Dynamic Changes in the Intraepithelial Lymphocyte Numbers Following Salmonella Typhimurium Infection in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Husbandry, Experimental Design, and Performance
2.3. Salmonella Recovery and Count
2.4. Isolation of Immune Cells and Flow Cytometry
2.5. RNA Isolation and Gene Expression
2.6. Statistical Analysis
3. Results
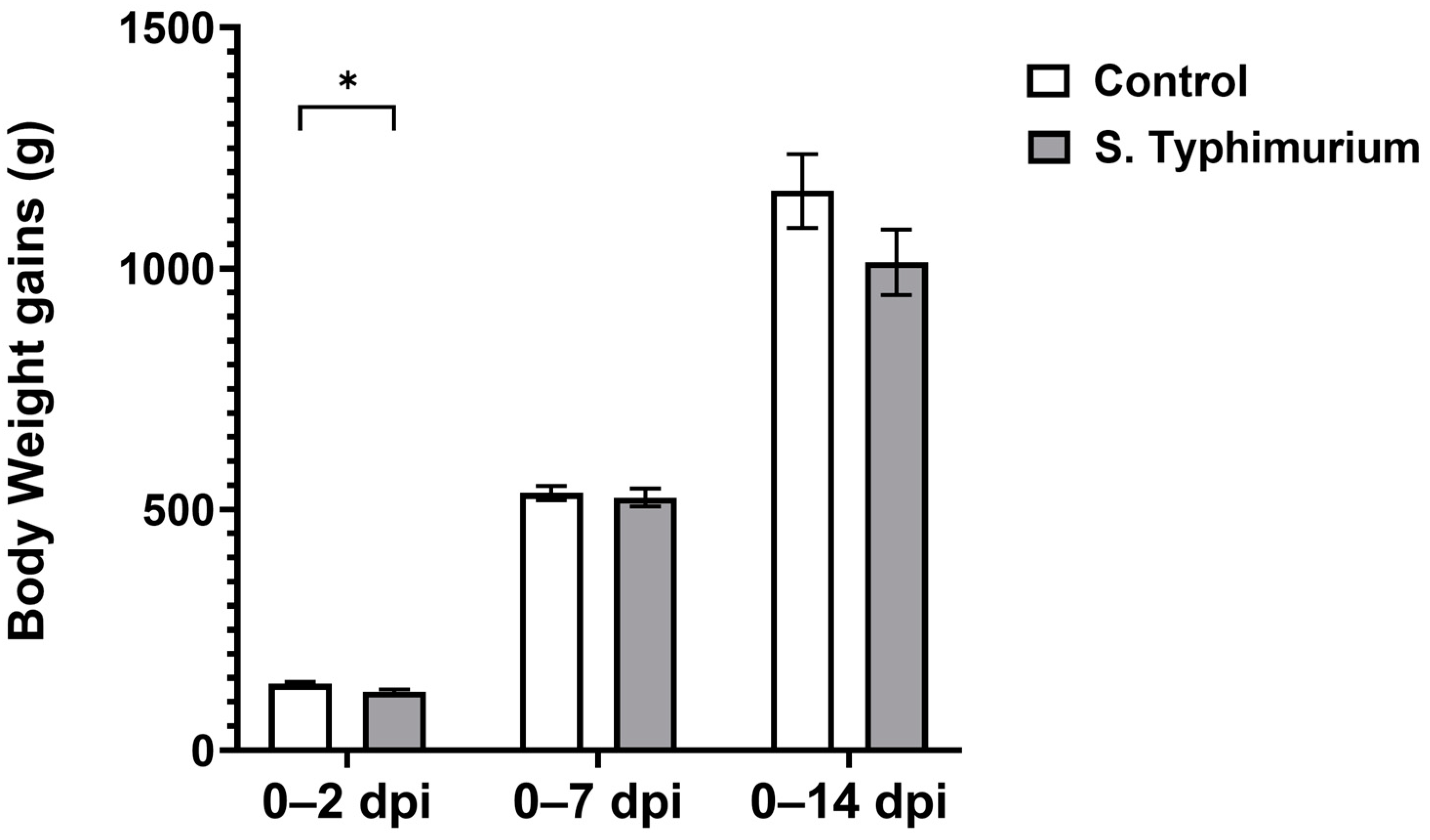
3.1. Chicken Performance
3.2. Salmonella Loads
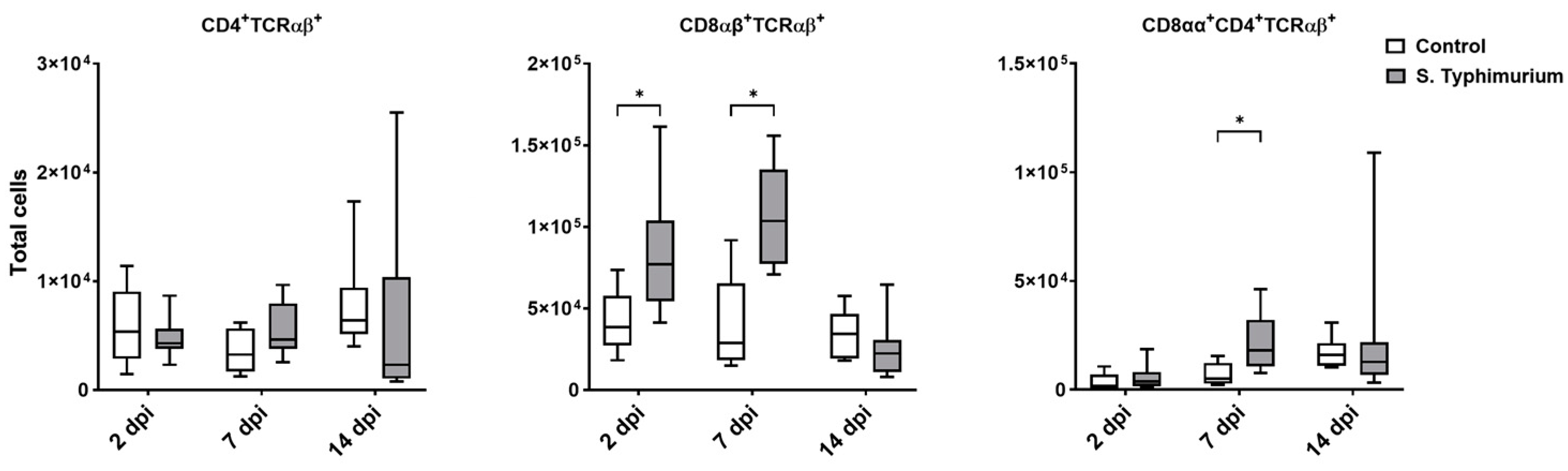
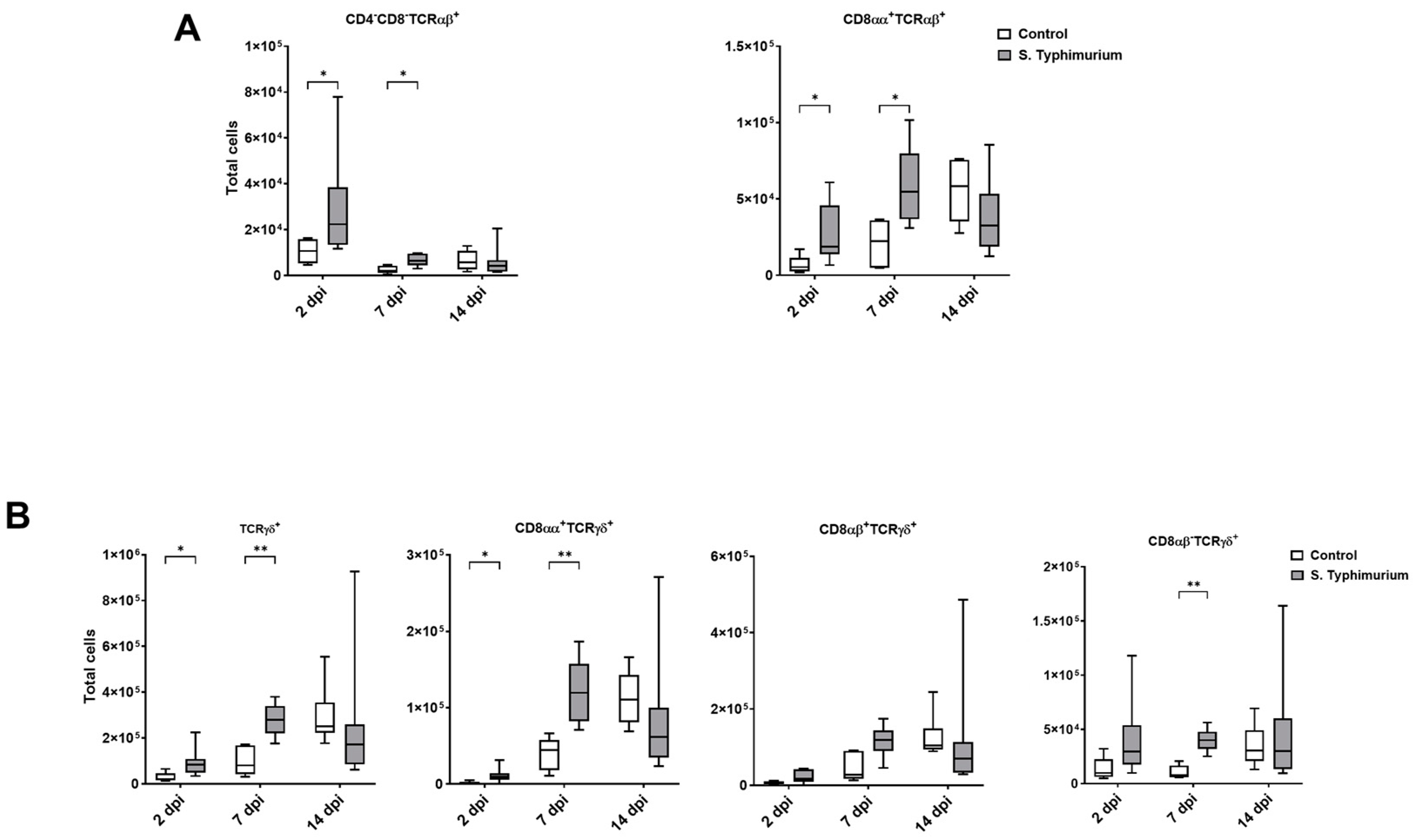
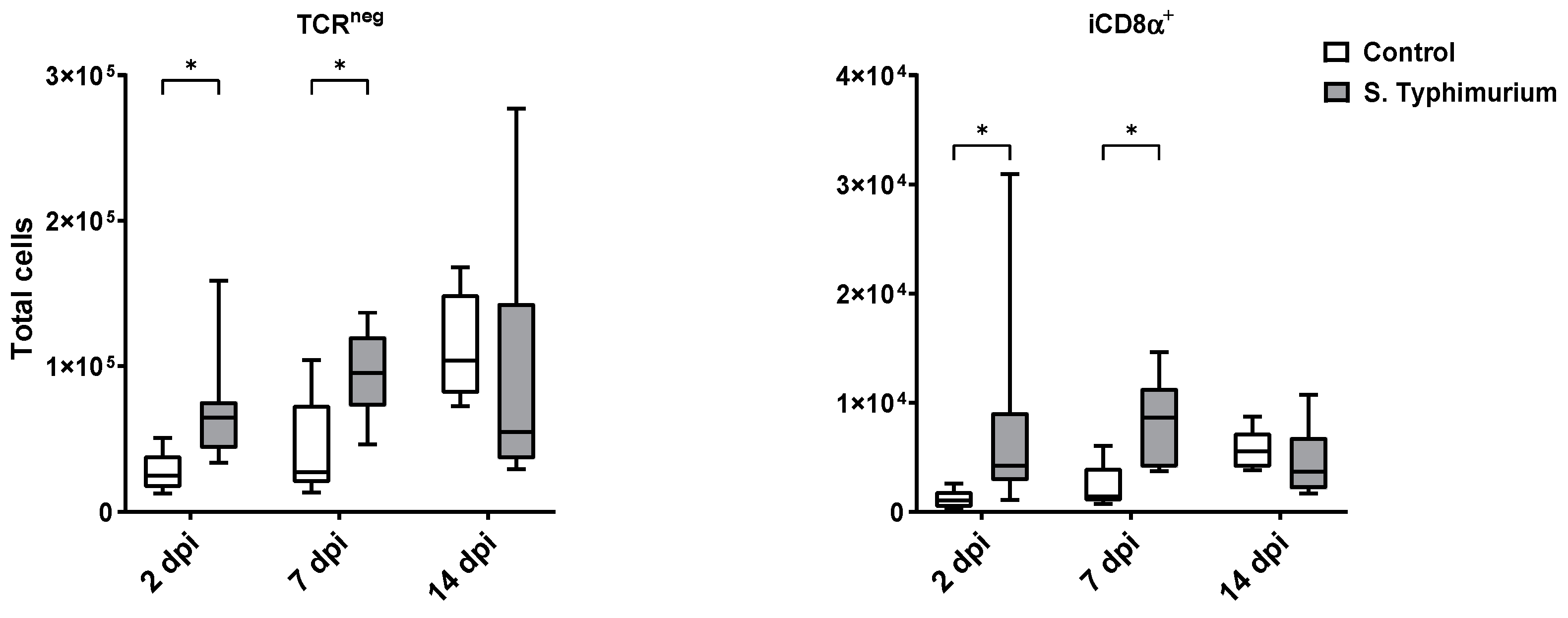
3.3. Effect of S. Typhimurium Challenge on IEL Populations
3.4. Spleen Lymphocytes
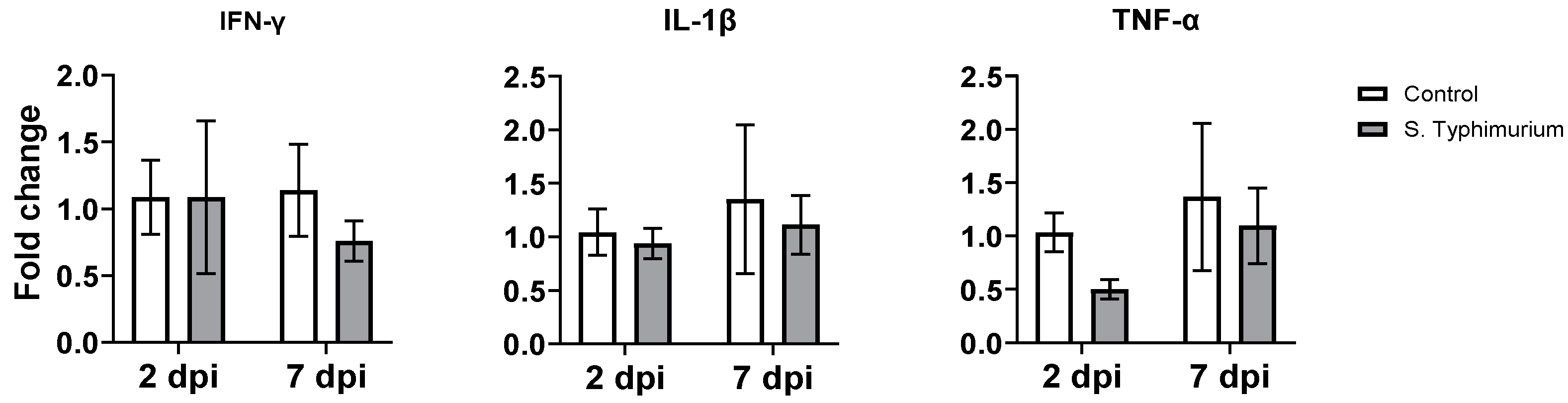
3.5. Gene Expression of Proinflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arsenault, R.J.; Napper, S.; Kogut, M.H. Salmonella Enterica Typhimurium Infection Causes Metabolic Changes in Chicken Muscle Involving AMPK, Fatty Acid and Insulin/mTOR Signaling. Vet. Res. 2013, 44, 35. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Ahmad, S.M.; Bhat, S.A.; Ahmed, R.; Urwat, U.; Mumtaz, P.T.; Bhat, S.A.; Dar, T.A.; Shah, R.A.; Ganai, N.A. Salmonella Typhimurium in Poultry: A Review. World’s Poult. Sci. J. 2017, 73, 345–354. [Google Scholar] [CrossRef]
- Sheela, R.R.; Babu, U.; Mu, J.; Elankumaran, S.; Bautista, D.A.; Raybourne, R.B.; Heckert, R.A.; Song, W. Immune Responses against Salmonella Enterica Serovar Enteritidis Infection in Virally Immunosuppressed Chickens. Clin. Diagn. Lab. Immunol. 2003, 10, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Matulova, M.; Varmuzova, K.; Sisak, F.; Havlickova, H.; Babak, V.; Stejskal, K.; Zdrahal, Z.; Rychlik, I. Chicken Innate Immune Response to Oral Infection with Salmonella Enterica Serovar Enteritidis. Vet. Res. 2013, 44, 37. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Wilhelm, A.; Jugert, C.; Pieper, J.; Sachse, K.; Methner, U. Chicken Cecum Immune Response to Salmonella Enterica Serovars of Different Levels of Invasiveness. Infect. Immun. 2007, 75, 5993–6007. [Google Scholar] [CrossRef]
- Cheroutre, H.; Lambolez, F.; Mucida, D. The Light and Dark Sides of Intestinal Intraepithelial Lymphocytes. Nat. Rev. Immunol. 2011, 11, 445–456. [Google Scholar] [CrossRef]
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Lawn, A.M.; Rose, M.E.; Bradley, J.W.A.; Rennie, M.C. Lymphocytes of the Intestinal Mucosa of Chickens. Cell Tissue Res. 1988, 251, 189–195. [Google Scholar] [CrossRef]
- Meijerink, N.; van Haarlem, D.A.; Velkers, F.C.; Stegeman, A.J.; Rutten, V.P.M.G.; Jansen, C.A. Analysis of Chicken Intestinal Natural Killer Cells, a Major IEL Subset during Embryonic and Early Life. Dev. Comp. Immunol. 2021, 114, 103857. [Google Scholar] [CrossRef]
- Choi, K.D.; Lillehoj, H.S.; Zalenga, D.S. Changes in Local IFN-Gamma and TGF-Beta4 mRNA Expression and Intraepithelial Lymphocytes Following Eimeria Acervulina Infection. Vet. Immunol. Immunopathol. 1999, 71, 263–275. [Google Scholar] [CrossRef]
- Hamisu, T.M.; Aliyu, H.B.; Hair-Bejo, M.; Omar, A.R.; Ideris, A. Alteration in the Population of Intraepithelial Lymphocytes and Virus Shedding in Specific-Pathogen-Free Chickens Following Inoculation with Lentogenic and Velogenic Newcastle Disease Virus Strains. Viral Immunol. 2022, 35, 328–337. [Google Scholar] [CrossRef]
- Majeed, S.; Hamad, S.K.; Shah, B.R.; Bielke, L.; Nazmi, A. Natural Intraepithelial Lymphocyte Populations Rise during Necrotic Enteritis in Chickens. Front. Immunol. 2024, 15, 1354701. [Google Scholar] [CrossRef] [PubMed]
- Kallapura, G.; Morgan, M.J.; Pumford, N.R.; Bielke, L.R.; Wolfenden, A.D.; Faulkner, O.B.; Latorre, J.D.; Menconi, A.; Hernandez-Velasco, X.; Kuttappan, V.A.; et al. Evaluation of the Respiratory Route as a Viable Portal of Entry for Salmonella in Poultry via Intratracheal Challenge of Salmonella Enteritidis and Salmonella Typhimurium. Poult. Sci. 2014, 93, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Nanno, M.; Matsumoto, S.; Koike, R.; Miyasaka, M.; Kawaguchi, M.; Masuda, T.; Miyawaki, S.; Cai, Z.; Shimamura, T.; Fujiura, Y. Development of Intestinal Intraepithelial T Lymphocytes Is Independent of Peyer’s Patches and Lymph Nodes in Aly Mutant Mice. J. Immunol. 1994, 153, 2014–2020. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Emami, N.K.; Calik, A.; White, M.B.; Kimminau, E.A.; Dalloul, R.A. Effect of Probiotics and Multi-Component Feed Additives on Microbiota, Gut Barrier and Immune Responses in Broiler Chickens During Subclinical Necrotic Enteritis. Front. Vet. Sci. 2020, 7, 572142. [Google Scholar] [CrossRef]
- Blue, C.E.C.; Suarez, M.G.; Nacer-Khodja, E.; Rodriguez, M.A.; Dalloul, R.A. Positive Impact of Dietary Marine Sulfated Polysaccharides Derived from Macroalgae during a Necrotic Enteritis Challenge. Poult. Sci. 2024, 103, 104502. [Google Scholar] [CrossRef]
- Ijaz, A.; Veldhuizen, E.J.A.; Broere, F.; Rutten, V.P.M.G.; Jansen, C.A. The Interplay between Salmonella and Intestinal Innate Immune Cells in Chickens. Pathogens 2021, 10, 1512. [Google Scholar] [CrossRef]
- Luhtala, M. Chicken CD4, CD8alphabeta, and CD8alphaalpha T Cell Co-Receptor Molecules. Poult. Sci. 1998, 77, 1858–1873. [Google Scholar] [CrossRef]
- Rigby, C.E.; Pettit, J.R. Some Factors Affecting Salmonella Typhimurium Infection and Shedding in Chickens Raised on Litter. Avian Dis. 1979, 23, 442–455. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Bowers, J.B.; Hess, J.B.; McKee, S.R. Effect of Dietary Glutamine Supplementation on Salmonella Colonization in the Ceca of Young Broiler Chicks. Poult. Sci. 2010, 89, 1042–1048. [Google Scholar] [CrossRef]
- Barrow, P.A.; Simpson, J.M.; Lovell, M.A. Intestinal Colonisation in the Chicken by Food-poisoning Salmonella Serotypes; Microbial Characteristics Associated with Faecal Excretion. Avian Pathol. 1988, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Sivula, C.P.; Bogomolnaya, L.M.; Andrews-Polymenis, H.L. A Comparison of Cecal Colonization of Salmonella Enterica Serotype Typhimurium in White Leghorn Chicks and Salmonella-Resistant Mice. BMC Microbiol. 2008, 8, 182. [Google Scholar] [CrossRef]
- Beal, R.K.; Wigley, P.; Powers, C.; Hulme, S.D.; Barrow, P.A.; Smith, A.L. Age at Primary Infection with Salmonella Enterica Serovar Typhimurium in the Chicken Influences Persistence of Infection and Subsequent Immunity to Re-Challenge. Vet. Immunol. Immunopathol. 2004, 100, 151–164. [Google Scholar] [CrossRef]
- Godinez, I.; Haneda, T.; Raffatellu, M.; George, M.D.; Paixão, T.A.; Rolán, H.G.; Santos, R.L.; Dandekar, S.; Tsolis, R.M.; Bäumler, A.J. T Cells Help To Amplify Inflammatory Responses Induced by Salmonella Enterica Serotype Typhimurium in the Intestinal Mucosa. Infect. Immun. 2008, 76, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, N.; van den Biggelaar, R.H.G.A.; van Haarlem, D.A.; Stegeman, J.A.; Rutten, V.P.M.G.; Jansen, C.A. A Detailed Analysis of Innate and Adaptive Immune Responsiveness upon Infection with Salmonella Enterica Serotype Enteritidis in Young Broiler Chickens. Vet. Res. 2021, 52, 109. [Google Scholar] [CrossRef]
- Walch, M.; Dotiwala, F.; Mulik, S.; Thiery, J.; Kirchhausen, T.; Clayberger, C.; Krensky, A.M.; Martinvalet, D.; Lieberman, J. Cytotoxic Cells Kill Intracellular Bacteria through Granulysin-Mediated Delivery of Granzymes. Cell 2014, 157, 1309–1323. [Google Scholar] [CrossRef]
- Labuda, J.C.; Depew, C.E.; Pham, O.H.; Benoun, J.M.; Ramirez, N.A.; McSorley, S.J. Unexpected Role of CD8 T Cells in Accelerated Clearance of Salmonella Enterica Serovar Typhimurium from H-2 Congenic Mice. Infect. Immun. 2019, 87, e00588-19. [Google Scholar] [CrossRef]
- Lee, S.-J.; Dunmire, S.; McSorley, S.J. MHC Class-I-Restricted CD8 T Cells Play a Protective Role during Primary Salmonella Infection. Immunol. Lett. 2012, 148, 138–143. [Google Scholar] [CrossRef]
- Sadeyen, J.-R.; Trotereau, J.; Velge, P.; Marly, J.; Beaumont, C.; Barrow, P.A.; Bumstead, N.; Lalmanach, A.-C. Salmonella Carrier State in Chicken: Comparison of Expression of Immune Response Genes between Susceptible and Resistant Animals. Microbes Infect. 2004, 6, 1278–1286. [Google Scholar] [CrossRef]
- Johanns, T.M.; Ertelt, J.M.; Rowe, J.H.; Way, S.S. Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Salmonella Infection. PLoS Pathog. 2010, 6, e1001043. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Kogut, M.H.; Arsenault, R.J.; Swaggerty, C.L.; Cole, K.; Reddish, J.M.; Selvaraj, R.K. Effect of Salmonella Infection on Cecal Tonsil Regulatory T Cell Properties in Chickens. Poult. Sci. 2015, 94, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Villagómez, D.; Van Kaer, L. TL and CD8αα: Enigmatic Partners in Mucosal Immunity. Immunol. Lett. 2010, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sujino, T.; London, M.; Hoytema van Konijnenburg, D.P.; Rendon, T.; Buch, T.; Silva, H.M.; Lafaille, J.J.; Reis, B.S.; Mucida, D. Tissue Adaptation of Regulatory and Intraepithelial CD4⁺ T Cells Controls Gut Inflammation. Science 2016, 352, 1581–1586. [Google Scholar] [CrossRef]
- Mucida, D.; Husain, M.M.; Muroi, S.; van Wijk, F.; Shinnakasu, R.; Naoe, Y.; Reis, B.S.; Huang, Y.; Lambolez, F.; Docherty, M.; et al. Transcriptional Reprogramming of Mature CD4+ T Helper Cells Generates Distinct MHC Class II-Restricted Cytotoxic T Lymphocytes. Nat. Immunol. 2013, 14, 281–289. [Google Scholar] [CrossRef]
- Reis, B.S.; Hoytema van Konijnenburg, D.P.; Grivennikov, S.I.; Mucida, D. Transcription Factor T-Bet Regulates Intraepithelial Lymphocyte Functional Maturation. Immunity 2014, 41, 244–256. [Google Scholar] [CrossRef]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual Expression of Runx3 and ThPOK Regulates Intestinal CD4+ T Cell Immunity. Nat. Immunol. 2013, 14, 271. [Google Scholar] [CrossRef]
- Konkel, J.E.; Maruyama, T.; Carpenter, A.C.; Xiong, Y.; Zamarron, B.F.; Hall, B.E.; Kulkarni, A.B.; Zhang, P.; Bosselut, R.; Chen, W. Control of the Development of CD8αα+ Intestinal Intraepithelial Lymphocytes by TGF-β. Nat. Immunol. 2011, 12, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Rabacal, W.A.S.; Scott Algood, H.M.; Parekh, V.V.; Olivares-Villagómez, D. In Vitro Induction of Regulatory CD4+CD8α+ T Cells by TGF-β, IL-7 and IFN-γ. PLoS ONE 2013, 8, e67821. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus Reuteri Induces Gut Intraepithelial CD4+CD8αα+ T Cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Carton, J.; Byrne, B.; Madrigal-Estebas, L.; O’Donoghue, D.P.; O’Farrelly, C. CD4+CD8+ Human Small Intestinal T Cells Are Decreased in Coeliac Patients, with CD8 Expression Downregulated on Intra-Epithelial T Cells in the Active Disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 961–968. [Google Scholar] [CrossRef]
- Senju, M.; Wu, K.C.; Mahida, Y.R.; Jewell, D.P. Coexpression of CD4 and CD8 on Peripheral Blood T Cells and Lamina Propria T Cells in Inflammatory Bowel Disease by Two Colour Immunofluorescence and Flow Cytometric Analysis. Gut 1991, 32, 918–922. [Google Scholar] [CrossRef]
- Das, G.; Augustine, M.M.; Das, J.; Bottomly, K.; Ray, P.; Ray, A. An Important Regulatory Role for CD4+CD8 Alpha Alpha T Cells in the Intestinal Epithelial Layer in the Prevention of Inflammatory Bowel Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 5324–5329. [Google Scholar] [CrossRef]
- Gangadharan, D.; Lambolez, F.; Attinger, A.; Wang-Zhu, Y.; Sullivan, B.A.; Cheroutre, H. Identification of Pre- and Postselection TCRalphabeta+ Intraepithelial Lymphocyte Precursors in the Thymus. Immunity 2006, 25, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, T.A.; Sandau, M.M.; Jameson, S.C.; Hogquist, K.A. The Timing of TCR Alpha Expression Critically Influences T Cell Development and Selection. J. Exp. Med. 2005, 202, 111–121. [Google Scholar] [CrossRef]
- McDonald, B.D.; Bunker, J.J.; Ishizuka, I.E.; Jabri, B.; Bendelac, A. Elevated T Cell Receptor Signaling Identifies a Thymic Precursor to the TCRαβ(+)CD4(-)CD8β(-) Intraepithelial Lymphocyte Lineage. Immunity 2014, 41, 219–229. [Google Scholar] [CrossRef]
- Ruscher, R.; Kummer, R.L.; Lee, Y.J.; Jameson, S.C.; Hogquist, K.A. CD8αα Intraepithelial Lymphocytes Arise from Two Main Thymic Precursors. Nat. Immunol. 2017, 18, 771–779. [Google Scholar] [CrossRef]
- Van Kaer, L.; Olivares-Villagómez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Denning, T.L.; Granger, S.W.; Mucida, D.; Graddy, R.; Leclercq, G.; Zhang, W.; Honey, K.; Rasmussen, J.P.; Cheroutre, H.; Rudensky, A.Y.; et al. Mouse TCRalphabeta+CD8alphaalpha Intraepithelial Lymphocytes Express Genes That Down-Regulate Their Antigen Reactivity and Suppress Immune Responses. J. Immunol. 2007, 178, 4230–4239. [Google Scholar] [CrossRef]
- Poussier, P.; Ning, T.; Banerjee, D.; Julius, M. A Unique Subset of Self-Specific Intraintestinal T Cells Maintains Gut Integrity. J. Exp. Med. 2002, 195, 1491–1497. [Google Scholar] [CrossRef]
- Hu, M.D.; Edelblum, K.L. Sentinels at the Frontline: The Role of Intraepithelial Lymphocytes in Inflammatory Bowel Disease. Curr. Pharmacol. Rep. 2017, 3, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ Regulatory T-Cell Development by a Commensal Bacterium of the Intestinal Microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.; Hinton, M.; Van Gils, B. Dietary Mannan-Oligosaccharides and Their Effect on Chicken Caecal Microflora in Relation to Salmonella Enteritidis Colonization. Avian Pathol. 2002, 31, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y. Regulatory T Cells and Infection: A Dangerous Necessity. Nat. Rev. Immunol. 2007, 7, 875–888. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Zhou, Z.; Zhang, J.; Zhang, J.; Tian, Z. Small Intestinal Intraepithelial Lymphocytes Expressing CD8 and T Cell Receptor Γδ Are Involved in Bacterial Clearance during Salmonella Enterica Serovar Typhimurium Infection. Infect. Immun. 2012, 80, 565–574. [Google Scholar] [CrossRef]
- Kao, J.Y.; Zhang, M.; Miller, M.J.; Mills, J.C.; Wang, B.; Liu, M.; Eaton, K.A.; Zou, W.; Berndt, B.E.; Cole, T.S.; et al. Helicobacter Pylori Immune Escape Is Mediated by Dendritic Cell-Induced Treg Skewing and Th17 Suppression in Mice. Gastroenterology 2010, 138, 1046–1054. [Google Scholar] [CrossRef]
- Berndt, A.; Methner, U. Gamma/Delta T Cell Response of Chickens after Oral Administration of Attenuated and Non-Attenuated Salmonella Typhimurium Strains. Vet Immunol. Immunopathol. 2001, 78, 143–161. [Google Scholar] [CrossRef]
- Hara, T.; Mizuno, Y.; Takaki, K.; Takada, H.; Akeda, H.; Aoki, T.; Nagata, M.; Ueda, K.; Matsuzaki, G.; Yoshikai, Y. Predominant Activation and Expansion of V Gamma 9-Bearing Gamma Delta T Cells in Vivo as Well as In Vitro in Salmonella Infection. J. Clin. Investig. 1992, 90, 204–210. [Google Scholar] [CrossRef]
- Mixter, P.F.; Camerini, V.; Stone, B.J.; Miller, V.L.; Kronenberg, M. Mouse T Lymphocytes That Express a Gamma Delta T-Cell Antigen Receptor Contribute to Resistance to Salmonella Infection In Vivo. Infect. Immun. 1994, 62, 4618–4621. [Google Scholar] [CrossRef]
- Pieper, J.; Methner, U.; Berndt, A. Characterization of Avian Γδ T-Cell Subsets after Salmonella Enterica Serovar Typhimurium Infection of Chicks. Infect. Immun. 2011, 79, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Talayero, P.; Mancebo, E.; Calvo-Pulido, J.; Rodríguez-Muñoz, S.; Bernardo, I.; Laguna-Goya, R.; Cano-Romero, F.L.; García-Sesma, A.; Loinaz, C.; Jiménez, C.; et al. Innate Lymphoid Cells Groups 1 and 3 in the Epithelial Compartment of Functional Human Intestinal Allografts. Am. J. Transplant. 2016, 16, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, A.; Gronke, K.; Biswas, A.; Martens, L.; Saeys, Y.; Filtjens, J.; Taveirne, S.; Van Ammel, E.; Kerre, T.; Matthys, P.; et al. A Murine Intestinal Intraepithelial NKp46-Negative Innate Lymphoid Cell Population Characterized by Group 1 Properties. Cell Rep. 2017, 19, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Ettersperger, J.; Montcuquet, N.; Malamut, G.; Guegan, N.; Lopez-Lastra, S.; Gayraud, S.; Reimann, C.; Vidal, E.; Cagnard, N.; Villarese, P.; et al. Interleukin-15-Dependent T-Cell-like Innate Intraepithelial Lymphocytes Develop in the Intestine and Transform into Lymphomas in Celiac Disease. Immunity 2016, 45, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Scott Algood, H.M.; Singh, K.; Parekh, V.V.; Greer, M.J.; Piazuelo, M.B.; Weitkamp, J.-H.; Matta, P.; Chaturvedi, R.; Wilson, K.T.; et al. CD8αα Innate-Type Lymphocytes in the Intestinal Epithelium Mediate Mucosal Immunity. Immunity 2014, 41, 451–464. [Google Scholar] [CrossRef]
- Göbel, T.W.; Kaspers, B.; Stangassinger, M. NK and T Cells Constitute Two Major, Functionally Distinct Intestinal Epithelial Lymphocyte Subsets in the Chicken. Int. Immunol. 2001, 13, 757–762. [Google Scholar] [CrossRef]


| Gene | Accession No. | Primer Sequence | Product Length (bp) | |
|---|---|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |||
| IL-1β [17] | XM_015297469.1 | CCCGCCTTCCGCTACA | CACGAAGCACTTCTGGTTGATG | 66 |
| IFN-γ [17] | NM_205149.1 | GCTCCCGATGAACGACTTGA | TGTAAGATGCTGAAGAGTTCATTCG | 63 |
| TNF-α [18] | MF000729.1 | CCCATCCCTGGTCCGTAAC | ATACGAAGTAAAGGCCGTCCC | 77 |
| GAPDH [18] | NM_204305 | CCTAGGATACACAGAGGACCAGGTT | GGTGGAGGAATGGCTGTCA | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, S.; Shah, B.R.; Khalid, N.; Bielke, L.; Nazmi, A. Dynamic Changes in the Intraepithelial Lymphocyte Numbers Following Salmonella Typhimurium Infection in Broiler Chickens. Animals 2024, 14, 3463. https://doi.org/10.3390/ani14233463
Majeed S, Shah BR, Khalid N, Bielke L, Nazmi A. Dynamic Changes in the Intraepithelial Lymphocyte Numbers Following Salmonella Typhimurium Infection in Broiler Chickens. Animals. 2024; 14(23):3463. https://doi.org/10.3390/ani14233463
Chicago/Turabian StyleMajeed, Shuja, Bikas R. Shah, Nimra Khalid, Lisa Bielke, and Ali Nazmi. 2024. "Dynamic Changes in the Intraepithelial Lymphocyte Numbers Following Salmonella Typhimurium Infection in Broiler Chickens" Animals 14, no. 23: 3463. https://doi.org/10.3390/ani14233463
APA StyleMajeed, S., Shah, B. R., Khalid, N., Bielke, L., & Nazmi, A. (2024). Dynamic Changes in the Intraepithelial Lymphocyte Numbers Following Salmonella Typhimurium Infection in Broiler Chickens. Animals, 14(23), 3463. https://doi.org/10.3390/ani14233463





