydrate level causes inflammation and influences glucose transport through SOCS3 in Japanese flounder (Paralichthys olivaceus). Sci. Rep. 2018, 8, 7415. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Y.J.; Yang, H.J.; Yuan, Y.; Liu, F.J.; Tian, L.X.; Liang, G.Y.; Yuan, R.M. Effect of dietary oxidized fish oil on growth performance, body composition, antioxidant defence mechanism and liver histology of juvenile largemouth bass Micropterus salmoides. Aquacult. Nutr. 2012, 18, 321–331. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Fish and Shrimp; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Ma, H.J.; Mou, M.M.; Pu, D.C.; Lin, S.M.; Chen, Y.J.; Luo, L. Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2019, 498, 482–487. [Google Scholar] [CrossRef]
- Xue, M.Y.; Yao, T.; Xue, M.; Francis, F.; Qin, Y.C.; Jia, M.; Li, J.G.; Gu, X. Mechanism analysis of metabolic fatty liver on largemouth bass (Micropterus salmoides) based on integrated lipidomics and proteomics. Metabolites 2022, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Xia, R.; Deng, X.; Yang, H.J.; Luo, L.; Lin, S.M.; Qin, C.J.; Chen, Y.J. Effects of dietary starch and metformin levels on the growth performance, body composition, hepatic glycolipid metabolism, and liver histology of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2023, 573, 739582. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.L.; Zhang, H.Q.; Zhang, W.D.; Li, M.H.; Huang, Y.; Yao, J.Y.; Huang, X.L.; Geng, Y.; Chen, D.F.; et al. High starch in diet leads to disruption of hepatic glycogen metabolism and liver fibrosis in largemouth bass (Micropterus salmoides), which is mediated by the PI3K/Akt signaling pathway. Front. Physiol. 2022, 13, 880513. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Gu, X.; Xue, M.; Ge, C.Y.; Liang, X.F. Proteomic analysis of hepatic fibrosis induced by a high starch diet in largemouth bass (Micropterus salmoides). Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101007. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.W.; Liu, Q.Q.; Zhu, J.; Deng, X.; Luo, L.; Lin, S.M.; Qin, C.J.; Chen, Y.J. Transcriptome analysis provides insights into the molecular mechanism of liver inflammation and apoptosis in juvenile largemouth bass (Micropterus salmoides) fed low protein high starch diets. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 45, 101047. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis; Association of Official Analytical Chemist: Arlington, TX, USA, 1995. [Google Scholar]
- Hall, M.B. Neutral detergent-soluble carbohydrates. In Nutritional Relevance and Analysis. A Laboratory Manual; Florida Cooperative Extension Service, Bulletin 330; University of Florida: Gainsville, FL, USA, 2000; 77p. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Shen, F.; Long, Y.; Li, F.; Ge, G.; Song, G.; Li, Q.; Qiao, Z.; Cui, Z. De novo transcriptome assembly and sex-biased gene expression in the gonads of Amur catfish (Silurus asotus). Genomics 2020, 112, 2603–2614. [Google Scholar] [CrossRef]
- Xin, Z.Z.; Liu, Q.N.; Liu, Y.; Zhang, D.Z.; Wang, Z.F.; Zhang, H.B.; Ge, B.M.; Zhou, C.H.; Chai, X.Y.; Tang, B.P. Transcriptome-wide identification of differentially expressed genes in Chinese oak silkworm Antheraea pernyi in response to lead challenge. J. Agric. Food Chem. 2017, 65, 9305–9314. [Google Scholar] [CrossRef]
- Zhu, J.; Du, R.Y.; Liu, Q.Q.; Luo, L.; Lin, S.M.; Zhang, H.Q.; Chen, Y.J. Transcriptome analysis provides insights into the molecular mechanism of hepatocyte apoptosis in response to feeding restriction in juvenile largemouth bass Micropterus salmoides. Aquaculture 2022, 548, 737550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gou, S.P.; Chen, N.S.; Xu, X.T.; Liu, Z.K.; Yin, J. Effects of dietary digestible starch levels on growth performance, body composition, and non-specific immunological index of largemouth bass (Micropterus salmoides). J. Fish. China 2015, 39, 1499–1510. (in Chinese). [Google Scholar]
- Li, X.Y.; Zheng, S.X.; Ma, X.K.; Cheng, K.M.; Wu, G.Y. Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, X.Y.; Zhang, Y.; Li, S.L.; Lu, L.Q.; Xu, D.; Liu, X.W. Effects of dietary carbohydrate levels on the growth, glycometabolism, antioxidant capacity and metabolome of largemouth bass (Micropterus salmoides). Aquac. Res. 2022, 53, 3748–3758. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.H. Effect of dietary glucose, dextrin and starch on growth and body composition of juvenile starry flounder (Platichthys stellatus). Fish. Sci. 2004, 70, 53–58. [Google Scholar] [CrossRef]
- Couto, A.; Enes, P.; Peres, H.; Oliva-Teles, A. Effect of water temperature and dietary starch on growth and metabolic utilization of diets in gilthead sea bream (Sparus aurata) juveniles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 45–50. [Google Scholar] [CrossRef]
- Brooks, G.; Fahey, T. Exercise Physiology; Macmillan Publishing, Co.: New York, NY, USA, 1984. [Google Scholar]
- Zhao, L.L.; Liao, L.; Tang, X.H.; Liang, J.; Liu, Q.; Luo, W.; Adam, A.A.; Luo, J.; Li, Z.Q.; Yang, S.; et al. High-carbohydrate diet altered conversion of metabolites, and deteriorated health in juvenile largemouth bass. Aquaculture 2022, 549, 737816. [Google Scholar] [CrossRef]
- Bruslé, J.; Anadon, G.G. The structure and function of fish liver. In Fish Morphology; Routledge: Oxfordshire, UK, 2017; pp. 77–93. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Phan, T.K.; Lay, F.T.; Hulett, M.D. Importance of phosphoinositide binding by human β-defensin 3 for Akt-dependent cytokine induction. Immunol. Cell Biol. 2018, 96, 54–67. [Google Scholar] [CrossRef]
- Keane, M.P.; Arenberg, D.A.; Lynch, J.P., 3rd; Whyte, R.I.; Iannettoni, M.D.; Burdick, M.D.; Wilke, C.A.; Morris, S.B.; Glass, M.C.; DiGiovine, B.; et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J. Immunol. 1997, 159, 1437–1443. [Google Scholar] [CrossRef]
- Wickremasinghe, M.I.; Thomas, L.H.; Friedland, J.S. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: Essential role of IL-1 from infected monocytes in a NF-κB-dependent network. J. Immunol. 1999, 163, 3936–3947. [Google Scholar] [CrossRef]
- Roda, J.M.; Parihar, R.; Magro, C.; Nuovo, G.J.; Tridandapani, S.; Carson, W.E., III. Natural killer cells produce T cell–recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006, 66, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 79, 357–384. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhu, H.; Xu, C.J.; Yuan, J.Y. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Kahana, S.; Finniss, S.; Cazacu, S.; Xiang, C.; Lee, H.K.; Brodie, S.; Goldstein, R.S.; Roitman, V.; Slavin, S.; Mikkelsen, T.; et al. Proteasome inhibitors sensitize glioma cells and glioma stem cells to TRAIL-induced apoptosis by PKCepsilon-dependent downregulation of AKT and XIAP expressions. Cell Signal. 2011, 23, 1348–1357. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.L.; Vaux, D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Malik, M.; Wen, G.; Chauhan, A.; Chauhan, V.; Gong, C.X.; Liu, F.; Brown, W.T.; Li, X. BDNF-Akt-Bcl2 antiapoptotic signaling pathway is compromised in the brain of autistic subjects. J. Neurosci. Res. 2010, 88, 2641–2647. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Dooley, S.; Ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Dijke, P.T.; IT-LIVER Consortium. TGF-β signaling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Diehl, A.M. Hedgehog signaling in liver pathophysiology. J. Hepatol. 2018, 68, 550–562. [Google Scholar] [CrossRef]
- Yan, J.Y.; Hu, B.W.; Shi, W.J.; Wang, X.Y.; Shen, J.Y.; Chen, Y.P.; Huang, H.R.; Jin, L.F. Gli2-regulated activation of hepatic stellate cells and liver fibrosis by TGF-β signaling. Am. J. Physiol.-Liver Physiol. 2021, 320, G720–G728. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Manon-Jensen, T.; Genovese, F.; Kristensen, J.H.; Nielsen, M.J.; Sand, J.M.B.; Hansen, N.U.B.; Bay-jensen, A.C.; Bager, C.L.; Krag, A.; et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Liver Physiol. 2015, 308, G807–G830. [Google Scholar] [CrossRef]


| Ingredients (%) | LS | MS | HS |
|---|---|---|---|
| Fish meal 1 | 34.0 | 34.0 | 34.0 |
| Soybean meal 1 | 7.50 | 7.50 | 7.50 |
| Wheat gluten 1 | 15.0 | 15.0 | 15.0 |
| Chicken meal 1 | 13.0 | 13.0 | 13.0 |
| Cassava starch 1 | 7.00 | 13.0 | 19.0 |
| Fish oil 2 | 2.40 | 2.40 | 2.40 |
| Soybean oil | 2.40 | 2.40 | 2.40 |
| Monocalcium phosphate 1 | 2.00 | 2.00 | 2.00 |
| Vitamin and mineral premix 3 | 2.00 | 2.00 | 2.00 |
| Microcrystalline cellulose | 6.85 | 6.00 | 1.69 |
| Vitamin C phosphate (35%) 1 | 0.20 | 0.20 | 0.20 |
| Methionine 1 | 0.25 | 0.25 | 0.25 |
| Choline chloride 1 | 0.50 | 0.50 | 0.50 |
| Zeolite powder 1 | 6.84 | 1.69 | 0.00 |
| Antiseptic 1 | 0.01 | 0.01 | 0.01 |
| Antioxidant 1 | 0.05 | 0.05 | 0.05 |
| Proximate composition (analyzed, % dry matter) | |||
| Moisture | 7.93 | 9.85 | 9.64 |
| Crude protein | 49.1 | 49.3 | 49.8 |
| Crude lipid | 11.4 | 11.6 | 11.6 |
| Starch | 8.13 | 14.1 | 20.1 |
| Ash | 18.3 | 13.6 | 12.0 |
| Genes | Forward Primer | Reverse Primer | Product Length (bp) | Tm (°C) | Amplification Efficiency (%) | Accession No. |
|---|---|---|---|---|---|---|
| atrs | CCTCTATTTCATCTCACCCTTCG | TCCCATACTGCTCAATCTCCTCT | 176 | 59.8 | 97.1 | XM_038694050.1 |
| caspase 8 | GAGTAGGGATGAAGTAAAGGC | CTGTAAGGAGAAATGAGGCT | 231 | 52.0 | 101 | XM_038718639.1 |
| col1a1 | GAGCGGCGAGTATTGGATTG | TGGACATGAGACGCAGGAAAG | 269 | 60.6 | 101 | XM_038692267.1 |
| diablo | CCAGACCACCTTGGCTGTCATAG | CTGCTCGGCTCCTGAAGTGTATG | 265 | 59.1 | 98.1 | XM_038723934.1 |
| gli | CCGACGGTCCTCTATGGTGT | TGTATTGCTGAGCGGGTGTT | 221 | 58.9 | 101 | XM_038696203.1 |
| igf-1 | TCTCCTGTAGCCACACCCTCT | GCCTCTATCTCCACACACAAACT | 134 | 57.8 | 102 | XM_038738328.1 |
| il-8 | TCCTGGCTGCTCTGGCTCTC | GGATGGCCCTCCTGTTAATGG | 111 | 63.1 | 98.5 | XM_038704089.1 |
| nlrp12 | AAGGAAAGGAGAGGATGACGAC | AAACCCGAACGCAGGATAGA | 268 | 59.1 | 99.3 | XM_038692024.1 |
| pi3k | TCTCAAGGGAGGAGGTCA | CCGAATGTCAGAGGGTC | 184 | 55.1 | 99.3 | XM_038730665.1 |
| eef1a1 | GTTGCTGCTGGTGTTGGTGAG | GAAACGCTTCTGGCTGTAAGG | 156 | 60.1 | 100 | XM_038695351 |
| Diets | LS | MS | HS |
|---|---|---|---|
| Feed efficiency ratio | 1.21 ± 0.01 a | 1.25 ± 0.01 ab | 1.30 ± 0.04 b |
| Protein efficiency ratio | 2.47 ± 0.01 a | 2.54 ± 0.02 ab | 2.61 ± 0.08 b |
| Feeding ratio (%) | 2.53 ± 0.01 b | 2.43 ± 0.09 ab | 2.28 ± 0.12 a |
| Condition factor (g/cm3) | 2.85 ± 0.07 | 2.81 ± 0.01 | 2.69 ± 0.11 |
| Viscerosomatic index (%) | 8.31 ± 0.39 a | 9.81 ± 0.78 b | 9.85 ± 0.30 b |
| Hepatosomatic index (%) | 2.42 ± 0.10 a | 3.52 ± 0.26 ab | 4.09 ± 0.77 b |
| Intraperitoneal fat ratio (%) | 1.76 ± 0.23 | 1.71 ± 0.16 | 1.75 ± 0.12 |
| Diets | LS | MS | HS |
|---|---|---|---|
| Glucose (mmol/L) | 2.94 ± 0.19 | 3.04 ± 0.01 | 2.60 ± 0.28 |
| Triglyceride (mmol/L) | 11.1 ± 1.5 | 13.3 ± 1.3 | 11.8 ± 0.7 |
| Cholesterol (mmol/L) | 10.8 ± 0.6 | 11.3 ± 1.2 | 12.0 ± 0.4 |
| Protein (g/L) | 24.0 ± 1.9 | 21.1 ± 0.8 | 21.9 ± 0.4 |
| Diets | LS | MS | HS |
|---|---|---|---|
| Whole body moisture (%) | 70.4 ± 0.0 | 70.3 ± 0.6 | 71.0 ± 1.0 |
| Whole body protein (%) | 17.4 ± 0.6 | 17.1 ± 0.1 | 16.9 ± 0.3 |
| Whole body lipid (%) | 9.45 ± 0.18 | 9.11 ± 0.81 | 8.89 ± 1.07 |
| Whole body ash (%) | 3.64 ± 0.03 | 3.75 ± 0.05 | 3.75 ± 0.06 |
| Liver moisture (%) | 67.1 ± 0.2 | 66.7 ± 0.4 | 67.0 ± 0.7 |
| Liver protein (%) | 11.2 ± 0.3 b | 8.55 ± 0.48 a | 7.83 ± 1.05 a |
| Liver lipid (%) | 4.77 ± 0.53 | 4.88 ± 0.36 | 4.68 ± 0.14 |
| Liver glycogen (mg/g) | 116 ± 10 a | 137 ± 8 ab | 162 ± 8 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, H.; Wang, K.; Qin, C.; He, Y.; Luo, L.; Lin, S.; Chen, Y. Transcriptome Profiling Unveils the Mechanisms of Inflammation, Apoptosis, and Fibrosis in the Liver of Juvenile Largemouth Bass Micropterus salmoides Fed High-Starch Diets. Animals 2024, 14, 3394. https://doi.org/10.3390/ani14233394
Liu X, Liu H, Wang K, Qin C, He Y, Luo L, Lin S, Chen Y. Transcriptome Profiling Unveils the Mechanisms of Inflammation, Apoptosis, and Fibrosis in the Liver of Juvenile Largemouth Bass Micropterus salmoides Fed High-Starch Diets. Animals. 2024; 14(23):3394. https://doi.org/10.3390/ani14233394
Chicago/Turabian StyleLiu, Xifeng, Hongkang Liu, Kangwei Wang, Chuanjie Qin, Yuanfa He, Li Luo, Shimei Lin, and Yongjun Chen. 2024. "Transcriptome Profiling Unveils the Mechanisms of Inflammation, Apoptosis, and Fibrosis in the Liver of Juvenile Largemouth Bass Micropterus salmoides Fed High-Starch Diets" Animals 14, no. 23: 3394. https://doi.org/10.3390/ani14233394
APA StyleLiu, X., Liu, H., Wang, K., Qin, C., He, Y., Luo, L., Lin, S., & Chen, Y. (2024). Transcriptome Profiling Unveils the Mechanisms of Inflammation, Apoptosis, and Fibrosis in the Liver of Juvenile Largemouth Bass Micropterus salmoides Fed High-Starch Diets. Animals, 14(23), 3394. https://doi.org/10.3390/ani14233394







