Quantification of Antimicrobial Use on Commercial Broiler Farms in Pakistan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. AMU Quantification
2.2.1. TI Based on DDDvetPK
2.2.2. TI Based on DCDvetPK
2.2.3. TI Based on UDDvetPK
2.2.4. Milligram/Kilogram Biomass
2.3. Data Analysis
3. Results
3.1. Farm Characteristics
3.2. AMU at the Farm Level
3.3. AMU per Antimicrobial Class
3.4. AMU per Indication
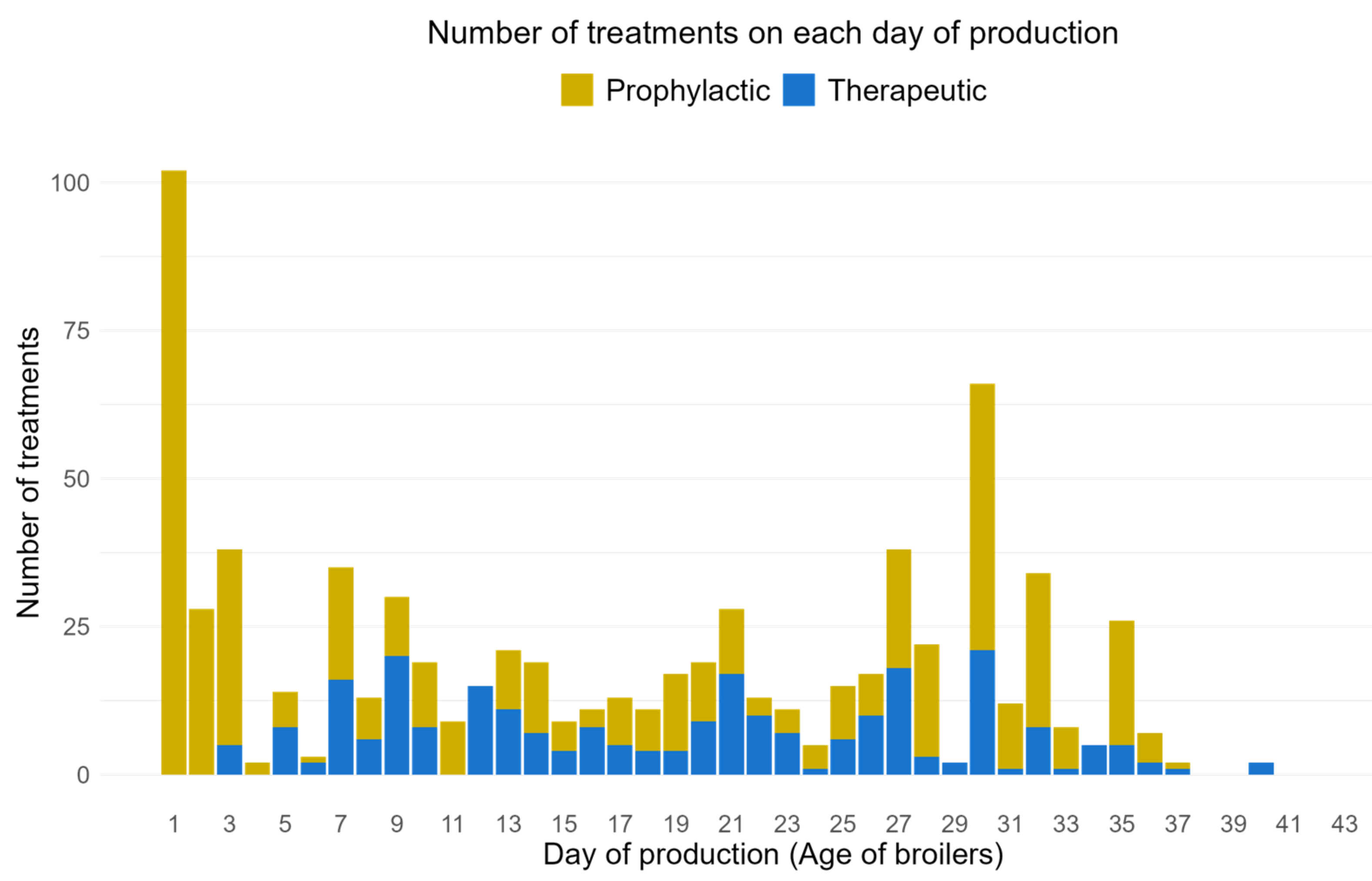
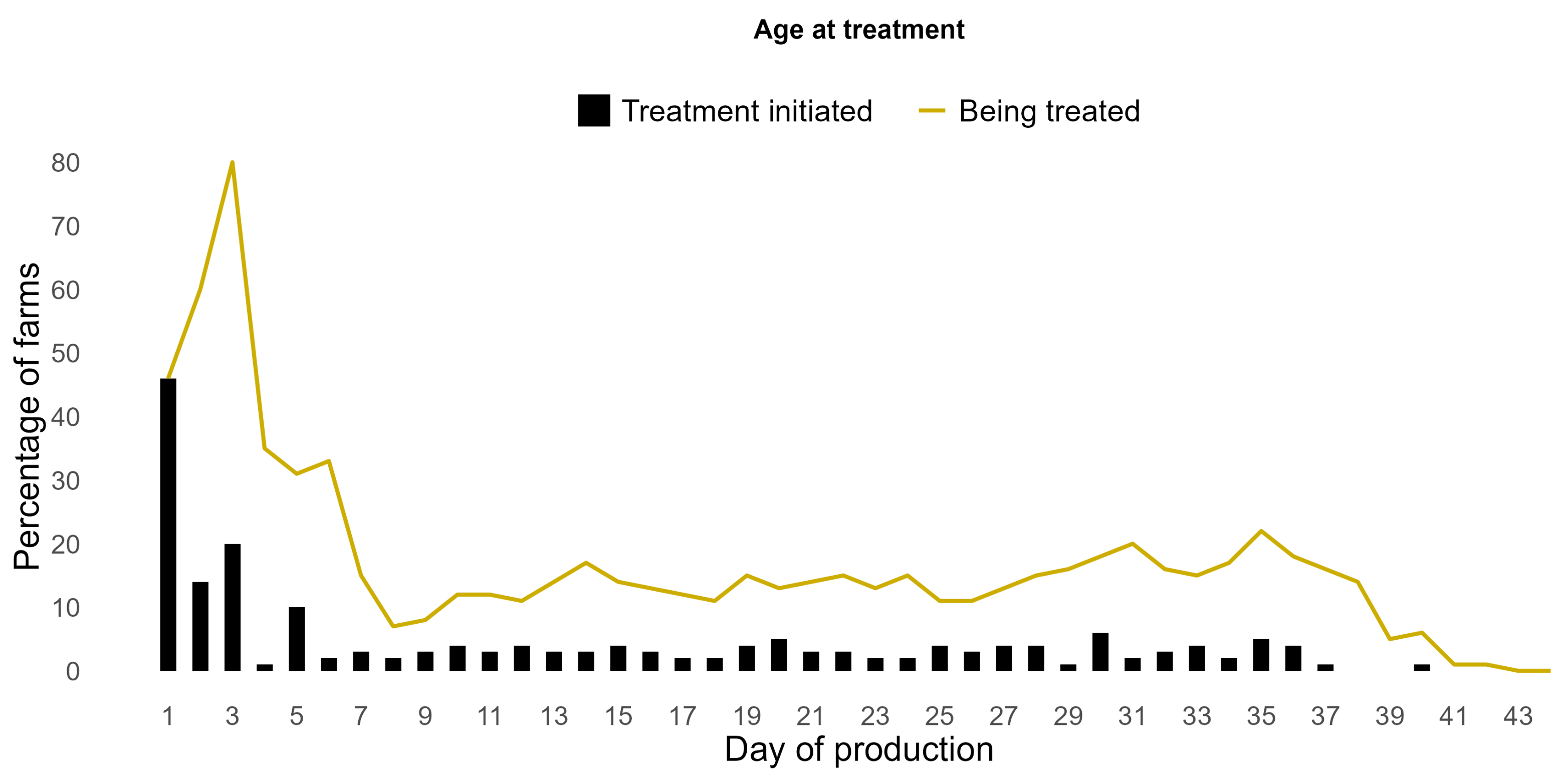
3.5. AMU per Production Cycle
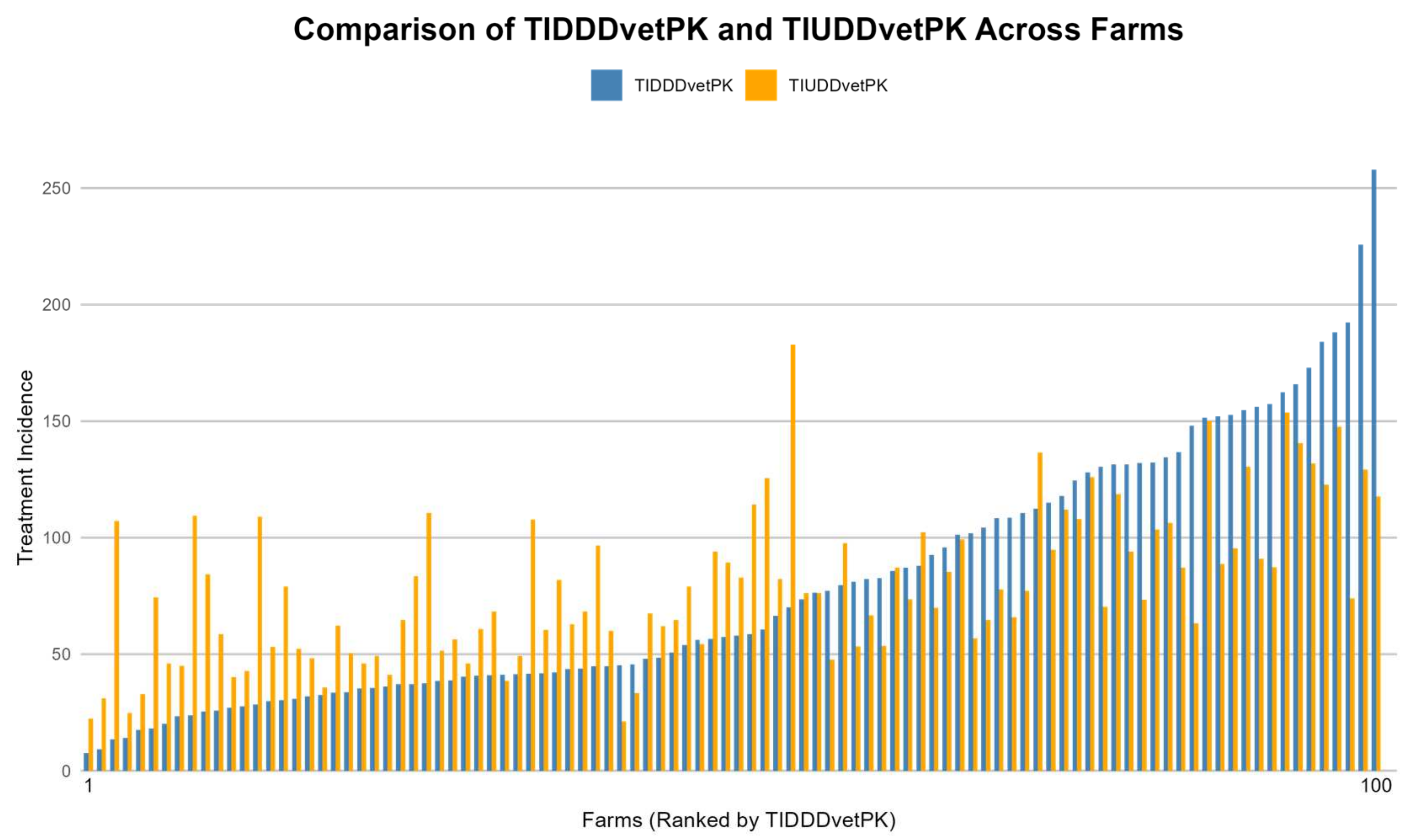
3.6. Comparison of UDDvetPK and DDDvetPK
3.7. Relationship Between AMU Metrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Iwu, C.D.; Patrick, S.M. An insight into the implementation of the global action plan on antimicrobial resistance in the WHO African region: A roadmap for action. Int. J. Antimicrob. Agents 2021, 58, 106411. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Int. J. Infect. Dis. 2020, 101, 19. [Google Scholar] [CrossRef]
- Qiu, Y.; Ferreira, J.P.; Ullah, R.W.; Flanagan, P.; Zaheer, M.U.; Tahir, M.F.; Alam, J.; Hoet, A.E.; Song, J.; Akram, M. Assessment of the Implementation of Pakistan’s National Action Plan on Antimicrobial Resistance in the Agriculture and Food Sectors. Antibiotics 2024, 13, 206. [Google Scholar] [CrossRef]
- Government of Pakistan. Pakistan Economic Survey 2023–24. 2024. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwj09M6XkIyKAxW7cfEDHT8SFN0QFnoECBYQAQ&url=https%3A%2F%2Fwww.finance.gov.pk%2Fsurvey_2024.html&usg=AOvVaw0jM2yONWacb7zmG7HcbwO_&opi=89978449 (accessed on 24 October 2024).
- Aslam, H.B.; Alarcon, P.; Yaqub, T.; Iqbal, M.; Häsler, B. A value chain approach to characterize the chicken sub-sector in Pakistan. Front. Vet. Sci. 2020, 7, 361. [Google Scholar] [CrossRef]
- Saleem, Z.; Sono, T.M.; Godman, B. Concerns with current Drug Laws regarding the purchasing antibiotics without a prescription in Pakistan; ways forward to assist the national action plan. Expert Rev. Anti Infect. Ther. 2023, 21, 1163–1165. [Google Scholar] [CrossRef]
- Alhouzani, T.; Saleem, M.; Sharma, A.; Khadka, S.; Gupta, R.P.; Aryal, P. Irrational Use of Antimicrobials in Poultry Farms and Antimicrobial Resistance: A Cross-Sectional Study. Eur. J. Med. Sci. 2021, 3, 10–14. [Google Scholar] [CrossRef]
- Umair, M.; Orubu, S.; Zaman, M.H.; Wirtz, V.J.; Mohsin, M. Veterinary consumption of highest priority critically important antimicrobials and various growth promoters based on import data in Pakistan. PLoS ONE 2022, 17, e0273821. [Google Scholar] [CrossRef]
- AACTING. Overview of Farm-Level AMU Monitiring Systems; Network on quantification, benchmarking and reporting of veterinary antimicrobial usage (AMU) at farm level. 2021. Available online: https://www.aacting.org/monitoring-systems/itoringsystems (accessed on 23 October 2024).
- EMA. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022; European Medicines Agency: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Schar, D.; Sommanustweechai, A.; Laxminarayan, R.; Tangcharoensathien, V. Surveillance of antimicrobial consumption in animal production sectors of low-and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018, 15, e1002521. [Google Scholar] [CrossRef]
- Collineau, L.; Belloc, C.; Stärk, K.D.; Hemonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health 2017, 64, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Bulut, E.; Nydam, D.V.; Ivanek, R. Standardization and evaluation of indicators for quantifying antimicrobial use on US dairy farms. Front. Antibiot. 2023, 2, 1176817. [Google Scholar] [CrossRef]
- Sanders, P.; Mevius, D.; Veldman, K.; van Geijlswijk, I.; Wagenaar, J.A.; Bonten, M.; Heederik, D. Comparison of different antimicrobial use indicators and antimicrobial resistance data in food-producing animals. JAC Antimicrob. Resist. 2021, 3, dlab172. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.; Sarrazin, S.; Van Gompel, L.; Luiken, R.E.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.; Dewulf, J. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J. Antimicrob. Chemother. 2019, 74, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.; van Geijlswijk, I.; Bonten, M.; Mughini-Gras, L.; van Hout, J.; Heederik, D. Comparing human and animal antimicrobial usage: A critical appraisal of the indicators used is needed. JAC Antimicrob. Resist. 2024, 6, dlae005. [Google Scholar] [CrossRef]
- WHOCC. ATCvet Index; WHO Collaborating Centre for Drug Statistics Methodology—WHOCC: Geneva, Switzerland, 2024. [Google Scholar]
- WHO. WHO List of Medically Important Antimicrobials; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D. Restriction in the use of antibiotics in food animals and antibiotic resistance in food animals and humans–a systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Anwar Sani, R.; Wagenaar, J.A.; Dinar, T.E.; Sunandar, S.; Nurbiyanti, N.; Suandy, I.; Pertela, G.; Jahja, E.J.; Purwanto, B.; Geijlswijk, I.M.v. The comparison and use of tools for quantification of antimicrobial use in Indonesian broiler farms. Front. Vet. Sci. 2023, 10, 1092302. [Google Scholar] [CrossRef]
- Mahmood, Q.; Chantziaras, I.; Yasir, A.; Dewulf, J. Establishing defined daily and course doses for antimicrobials used in Pakistani broilers to enable farm-level quantification and comparison of antimicrobial use. Prev. Vet. Med. 2024, 233, 106348. [Google Scholar] [CrossRef]
- EMA. Principles on Assignment of Defined Daily Dose for Animals (DDDvet) and Defined Course Dose for Animals (DCDvet); European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Mohsin, M.; Umair, M. Trends in antimicrobial use in livestock animals in Pakistan. Int. J. Infect. Dis. 2020, 101, 17–18. [Google Scholar] [CrossRef]
- Mohsin, M.; Van Boeckel, T.P.; Saleemi, M.K.; Umair, M.; Naseem, M.N.; He, C.; Khan, A.; Laxminarayan, R. Excessive use of medically important antimicrobials in food animals in Pakistan: A five-year surveillance survey. Glob. Health Action 2019, 12, 1697541. [Google Scholar] [CrossRef]
- Umair, M.; Tahir, M.F.; Ullah, R.W.; Ali, J.; Siddique, N.; Rasheed, A.; Akram, M.; Zaheer, M.U.; Mohsin, M. Quantification and trends of antimicrobial use in commercial broiler chicken production in Pakistan. Antibiotics 2021, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Borck Høg, B.; Dalhoff Andersen, V.; Chauvin, C. Monitoring of farm-level antimicrobial use to guide stewardship: Overview of existing systems and analysis of key components and processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Chantziaras, I.; Mohsin, M.A.S.; Boyen, F.; Fournié, G.; Islam, S.S.; Berge, A.C.; Caekebeke, N.; Joosten, P.; Dewulf, J. Quantitative and qualitative analysis of antimicrobial usage and biosecurity on broiler and Sonali farms in Bangladesh. Prev. Vet. Med. 2023, 217, 105968. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.e.; Khan, A.; Mmbaga, E.J.; Green, I.R.; Asaduzzaman, M. Use of antibiotics in poultry and poultry farmers-a cross-sectional survey in Pakistan. Front. Public Health 2023, 11, 1154668. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Chaudhry, M.I.; Katchova, A.; Miranda, M.J. Examining pricing mechanics in the poultry value chain-empirical evidence from Pakistan. Res. Agric. Appl. Econ. 2016. [Google Scholar] [CrossRef]
- Mallioris, P.; Teunis, G.; Lagerweij, G.; Joosten, P.; Dewulf, J.; Wagenaar, J.A.; Stegeman, A.; Mughini-Gras, L. Biosecurity and antimicrobial use in broiler farms across nine European countries: Toward identifying farm-specific options for reducing antimicrobial usage. Epidemiol. Infect. 2023, 151, e13. [Google Scholar] [CrossRef]
- Boamah, V.E.; Agyare, C.; Odoi, H.; Dalsgaard, A. Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J. Antimicrob. Agents 2016, 2, 1000120. [Google Scholar] [CrossRef]
- Hassan, M.M.; Kalam, M.A.; Alim, M.A.; Shano, S.; Nayem, M.R.K.; Badsha, M.R.; Al Mamun, M.A.; Hoque, A.; Tanzin, A.Z.; Nath, C. Knowledge, attitude, and practices on antimicrobial use and antimicrobial resistance among commercial poultry farmers in Bangladesh. Antibiotics 2021, 10, 784. [Google Scholar] [CrossRef]
- Jacobs, L.; Delezie, E.; Duchateau, L.; Goethals, K.; Ampe, B.; Lambrecht, E.; Gellynck, X.; Tuyttens, F.A.M. Effect of post-hatch transportation duration and parental age on broiler chicken quality, welfare, and productivity. Poult. Sci. 2016, 95, 1973–1979. [Google Scholar] [CrossRef]
- Shynkaruk, T.; Long, K.; LeBlanc, C.; Schwean-Lardner, K. Impact of stocking density on the welfare and productivity of broiler chickens reared to 34 d of age. J. Appl. Poult. Res. 2023, 32, 100344. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Nahed, A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F. Necrotic enteritis in broiler chickens: Disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef] [PubMed]
- Sadeeq-ur-Rahman, S.-U.-R.; Mashkoor Mohsin, M.M. The under reported issue of antibiotic-resistance in food-producing animals in Pakistan. Pak. Vet. J. 2019, 39, 323–328. [Google Scholar] [CrossRef]
- The Government of Punjab, P. The Punjab Poultry Production Act 2016 (Act XLVII of 2016). 2016, No.PAP/Legis·2(129)/2016/1494. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiNq6HtkYyKAxXAVfEDHSHYCq4QFnoECCIQAQ&url=https%3A%2F%2Fwww.fao.org%2Ffaolex%2Fresults%2Fdetails%2Fen%2Fc%2FLEX-FAOC161819%2F&usg=AOvVaw2mi-JRjYld5JS3L4zWJiKu&opi=89978449 (accessed on 23 October 2021).



| Term | Definition |
|---|---|
| Therapeutic antimicrobial use | Administration of antimicrobials to treat animals with clinical evidence of a disease; e.g., amoxicillin, ampicillin, enrofloxacin. |
| Prophylaxis | Administration of antimicrobials to animals without evidence of clinical signs of a disease. The animals might be at high risk of infectious disease but there is no known disease in the flock (for example, conditions due to environmental change, crowded space, and transport of animals); e.g., bacitracin, benzylpenicillin, virginiamycin. |
| Antimicrobial growth promoters (AGPs) | Administration of sub-therapeutic doses of antimicrobials through feed to stimulate growth and weight gain in animals or to increase feed efficiency; e.g., enramycin, avilamycin, lincomycin. |
| Antimicrobial Class (Active Substance) | Medical Importance | Percentage |
|---|---|---|
| Polymyxins (Colistin) | HPCIA | 17% |
| Aminoglycosides (Neomycin, Streptomycin, Gentamicin, Apramycin, Dihydrostreptomycin) | CIA | 15% |
| Quinolones (Enrofloxacin, Norfloxacin, Pefloxacine, Flumequine, Ofloxacin) | HPCIA | 12% |
| Macrolides (Tylosin, Tilmicosin, Spiramycin, Erythromycin) | CIA | 12% |
| Tetracyclines (Doxycycline, Chlortetracycline, Oxytetracycline) | HIA | 9% |
| Aminopenicillins (Amoxicillin, Ampicillin) | HIA | 7% |
| NS Penicillins (Procaine Penicillin) | HIA | 6% |
| Amphenicols (Florfenicol, Chloramphenicol) | HIA | 6% |
| Lincosamides (Lincomycin) | HIA | 5% |
| Polypeptides (Bacitracin, Enramycin) | IA | 5% |
| Sulfonamides (Sulfadiazine, Sulfamethoxypyridazine, Sulfadimerazine, Sulfamethoxine, Sulfachlorpyridazine, Sulfadiamidine, Sulfamethoxazole) | HIA | 4% |
| Nitrofurans derivatives (Furaltadone) | IA | 2% |
| Streptogramins (Virginiamycin) | HIA | 1% |
| Phosphonic acid derivatives (Fosfomycin) | HPCIA | 1% |
| Category | Indication | Treatment (%, n = 252) | Prophylaxis (%, n = 489) | Total (%, n = 741) |
|---|---|---|---|---|
| Enteric Diseases | Feed supplement for necrotic enteritis | 0 | 21.9 | 14.4 |
| Enteritis | 4.4 | 4.7 | 4.6 | |
| Necrotic enteritis | 0.8 | 0.8 | 0.8 | |
| Diarrhea | 2 | 0.6 | 1.1 | |
| Salmonellosis | 6 | 1.2 | 7.2 | |
| Shigellosis | 0.8 | 0 | 0.3 | |
| Respiratory Diseases | Chronic respiratory disease (CRD) | 24.2 | 9.2 | 14.3 |
| Feed supplement for CRD | 0 | 10.2 | 6.7 | |
| Respiratory distress | 5.2 | 3.7 | 4.2 | |
| Infectious coryza | 5.6 | 1.4 | 2.8 | |
| Infectious sinusitis | 7.1 | 0 | 2.4 | |
| Complex chronic respiratory disease | 1.6 | 2.9 | 2.4 | |
| Avian influenza | 0 | 2.9 | 1.9 | |
| Airsacculitis | 1.6 | 1.6 | 1.6 | |
| Pneumonia | 0.4 | 0.6 | 0.5 | |
| Avian coryza | 0 | 0.4 | 0.3 | |
| Systematic Diseases | Colibacillosis | 21 | 7.8 | 12.3 |
| Fowl cholera | 4 | 2.5 | 3 | |
| Acute fatal septicemia | 0.4 | 1.2 | 0.9 | |
| Colisepticemia | 1.6 | 0.4 | 0.8 | |
| Septicemia | 1.6 | 0.4 | 0.8 | |
| Pericarditis | 1.2 | 0.4 | 0.7 | |
| Peritonitis | 0.4 | 0.6 | 0.5 | |
| General Diseases/Conditions | Early chick mortality | 0 | 18.4 | 12.1 |
| Omphalitis | 1.2 | 0.4 | 1.6 | |
| Cellulitius | 0 | 0.2 | 0.1 | |
| Infectious sinuvitis | 1.6 | 0.6 | 0.9 | |
| Locomotive Diseases | Arthritis | 1.6 | 0.4 | 0.8 |
| Bumblefoot | 0.4 | 0 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, Q.; Chantziaras, I.; Dewulf, J. Quantification of Antimicrobial Use on Commercial Broiler Farms in Pakistan. Animals 2024, 14, 3510. https://doi.org/10.3390/ani14233510
Mahmood Q, Chantziaras I, Dewulf J. Quantification of Antimicrobial Use on Commercial Broiler Farms in Pakistan. Animals. 2024; 14(23):3510. https://doi.org/10.3390/ani14233510
Chicago/Turabian StyleMahmood, Qamer, Ilias Chantziaras, and Jeroen Dewulf. 2024. "Quantification of Antimicrobial Use on Commercial Broiler Farms in Pakistan" Animals 14, no. 23: 3510. https://doi.org/10.3390/ani14233510
APA StyleMahmood, Q., Chantziaras, I., & Dewulf, J. (2024). Quantification of Antimicrobial Use on Commercial Broiler Farms in Pakistan. Animals, 14(23), 3510. https://doi.org/10.3390/ani14233510







