Dietary Energy Sources Affect Cecal and Fecal Microbiota of Healthy Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Sample Collection
2.3. Phenotypes
2.4. 16S rRNA Sequencing
2.5. Bioinformatics Analysis
2.6. Quantification of Archaea and Protozoa
2.7. Statistical Analysis
3. Results
3.1. Phenotypes
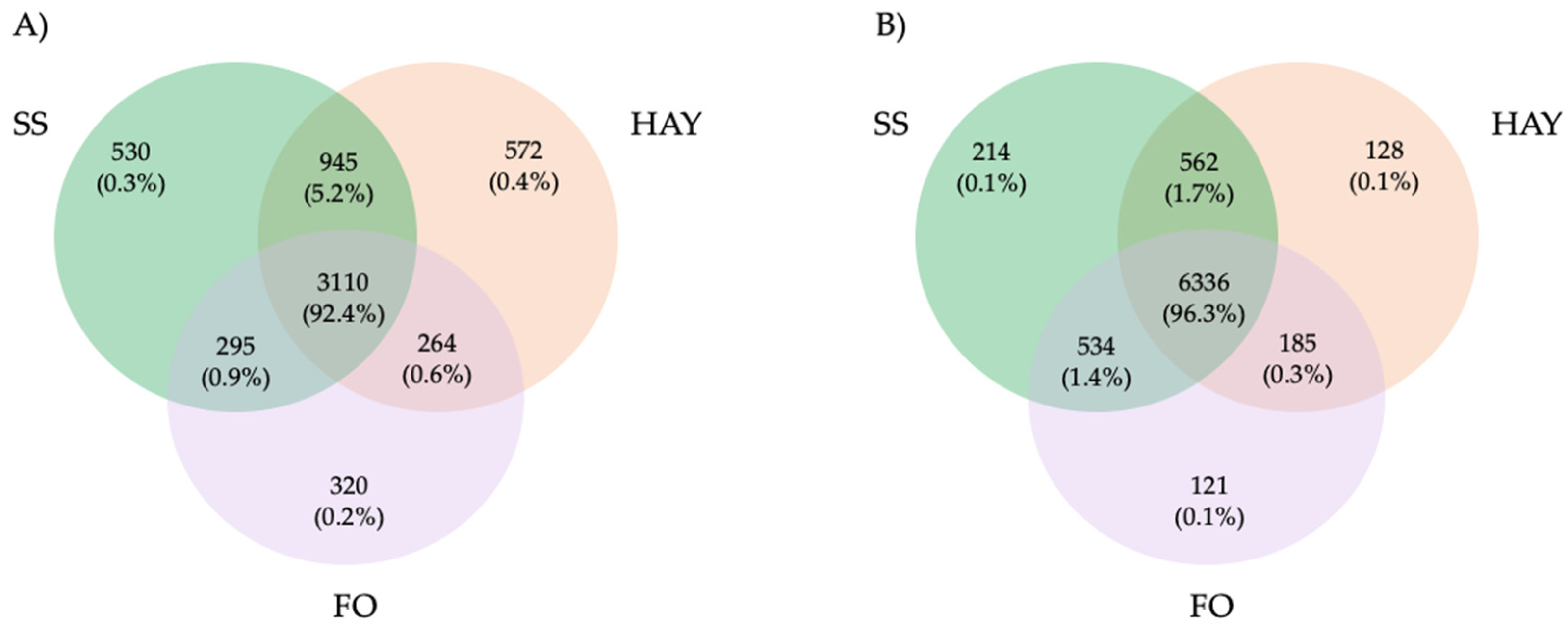
3.2. Microbiome Composition Analysis
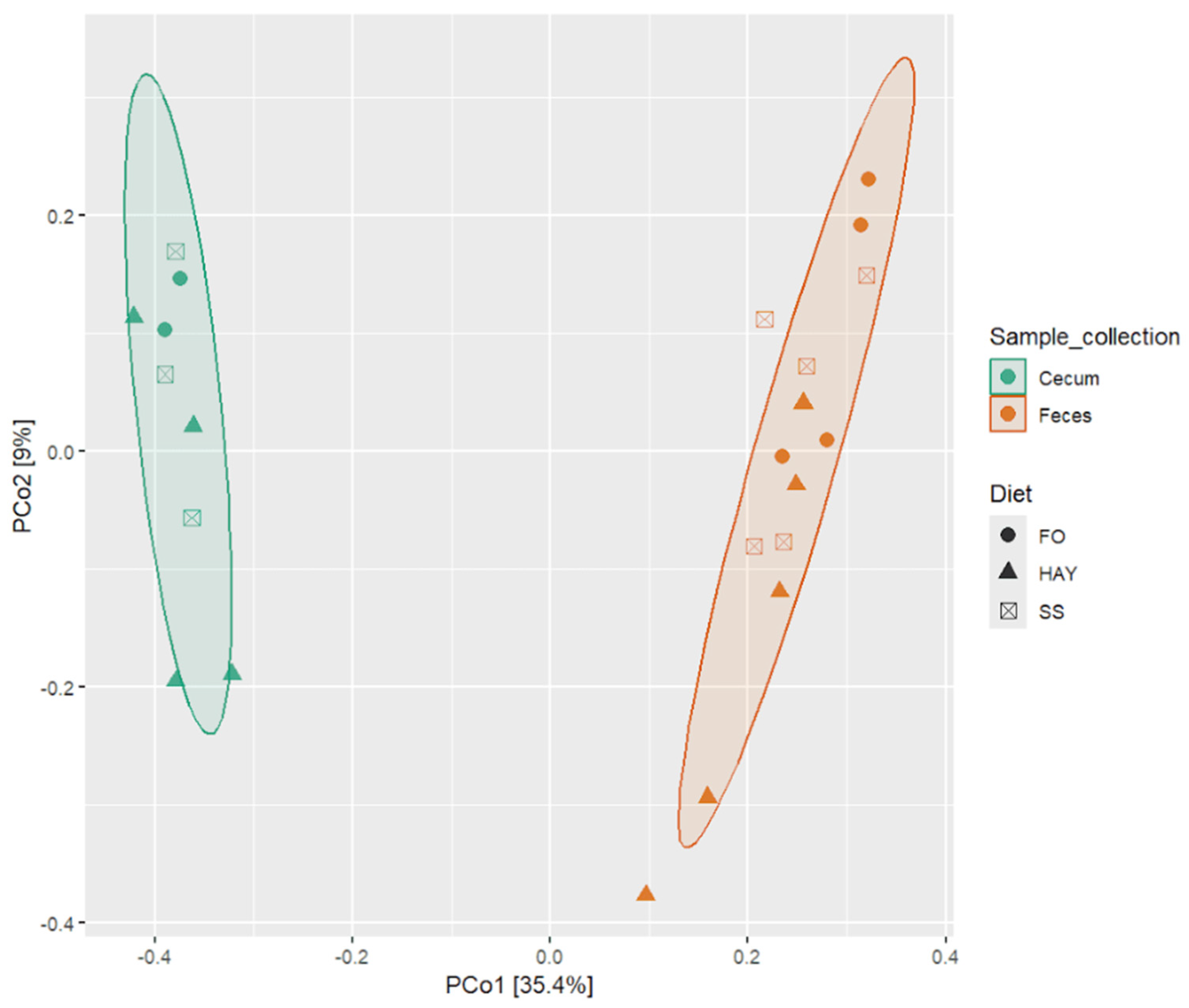
3.3. Alpha and Beta Diversity
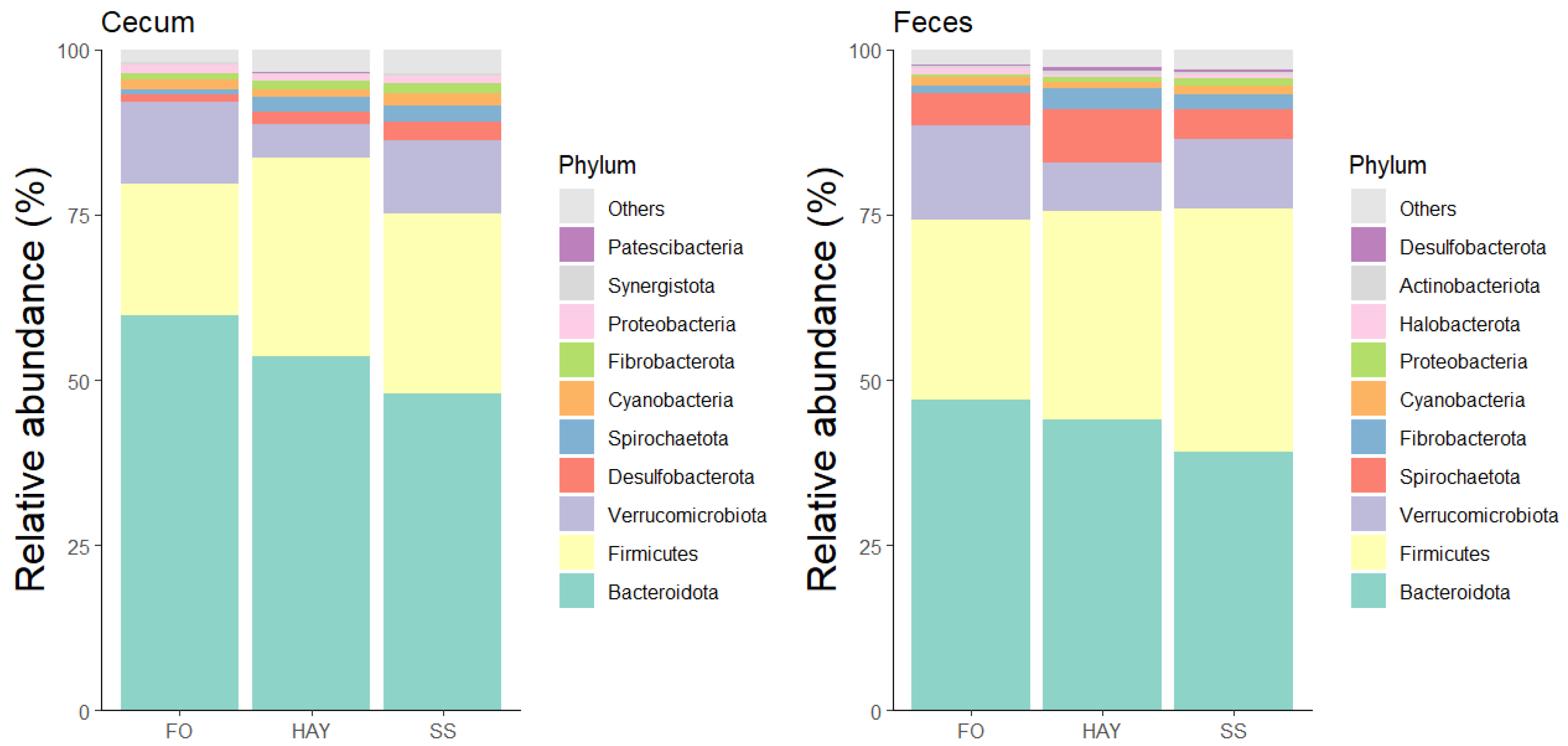
3.4. Analysis of the Bacterial Community Composition at the Phylum Level
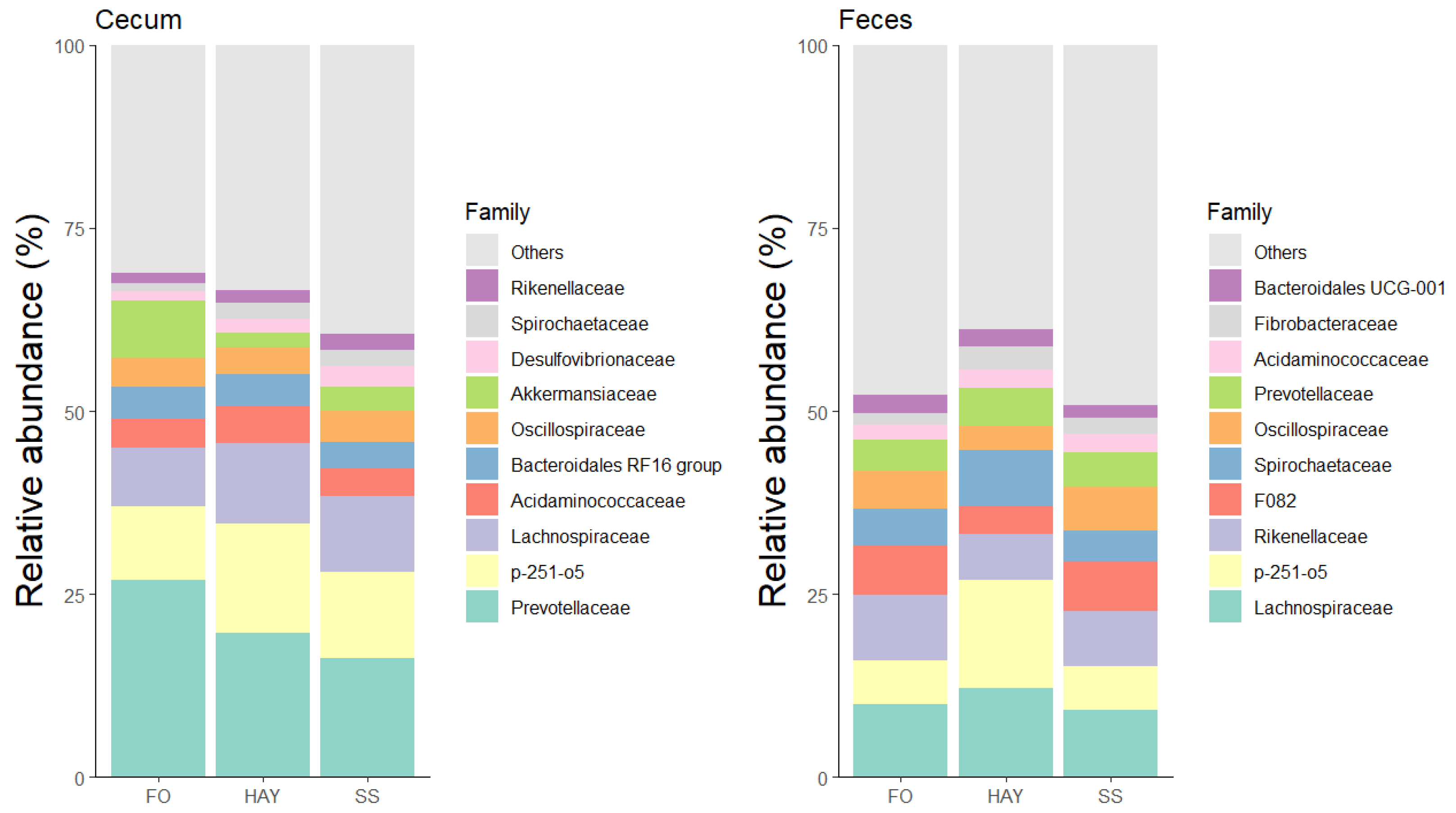
3.5. Analysis of the Bacterial Community Composition at the Family Level
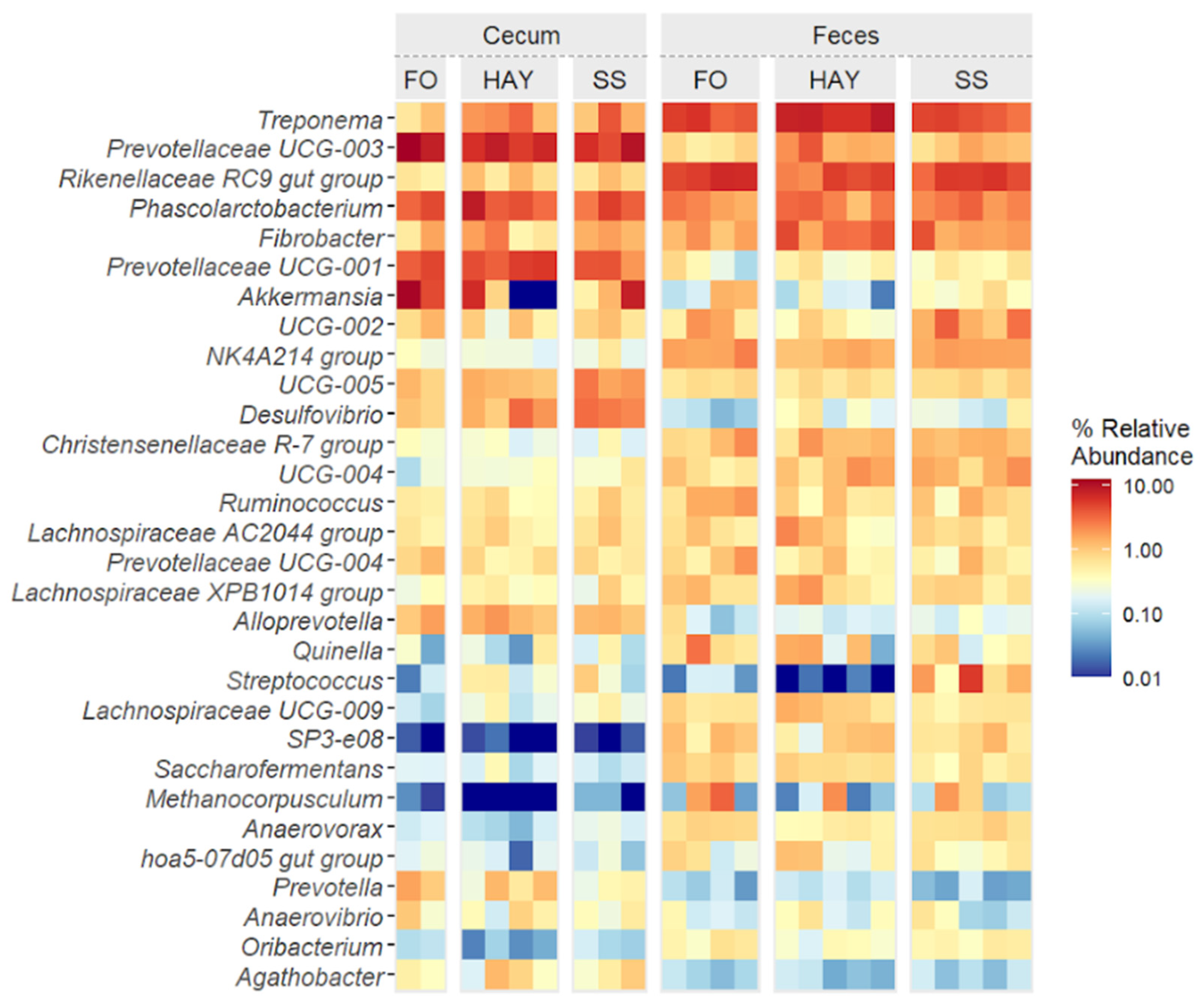
3.6. Analysis of the Bacterial Community Composition at the Genus Level
3.7. Relative DNA Quantification of Total Bacteria, Archaea, and Protozoans by qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council (U.S.). Committee on Nutrient Requirements of Horses; Nutrient requirements of horses; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Dougal, K.; De La Fuente, G.; Harris, P.A.; Girdwood, S.E.; Pinloche, E.; Geor, R.J.; Nielsen, B.D.; Schott, H.C.; Elzinga, S.; Newbold, C.J. Characterisation of the Faecal Bacterial Community in Adult and Elderly Horses Fed a High Fibre, High Oil or High Starch Diet Using 454 Pyrosequencing. PLoS ONE 2014, 9, e87424. [Google Scholar] [CrossRef]
- Kristoffersen, C.; Jensen, R.B.; Avershina, E.; Austbø, D.; Tauson, A.H.; Rudi, K. Diet-Dependent Modular Dynamic Interactions of the Equine Cecal Microbiota. Microbes Environ. 2016, 31, 378–386. [Google Scholar] [CrossRef]
- Warzecha, C.M.; Coverdale, J.A.; Janecka, J.E.; Leatherwood, J.L.; Pinchak, W.E.; Wickersham, T.A.; McCann, J.C. Influence of short-term dietary starch inclusion on the equine cecal microbiome. J. Anim. Sci. 2017, 95, 5077–5090. [Google Scholar] [CrossRef]
- Dunnett, C.E.; Marlin, D.J.; Harris, R.C. Effect of dietary lipid on response to exercise: Relationship to metabolic adaptation. Equine Vet. J. 2002, 34, 75–80. [Google Scholar] [CrossRef]
- Vervuert, I.; Klein, S.; Coenen, M. Short-term effects of a moderate fish oil or soybean oil supplementation on postprandial glucose and insulin responses in healthy horses. Vet. J. 2010, 184, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Bulmer, L.S.; Murray, J.A.; Burns, N.M.; Garber, A.; Wemelsfelder, F.; McEwan, N.R.; Hastie, P.M. High-starch diets alter equine faecal microbiota and increase behavioural reactivity. Sci. Rep. 2019, 9, 18621. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Rodrigues, M.A.M.; Bessa, R.J.B.; Ferreira, L.M.; Martin-Rosset, W. Understanding the equine cecum-colon ecosystem: Current knowledge and future perspectives. Animal 2011, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.M.; de Almeida, F.Q.; de Godoi, F.N.; Silva, V.P.; França, A.B.; Santiago, J.M.; dos Santos, C.S. Buffer capacity, pH and faeces consistency in horses submitted to dietetic starch overload. Ciência Rural 2009, 39, 1782–1788. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. The Impact of Diet on the Hindgut Microbiome. J. Equine Vet. Sci. 2017, 52, 23–28. [Google Scholar] [CrossRef]
- McLean, B.M.L.; Hyslop, J.J.; Longland, A.C.; Cuddeford, D.; Hollands, T. Physical processing of barley and its effects on intra-caecal fermentation parameters in ponies. Anim. Feed Sci. Technol. 2000, 85, 79–87. [Google Scholar] [CrossRef]
- Harlow, B.E.; Lawrence, L.M.; Hayes, S.H.; Crum, A.; Flythe, M.D. Effect of Dietary Starch Source and Concentration on Equine Fecal Microbiota. PLoS ONE 2016, 11, e0154037. [Google Scholar] [CrossRef] [PubMed]
- Julliand, V.; De Fombelle, A.; Drogoul, C.; Jacotot, E. Feeding and microbial disorders in horses: Part 3—Effects of three hay:grain ratios on microbial profile and activities. J. Equine Vet. Sci. 2001, 21, 543–546. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Sacy, A.; Karges, K.; Apper, E. Gastro-Intestinal Microbiota in Equines and Its Role in Health and Disease: The Black Box Opens. Microorganisms 2022, 10, 2517. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.C.K.; Avershina, E.; Mydland, L.T.; Næsset, J.A.; Austbø, D.; Moen, B.; Måge, I.; Rudi, K. High nutrient availability reduces the diversity and stability of the equine caecal microbiota. Microb. Ecol. Health Dis. 2015, 26, 27216. [Google Scholar] [CrossRef]
- Richards, N.; Rowe, J.; Hinch, G. Enhancing Starch Digestion in the Equine Small Intestine. Ph.D. Thesis, University of New England, Portland, ME, USA, 2004. [Google Scholar]
- Potter, G.D.; Webb, S.P.; Evans, J.W.; Webb, G.W. Digestible energy requirements for work and maintenance of horses fed conventional and fat-supplemented diets. J. Equine Vet. Sci. 1990, 10, 214–218. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International. In Official Methods of Analysis of the Association of Official Analytical Chemists International; Cunnif, P., Ed.; AOAC International: Rockville, MD, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Hendrix, D.L. Rapid Extraction and Analysis of Nonstructural Carbohydrates in Plant Tissues. Crop Sci. 1993, 33, 1306–1311. [Google Scholar] [CrossRef]
- Luthersson, N.; Hou Nielsen, K.; Harris, P.; Parkin, T.D.H. Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet. J. 2009, 41, 625–630. [Google Scholar] [CrossRef]
- Braga, A.C.; Araújo, K.V.; Leite, G.G.; Mascarenhas, A.G. Neutral detergent fiber levels in diet of equines. Rev. Bras. Zootec. 2008, 37, 1965–1972. [Google Scholar] [CrossRef]
- Diaz, A.D.P.U.; Santana, A.E.; Valadão, C.A.A.; de Souza, A.H. Canulação Cecal Em Equinos. Braz. Anim. Sci. 2010, 11, 357–362. [Google Scholar] [CrossRef]
- Grimm, P.; Combes, S.; Pascal, G.; Cauquil, L.; Julliand, V. Dietary composition and yeast/microalgae combination supplementation modulate the microbial ecosystem in the caecum, colon and faeces of horses. Br. J. Nutr. 2020, 123, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Vogedes, L.A.; Fernandez, G.C.J.; Frankeny, R.L. Effects of cereal grain supplementation on apparent digestibility of nutrients and concentrations of fermentation end-products in the feces and serum of horses consuming alfalfa cubes. J. Anim. Sci. 2004, 82, 1986–1996. [Google Scholar] [CrossRef]
- Zeyner, A.; Geißler, C.; Dittrich, A. Effects of hay intake and feeding sequence on variables in faeces and faecal water (dry matter, pH value, organic acids, ammonia, buffering capacity) of horses. J. Anim. Physiol. Anim. Nutr. 2004, 88, 7–19. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Regueira-Iglesias, A.; Balsa-Castro, C.; Blanco-Pintos, T.; Tomás, I. Critical review of 16S rRNA gene sequencing workflow in microbiome studies: From primer selection to advanced data analysis. Mol. Oral Microbiol. 2023, 38, 347–399. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2, High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Huse, S.M.; Huber, J.A.; Morrison, H.G.; Sogin, M.L.; Welch, D.M. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol. 2007, 8, R143. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, 255. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Poulsen, M.; Schwab, C.; Borg Jensen, B.; Engberg, R.M.; Spang, A.; Canibe, N.; Højberg, O.; Milinovich, G.; Fragner, L.; Schleper, C.; et al. Methylotrophic methanogenic Thermoplasmata implicated in reduced methane emissions from bovine rumen. Nat. Commun. 2013, 4, 1428. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Karnati, S.K.R.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Deng, L.; Chen, S.; Zhu, C.; Li, J. Effects of Pasture Grass, Silage, and Hay Diet on Equine Fecal Microbiota. Animals 2021, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Muhonen, S.; Sadet-Bourgeteau, S.; Julliand, V. Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces. Animals 2021, 11, 2337. [Google Scholar] [CrossRef]
- Nadeau, J.A.; Andrews, F.M.; Mathew, A.G.; Argenzio, R.A.; Blackford, J.T.; Sohtell, M.; Saxton, A.M. Evaluation of diet as a cause of gastric ulcers in horses. Am. J. Vet. Res. 2000, 61, 784–790. [Google Scholar] [CrossRef]
- Hydock, K.L.; Nissley, S.G.; Staniar, W.B. A standard protocol for fecal pH measurement in the horse. Prof. Anim. Sci. 2014, 30, 643–648. [Google Scholar] [CrossRef]
- Ermers, C.; McGilchrist, N.; Fenner, K.; Wilson, B.; McGreevy, P. The Fibre Requirements of Horses and the Consequences and Causes of Failure to Meet Them. Animals 2023, 13, 1414. [Google Scholar] [CrossRef]
- Luthersson, N.; Nadeau, J.A. Gastric ulceration. In Equine Applied and Clinical Nutrition; Geor, R.J., Harris, P.A., Coenen, M., Eds.; W. B. Saunders: London, UK, 2013; pp. 558–567. [Google Scholar]
- Sorensen, R.J.; Drouillard, J.S.; Douthit, T.L.; Ran, Q.; Marthaler, D.G.; Kang, Q.; Vahl, C.I.; Lattimer, J.M. Effect of hay type on cecal and fecal microbiome and fermentation parameters in horses. J. Anim. Sci. 2021, 99, skaa407. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.K.; Newbold, C.J.; Jones, E.; Worgan, H.J.; Grove-White, D.H.; Dugdale, A.H.; Barfoot, C.; Harris, P.A.; Argo, C.M. The Equine Gastrointestinal Microbiome: Impacts of Age and Obesity. Front. Microbiol. 2018, 9, 3017. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, S.E.; Weese, J.S.; Adams, A.A. Comparison of the Fecal Microbiota in Horses With Equine Metabolic Syndrome and Metabolically Normal Controls Fed a Similar All-Forage Diet. J. Equine Vet. Sci. 2016, 44, 9–16. [Google Scholar] [CrossRef]
- Raspa, F.; Chessa, S.; Bergero, D.; Sacchi, P.; Ferrocino, I.; Cocolin, L.; Corvaglia, M.R.; Moretti, R.; Cavallini, D.; Valle, E. Microbiota characterization throughout the digestive tract of horses fed a high-fiber vs. a high-starch diet. Front. Vet. Sci. 2024, 11, 1386135. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Johnson, P.J.; Lopes, M.A.; Perry, S.C.; Lanter, H.R. A Microbiological Map of the Healthy Equine Gastrointestinal Tract. PLoS ONE 2016, 11, e0166523. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef]
- Stothart, M.R.; McLoughlin, P.D.; Medill, S.A.; Greuel, R.J.; Wilson, A.J.; Poissant, J. Methanogenic patterns in the gut microbiome are associated with survival in a population of feral horses. Nat. Commun. 2024, 15, 6012. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef]
- Lindenberg, F.; Krych, L.; Fielden, J.; Kot, W.; Frøkiær, H.; van Galen, G.; Nielsen, D.S.; Hansen, A.K. Expression of immune regulatory genes correlate with the abundance of specific Clostridiales and Verrucomicrobia species in the equine ileum and cecum. Sci. Rep. 2019, 9, 12674. [Google Scholar] [CrossRef]
- Ayoub, C.; Arroyo, L.G.; Renaud, D.; Weese, J.S.; Gomez, D.E. Fecal Microbiota Comparison Between Healthy Teaching Horses and Client-Owned Horses. J. Equine Vet. Sci. 2022, 118, 104105. [Google Scholar] [CrossRef] [PubMed]
- Paßlack, N.; Vahjen, W.; Zentek, J. Impact of Dietary Cellobiose on the Fecal Microbiota of Horses. J. Equine Vet. Sci. 2020, 91, 103106. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Lansade, L.; Bars-Cortina, D.; Dhorne-Pollet, S.; Foury, A.; Moisan, M.P.; Ruet, A. Gut microbiota resilience in horse athletes following holidays out to pasture. Sci. Rep. 2021, 11, 5007. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Lantz, H.; Munyaka, P.M.; Willing, B.P. Prevotella in Pigs: The Positive and Negative Associations with Production and Health. Microorganisms 2020, 8, 1584. [Google Scholar] [CrossRef]
- Park, T.; Yoon, J.; Yun, Y.M.; Unno, T. Comparison of the fecal microbiota with high- and low performance race horses. J. Anim. Sci. Technol. 2024, 66, 425–437. [Google Scholar] [CrossRef]
- Willette, J.A.; Pitta, D.; Indugu, N.; Vecchiarelli, B.; Hennessy, M.L.; Dobbie, T.; Southwood, L.L. Experimental crossover study on the effects of withholding feed for 24 h on the equine faecal bacterial microbiota in healthy mares. BMC Vet. Res. 2021, 17, 3. [Google Scholar] [CrossRef]
- Wu, Y.; Yue, X.; Zhou, A.; Song, X.; Su, B.; Cao, F.; Ding, J. Simultaneous recovery of short-chain fatty acids and phosphorus during lipid-rich anaerobic fermentation with sodium hydroxide conditioning. Chemosphere 2023, 312, 137227. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Park, T.; Cheong, H.; Yoon, J.; Kim, A.; Yun, Y.; Unno, T. Comparison of the fecal microbiota of horses with intestinal disease and their healthy counterparts. Vet. Sci. 2021, 8, 113. [Google Scholar] [CrossRef]
- Cipriano-Salazar, M.; Adegbeye, M.J.; Elghandour, M.M.; Barbabosa-Pilego, A.; Mellado, M.; Hassan, A.; Salem, A.Z. The Dietary Components and Feeding Management as Options to Offset Digestive Disturbances in Horses. J. Equine Vet. Sci. 2019, 74, 103–110. [Google Scholar] [CrossRef]
- Steelman, S.M.; Chowdhary, B.P.; Dowd, S.; Suchodolski, J.; Janečka, J.E. Pyrosequencing of 16S rRNA genes in fecal samples reveals high diversity of hindgut microflora in horses and potential links to chronic laminitis. BMC Vet. Res. 2012, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Weinert-Nelson, J.R.; Biddle, A.S.; Williams, C.A. Fecal microbiome of horses transitioning between warm-season and cool-season grass pasture within integrated rotational grazing systems. Anim. Microbiome 2022, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Caruso, V.; Song, X.; Asquith, M.; Karstens, L. Performance of Microbiome Sequence Inference Methods in Environments with Varying Biomass. MSystems 2019, 4, 10.1128. [Google Scholar] [CrossRef] [PubMed]
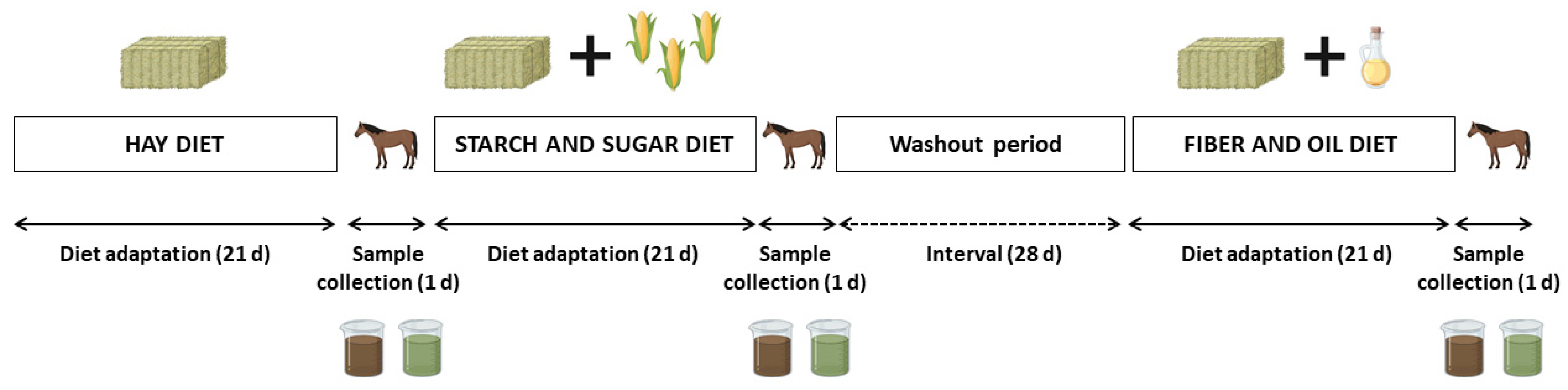
| Item | Hay Diet | SS Diet | FO Diet |
|---|---|---|---|
| Dry matter (DM) (% as-fed) | 93.6 | 92.3 | 93.2 |
| Ash (% DM) | 8.4 | 9.3 | 6.6 |
| Crude protein (% DM) | 12.9 | 13.9 | 13.7 |
| Ether extract (% DM) | 1.4 | 2.7 | 5.4 |
| Neutral detergent fiber (% DM) | 74.0 | 59.8 | 61.9 |
| Acid detergent fiber (% DM) | 39.8 | 31.1 | 34.2 |
| Crude fiber (% DM) | 36.9 | 28.4 | 31.5 |
| Hemicellulose (% DM) 1 | 34.2 | 28.7 | 27.7 |
| Starch (% DM) | 0.1 | 6.7 | 2.3 |
| Estimated digestible energy * (Mcal kg−1) | 1.6 | 1.9 | 2.2 |
| Crude energy (Mcal g−1) | 4.2 | 4.0 | 4.0 |
| Primer | 5′→3′ Sequence | Amplicon Size | References |
|---|---|---|---|
| 1114F (total bacteria) | CGGCAACGAGCGCAACCC | 130 bp | Denman and McSweeney [35] |
| 1275R (total bacteria) | CCATTGTAGCACGTGTGTAGCC | ||
| 958F (archaea) | AATTGGAKTCAACGCCGGR | 142 bp | Poulsen et al. [36] |
| 1100R (archaea) | TGGGTCTCGCTCGTTG | ||
| 316F (protozoa) | GCTTTCGWTGGTAGTGTATT | 223 bp | Sylvester et al. [37] |
| 539R (protozoa) | CTTGCCCTCYAATCGTWCT |
| HAY Diet | SS Diet 1 | FO Diet 2 | Diet (p-Value) | |
|---|---|---|---|---|
| SCFA cecum (mmol/L) * | ||||
| Acetate | 27.62 ± 2.69 | 28.56 ± 3.11 | 21.26 ± 3.81 | 0.4029 |
| Propionate | 9.82 ± 0.59 | 10.05 ± 0.68 | 7.37 ± 0.84 | 0.1605 |
| Butyrate | 2.69 ± 0.25 | 2.63 ± 0.28 | 1.65 ± 0.33 | 0.1059 |
| Isobutyrate | 0.19 ± 0.06 | 0.52 ± 0.07 | 0.31 ± 0.08 | 0.0576 |
| Valerate | 0.15 ± 0.03 | 0.26 ± 0.04 | 0.10 ± 0.05 | 0.1467 |
| Isovalerate | 0.15 ± 0.04 | 0.34 ± 0.04 | 0.15 ± 0.05 | 0.0805 |
| Total SCFA | 40.62 ± 3.53 | 42.36 ± 4.07 | 30.84 ± 4.99 | 0.3052 |
| (acetate/butyrate)/propionate | 3.05 ± 0.13 | 3.11 ± 0.15 | 3.10 ± 0.18 | 0.9459 |
| SCFA feces (mmol/L) * | ||||
| Acetate | 6.62 ± 0.89 | 7.88 ± 0.90 | 7.32 ± 0.97 | 0.4400 |
| Propionate | 2.75 ± 0.29 | 3.26 ± 0.29 | 3.14 ± 0.32 | 0.3822 |
| Butyrate | 0.56 ± 0.10 | 0.71 ± 0.11 | 0.63 ± 0.11 | 0.3411 |
| Isobutyrate | 0.21 ± 0.03 | 0.25 ± 0.03 | 0.22 ± 0.03 | 0.5022 |
| Valerate | ||||
| Isovalerate | 0.12 ± 0.05 | 0.25 ± 0.07 | 0.35 ± 0.13 | 0.3383 |
| Total SCFA | 10.26 ± 1.34 | 12.35 ± 1.34 | 11.65 ± 1.46 | 0.4141 |
| (acetate/butyrate)/propionate | 2.61 ± 0.11 | 2.57 ± 0.11 | 2.49 ± 0.12 | 0.6127 |
| HAY Diet | SS Diet 1 | FO Diet 2 | Diet (p-Value) | |
|---|---|---|---|---|
| pH | ||||
| Cecal | 7.18 ± 0.10 | 7.45 ± 0.11 | 7.60 ± 0.14 | 0.1454 |
| Fecal | 6.70 ± 0.10 a | 6.22 ± 0.10 b | 6.14 ± 0.11 b | 0.0052 |
| BC5 (mmol/L) | ||||
| Cecal | 68.04 ± 5.04 | 70.93 ± 5.49 | 66.89 ± 6.19 | 0.7513 |
| Fecal | 19.81 ± 1.80 b | 25.99 ± 1.80 a | 17.65 ± 1.97 b | 0.0150 |
| BC6 (mmol/L) | ||||
| Cecal | 29.37 ± 2.48 b | 36.94 ± 2.67 a | 41.87 ± 2.96 a | 0.0301 |
| Fecal | 6.93 ± 0.76 | 5.37 ± 0.76 | 3.66 ± 0.85 | 0.0660 |
| Index | Hay Diet | SS Diet 1 | FO Diet 2 | Diet (p-Value) | |||
|---|---|---|---|---|---|---|---|
| Mean | SE 3 | Mean | SE 3 | Mean | SE 3 | ||
| Cecal samples | |||||||
| Shannon index | 3.18 | 0.07 | 3.22 | 0.08 | 2.97 | 0.10 | 0.2700 |
| Chao1 | 159.03 | 2.28 | 161.28 | 2.63 | 158.52 | 3.22 | 0.7662 |
| Fecal samples | |||||||
| Shannon index | 3.40 b | 0.03 | 3.58 a | 0.03 | 3.48 ab | 0.04 | 0.0123 |
| Chao1 | 172.74 | 6.03 | 174.04 | 6.03 | 168.95 | 6.73 | 0.8350 |
| Organism | Hay Diet | SS Diet 1 | FO Diet 2 | |||
|---|---|---|---|---|---|---|
| Mean | SE 3 | Mean | SE 3 | Mean | SE 3 | |
| Cecal samples | ||||||
| Archaea | 2.02 | 0.07 | 1.95 | 0.07 | 1.80 | 0.08 |
| Protozoa | 2.11 | 0.07 | 2.08 | 0.07 | 2.03 | 0.08 |
| Fecal samples | ||||||
| Archaea | 2.26 | 0.09 | 2.04 | 0.11 | 2.08 | 0.13 |
| Protozoa | 1.91 | 0.09 | 1.94 | 0.11 | 1.64 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandi, L.A.; Nunes, A.T.; Faleiros, C.A.; Poleti, M.D.; Oliveira, E.C.d.M.; Schmidt, N.T.; Sousa, R.L.M.; Fukumasu, H.; Balieiro, J.C.C.; Brandi, R.A. Dietary Energy Sources Affect Cecal and Fecal Microbiota of Healthy Horses. Animals 2024, 14, 3494. https://doi.org/10.3390/ani14233494
Brandi LA, Nunes AT, Faleiros CA, Poleti MD, Oliveira ECdM, Schmidt NT, Sousa RLM, Fukumasu H, Balieiro JCC, Brandi RA. Dietary Energy Sources Affect Cecal and Fecal Microbiota of Healthy Horses. Animals. 2024; 14(23):3494. https://doi.org/10.3390/ani14233494
Chicago/Turabian StyleBrandi, Laura A., Alanne T. Nunes, Camila A. Faleiros, Mirele D. Poleti, Elisângela C. de M. Oliveira, Natalia T. Schmidt, Ricardo L. M. Sousa, Heidge Fukumasu, Julio C. C. Balieiro, and Roberta A. Brandi. 2024. "Dietary Energy Sources Affect Cecal and Fecal Microbiota of Healthy Horses" Animals 14, no. 23: 3494. https://doi.org/10.3390/ani14233494
APA StyleBrandi, L. A., Nunes, A. T., Faleiros, C. A., Poleti, M. D., Oliveira, E. C. d. M., Schmidt, N. T., Sousa, R. L. M., Fukumasu, H., Balieiro, J. C. C., & Brandi, R. A. (2024). Dietary Energy Sources Affect Cecal and Fecal Microbiota of Healthy Horses. Animals, 14(23), 3494. https://doi.org/10.3390/ani14233494





