The Combined Use of Triamcinolone and Platelet-Rich Plasma in Equine Metacarpophalangeal Joint Osteoarthritis Treatments: An In Vivo and In Vitro Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Primary Cultures of Equine Chondrocytes
2.1.2. PRP Preparation
2.1.3. Cell Viability Analysis
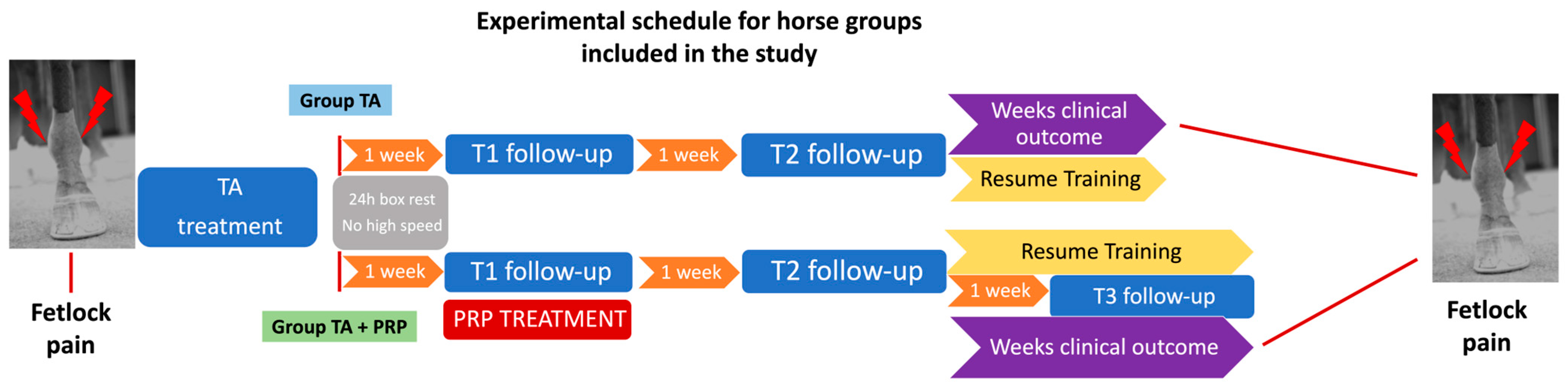
2.2. In Vivo Study
2.2.1. Cases Selection
2.2.2. Treatments
2.3. Statistical Analyses
3. Results
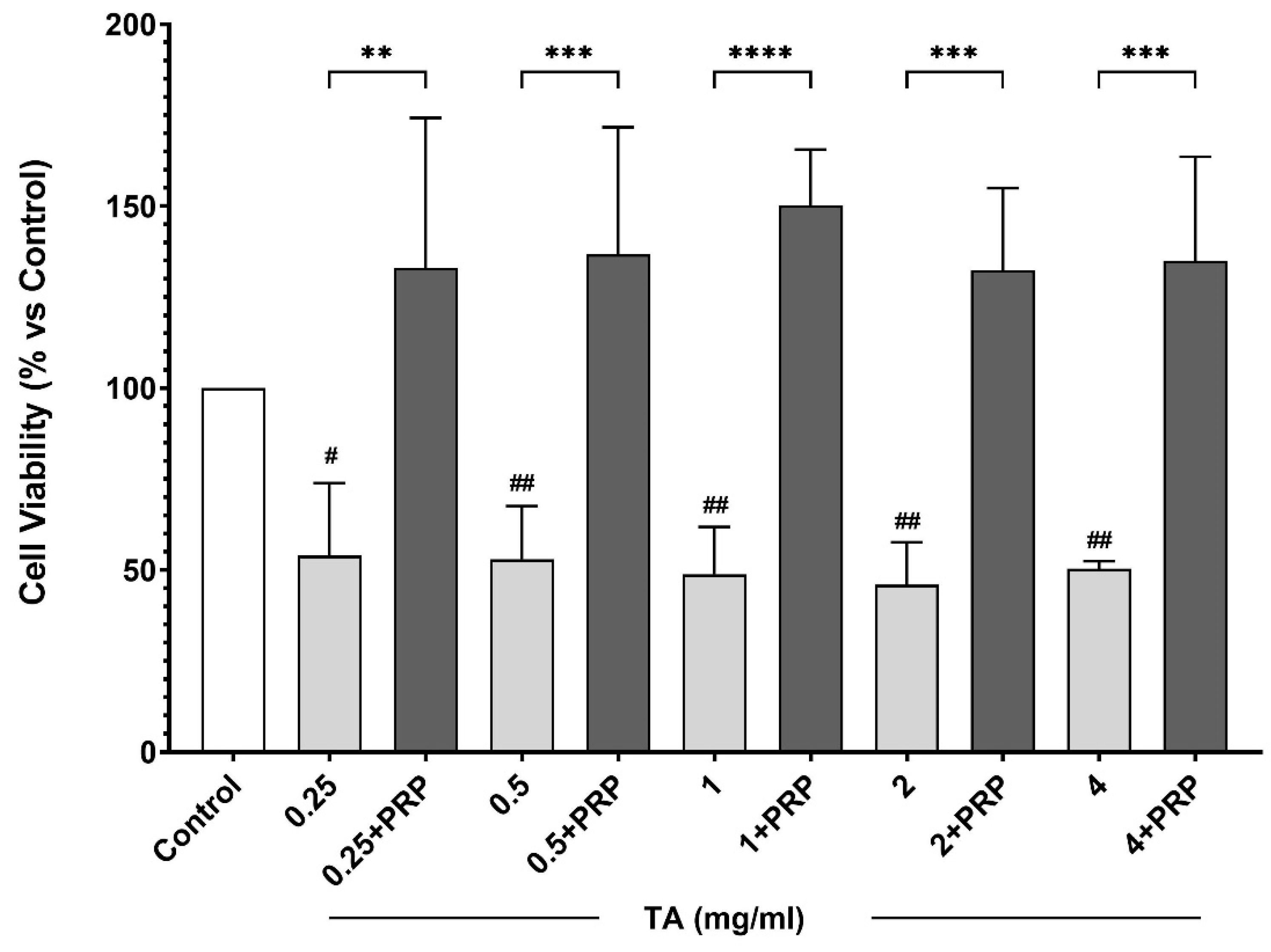
3.1. In Vitro Study
Effect of PRP on Chondrocyte Culture
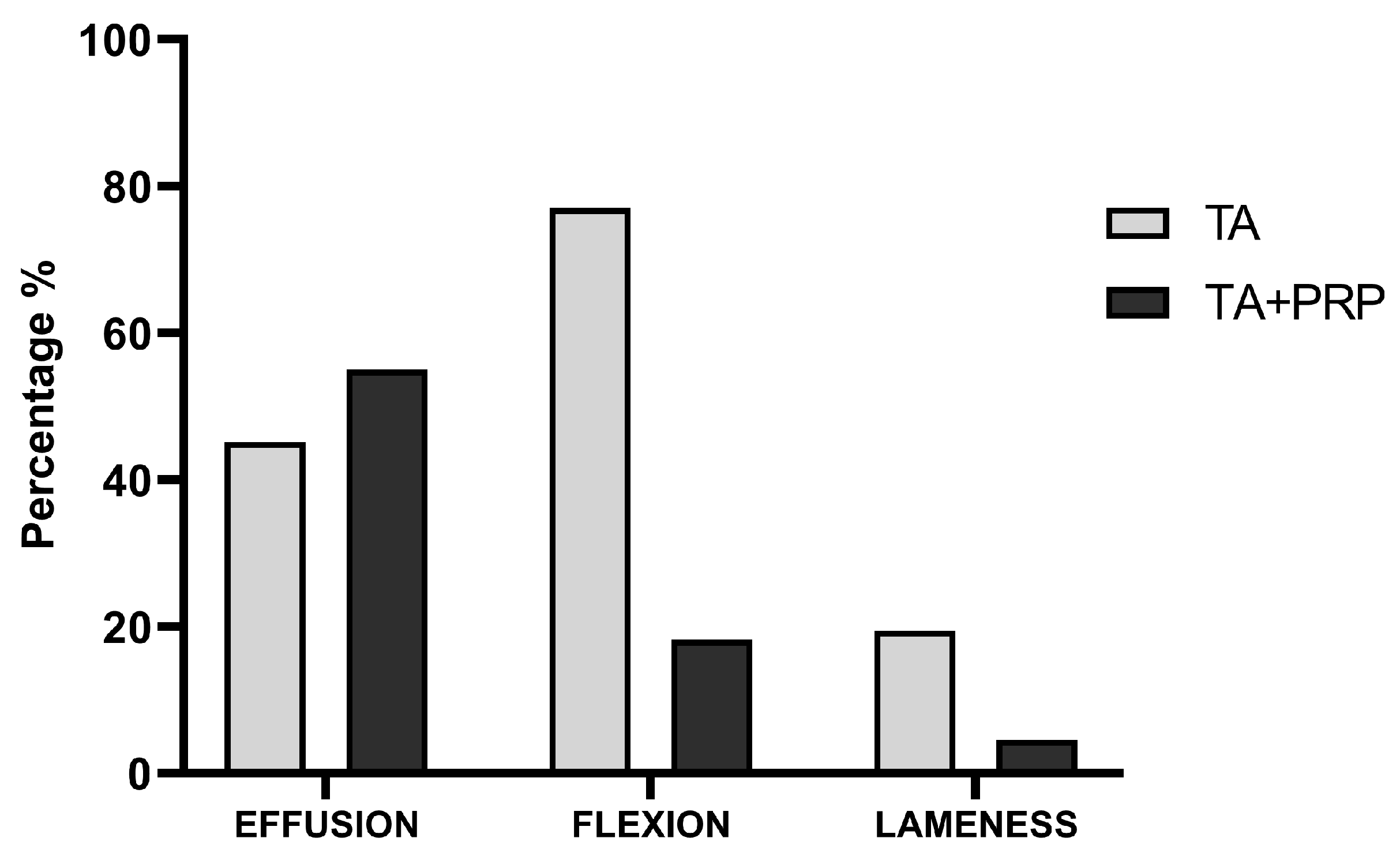
3.2. In Vivo Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawcak, C.E.; McIlwraith, C.W.; Norrdin, R.W.; Park, R.D.; Steyn, P.S. Clinical Effects of Exercise on Subchondral Bone of Carpal and Metacarpophalangeal Joints in Horses. Am. J. Vet. Res. 2000, 61, 1252–1258. [Google Scholar] [CrossRef]
- Neundorf, R.H.; Lowerison, M.B.; Cruz, A.M.; Thomason, J.J.; McEwen, B.J.; Hurtig, M.B. Determination of the Prevalence and Severity of Metacarpophalangeal Joint Osteoarthritis in Thoroughbred Racehorses via Quantitative Macroscopic Evaluation. Am. J. Vet. Res. 2010, 71, 1284–1293. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Nixon, A.J. Medical Treatment of Osteoarthritis in the Horse—A Review. Vet. J. 2006, 171, 51–69. [Google Scholar] [CrossRef] [PubMed]
- de Grauw, J.C.; Visser-Meijer, M.C.; Lashley, F.; Meeus, P.; van Weeren, P.R. Intra-Articular Treatment with Triamcinolone Compared with Triamcinolone with Hyaluronate: A Randomised Open-Label Multicentre Clinical Trial in 80 Lame Horses. Equine Vet. J. 2016, 48, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.T.; Bolt, D.M.; Ishihara, A.; Rajala-Schultz, P.J.; Bertone, A.L. Anti-Inflammatory and Analgesic Effects of Intra-Articular Injection of Triamcinolone Acetonide, Mepivacaine Hydrochloride, or Both on Lipopolysaccharide-Induced Lameness in Horses. Am. J. Vet. Res. 2008, 69, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Danial, C.M.; Braun, H.J.; Pouliot, M.A.; Kim, H.J. The Chondrotoxicity of Single-Dose Corticosteroids. Knee Surgery, Sports Traumatology. Arthroscopy 2012, 20, 1809–1814. [Google Scholar] [CrossRef]
- Van Pelt, R.W.; Riley, W.F. Tarsal Hydrarthrosis in the Horse: Response to Intra-Articular Injection of Synthetic Steroids. Can. Vet. J. 1969, 10, 130–135. [Google Scholar] [PubMed]
- Frisbie, D.D.; Kawcak, C.E.; Trotter, G.W.; Powers, B.E.; Walton, R.M.; McIlwraith, C.W. Effects of Triamcinolone Acetonide on an in Vivo Equine Osteochondral Fragment Exercise Model. Equine Vet. J. 1997, 29, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of Intra-Articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop. J. Sports Med. 2015, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, A.M.; Kosel, K. Effects of Intra-Articularly Administered Corticosteroids and Salicylates on the Surface Structure of Articular Cartilage. J. Anat. 1978, 127, 393–402. [Google Scholar]
- McIlwraith, C.W. The Use of Intra-Articular Corticosteroids in the Horse: What Is Known on a Scientific Basis? Equine Vet. J. 2010, 42, 563–571. [Google Scholar] [CrossRef]
- Dechant, J.E.; Baxter, G.M.; Frisbie, D.D.; Trotter, G.W.; McIlwraith, C.W. Effects of Dosage Titration of Methylprednisolone Acetate and Triamcinolone Acetonide on Interleukin-1-Conditioned Equine Articular Cartilage Explants in Vitro. Equine Vet. J. 2003, 35, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.A.; Tablin, F. Intra-Articular Use of a Platelet-Rich Product in Normal Horses: Clinical Signs and Cytologic Responses. Vet. Surg. 2013, 42, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Dório, M.; Pereira, R.M.R.; Luz, A.G.B.; Deveza, L.A.; de Oliveira, R.M.; Fuller, R. Efficacy of Platelet-Rich Plasma and Plasma for Symptomatic Treatment of Knee Osteoarthritis: A Double-Blinded Placebo-Controlled Randomized Clinical Trial. BMC Musculoskelet. Disord. 2021, 22, 822. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Roffi, A.; Di Matteo, B.; Merli, M.L.; Marcacci, M. Platelet-Rich Plasma: Why Intra-Articular? A Systematic Review of Preclinical Studies and Clinical Evidence on PRP for Joint Degeneration. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2459–2474. [Google Scholar] [CrossRef]
- Hur, C.I.; Park, C.; Li, H.; Seon, J.K.; Kim, H.K.; Yoon, T.R.; Song, E.-K. Effect of Autologus Platelet-Rich Plasma on IL-6, MMP-3 and MCP-1 Expression in Synoviocytes from Osteoarthritic Patients Knees. Open J. Regen. Med. 2014, 3, 64–72. [Google Scholar] [CrossRef]
- Bonilla-Gutiérrez, A.F.; López, C.; Carmona, J.U. Regenerative Therapies for the Treatment of Tenodesmic Injuries in Horses. J. Equine Vet. Sci. 2019, 73, 139–147. [Google Scholar] [CrossRef]
- Muto, T.; Kokubu, T.; Mifune, Y.; Sakata, R.; Nagura, I.; Nishimoto, H.; Harada, Y.; Nishida, K.; Kuroda, R.; Kurosaka, M. Platelet-Rich Plasma Protects Rotator Cuff-Derived Cells from the Deleterious Effects of Triamcinolone Acetonide. J. Orthop. Res. 2013, 31, 976–982. [Google Scholar] [CrossRef]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Apostolakos, J.; Russell, R.P.; Bradley, J.; ElAttrache, N.S.; Romeo, A.A.; Arciero, R.A.; Mazzocca, A.D. The Effect of Ketorolac Tromethamine, Methylprednisolone, and Platelet-Rich Plasma on Human Chondrocyte and Tenocyte Viability. Arthrosc.-J. Arthrosc. Relat. Surg. 2013, 29, 1164–1174. [Google Scholar] [CrossRef]
- Baboldashti, N.Z.; Poulsen, R.C.; Franklin, S.L.; Thompson, M.S.; Hulley, P.A. Platelet-Rich Plasma Protects Tenocytes From Adverse Side Effects of Dexamethasone and Ciprofloxacin. Am. J. Sports Med. 2011, 39, 1929–1935. [Google Scholar] [CrossRef]
- Durant, T.J.S.; Dwyer, C.R.; McCarthy, M.B.R.; Cote, M.P.; Bradley, J.P.; Mazzocca, A.D. Protective Nature of Platelet-Rich Plasma Against Chondrocyte Death When Combined with Corticosteroids or Local Anesthetics. Am. J. Sports Med. 2017, 45, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.N.; Tang, Y.Y.N.; Lee, S.K.M.; Fu, B.S.C.; Chan, B.P.; Chan, C.K.M. Effect of Dexamethasone on Cultured Human Tenocytes and Its Reversibility by Platelet-Derived Growth Factor. J. Bone Jt. Surg. Am. 2003, 85, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of Platelet-Rich Plasma and Its Clinical Application in Cartilage Repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Palma, H.E.; Gallio, M.; da Silva, G.B.; Cantarelli, C.; Bertolin, K.; Wolkmer, P.; Wergutz, J.; Krause, L.M.F.; Krause, A.; Antoniazzi, A.Q.; et al. Comparison of the Effects of Triamcinolone Acetonide or Platelet-Rich Plasma on Expression of Extracellular Matrix-Related Genes in Equine Healthy Chondrocytes in Vitro. Ciência Rural. 2019, 49. [Google Scholar] [CrossRef]
- Mancini, F.; Nannarone, S.; Buratta, S.; Ferrara, G.; Stabile, A.M.; Vuerich, M.; Santinelli, I.; Pistilli, A.; Chiaradia, E. Effects of Xylazine and Dexmedetomidine on Equine Articular Chondrocytes in Vitro. Vet. Anaesth. Analg. 2017, 44, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Tognoloni, A.; Bartolini, D.; Pepe, M.; Di Meo, A.; Porcellato, I.; Guidoni, K.; Galli, F.; Chiaradia, E. Platelets Rich Plasma Increases Antioxidant Defenses of Tenocytes via Nrf2 Signal Pathway. Int. J. Mol. Sci. 2023, 24, 13299. [Google Scholar] [CrossRef]
- AAEP Horse Show Committee. Guide to Veterinary Services for Horse Shows, 7th ed.; American Association of Equine Practitioners: Lexington, KY, USA, 1999. [Google Scholar]
- Santschi, E.M. How to Interpret Radiographs of the Fetlock and Pastern Joint of the Young Performance Horse. In Proceedings of the AAEP Annual Convention, Nashville, TN, USA, 7–11 December 2013; Volume 59, pp. 395–401. [Google Scholar]
- Sherman, S.L.; Khazai, R.S.; James, C.H.; Stoker, A.M.; Flood, D.L.; Cook, J.L. In Vitro Toxicity of Local Anesthetics and Corticosteroids on Chondrocyte and Synoviocyte Viability and Metabolism. Cartilage 2015, 6, 233–240. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Li, Z.; Zhang, F.; Chen, L. Glucocorticoid Caused Lactic Acid Accumulation and Damage in Human Chondrocytes via ROS-Mediated Inhibition of Monocarboxylate Transporter 4. Bone 2022, 155, 116299. [Google Scholar] [CrossRef] [PubMed]
- Farkas, B.; Kvell, K.; Czömpöly, T.; Illés, T.; Bárdos, T. Increased Chondrocyte Death after Steroid and Local Anesthetic Combination. Clin. Orthop. Relat. Res. 2010, 468, 3112–3120. [Google Scholar] [CrossRef]
- Tu, Y.; Xue, H.; Francis, W.; Davies, A.P.; Pallister, I.; Kanamarlapudi, V.; Xia, Z. Lactoferrin Inhibits Dexamethasone-Induced Chondrocyte Impairment from Osteoarthritic Cartilage through up-Regulation of Extracellular Signal-Regulated Kinase 1/2 and Suppression of FASL, FAS, and Caspase 3. Biochem. Biophys. Res. Commun. 2013, 441, 249–255. [Google Scholar] [CrossRef]
- Sharma, V.; Sakhalkar, U.; Nadkarni, P.; Mishal, R.; Parandhaman, D.; Vichare, K.; Francis, A.; Khanna, M.; Kukreja, M.; Sharma, A. Cytoprotective Effect of Growth Factors Derived From Platelets on Corticosteroid-Treated Primary Anterior Cruciate Ligament-Derived Stromal Cells and Chondrocytes. Cureus 2024, 16, e65566. [Google Scholar] [CrossRef]
- Shapiro, P.S.; Rohde, R.S.; Froimson, M.I.; Lash, R.H.; Postak, P.; Greenwald, A.S. The Effect of Local Corticosteroid or Ketorolac Exposure on Histologic and Biomechanical Properties of Rabbit Tendon and Cartilage. HAND 2007, 2, 165–172. [Google Scholar] [CrossRef]
- Albano, M.B.; Skroch, G.P.; Ioshii, S.O.; Grahels, X.S.; de Alencar, P.G.C.; Matias, J.E.F. Computerized Photocolorimetric Analysis of the Effects of Intraarticular Betamethasone on the Proteoglycan Concentration of Leporine Knee Cartilage Matrix: Influence of the Number of Intraarticular Injections. Rev. Col. Bras. Cir. 2009, 36, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Euppayo, T.; Siengdee, P.; Buddhachat, K.; Pradit, W.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. In Vitro Effects of Triamcinolone Acetonide and in Combination with Hyaluronan on Canine Normal and Spontaneous Osteoarthritis Articular Cartilage. Vitr. Cell Dev. Biol. Anim. 2016, 52, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Tognoloni, A.; Pellegrini, M.; Di Salvo, A.; Sforna, M.; Cagiola, M.; Seccaroni, M.; Nannarone, S.; Beccati, F.; Pressanto, M.C.; Di Meo, A.; et al. Cytotoxicity of Local Anaesthetics and Protective Effects of Platelet Rich Plasma on Equine Tenocytes: An in Vitro Study. Vet. J. 2024, 306, 106159. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, A.; Li, Q.; Wu, J. Platelet-Rich Plasma Attenuates Interleukin-1β-Induced Apoptosis and Inflammation in Chondrocytes through Targeting Hypoxia-Inducible Factor-2α. Tissue Cell 2021, 73, 101646. [Google Scholar] [CrossRef] [PubMed]
- Gilbertie, J.M.; Long, J.M.; Schubert, A.G.; Berglund, A.K.; Schaer, T.P.; Schnabel, L.V. Pooled Platelet-Rich Plasma Lysate Therapy Increases Synoviocyte Proliferation and Hyaluronic Acid Production While Protecting Chondrocytes from Synoviocyte-Derived Inflammatory Mediators. Front. Vet. Sci. 2018, 5, 00150. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.-S.; Wu, C.-M.; Dubey, N.K.; Lo, W.-C.; Tsai, F.-C.; Tung, T.D.X.; Hung, W.-C.; Hsu, W.-C.; Chen, W.-H.; Deng, W.-P. Mechanistic Insight into Hyaluronic Acid and Platelet-Rich Plasma-Mediated Anti-Inflammatory and Anti-Apoptotic Activities in Osteoarthritic Mice. Aging 2018, 10, 4152–4165. [Google Scholar] [CrossRef]
- Muto, T.; Kokubu, T.; Mifune, Y.; Inui, A.; Sakata, R.; Harada, Y.; Takase, F.; Kurosaka, M. Effects of Platelet-Rich Plasma and Triamcinolone Acetonide on Interleukin-1ß-Stimulated Human Rotator Cuff-Derived Cells. Bone Jt. Res. 2016, 5, 602–609. [Google Scholar] [CrossRef]
- Suntiparpluacha, M.; Tammachote, N.; Tammachote, R. Triamcinolone Acetonide Reduces Viability, Induces Oxidative Stress, and Alters Gene Expressions of Human Chondrocytes. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4985–4992. [Google Scholar] [PubMed]
- Tohidnezhad, M.; Varoga, D.; Wruck, C.J.; Brandenburg, L.O.; Seekamp, A.; Shakibaei, M.; Sönmez, T.T.; Pufe, T.; Lippross, S. Platelet-Released Growth Factors Can Accelerate Tenocyte Proliferation and Activate the Anti-Oxidant Response Element. Histochem. Cell Biol. 2011, 135, 453–460. [Google Scholar] [CrossRef]
- Peng, C.; Yang, L.; Labens, R.; Gao, Y.; Zhu, Y.; Li, J. A Systematic Review and Meta-Analysis of the Efficacy of Platelet-Rich Plasma Products for Treatment of Equine Joint Disease. Equine Vet. J. 2024, 56, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, S.Y.; Yoon, K.S.; Shin, S. Effects of Platelet-Rich Plasma With Concomitant Use of a Corticosteroid on Tenocytes From Degenerative Rotator Cuff Tears in Interleukin 1β-Induced Tendinopathic Conditions. Am. J. Sports Med. 2017, 45, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yao, X.; Shan, N.; Cai, Y.; Fan, Y. Platelet-Rich Plasma Ameliorates Cartilage Degradation in Rat Models of Osteoarthritis via the OPG/RANKL/RANK System. Folia Histochem. Cytobiol. 2024, 62, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jang, J.M.; Yang, G.; Ha, H.C.; Fu, Z.; Kim, D.K. A Novel Role of Hyaluronic Acid and Proteoglycan Link Protein 1 (HAPLN1) in Delaying Vascular Endothelial Cell Senescence. Biomol. Ther. 2023, 31, 629–639. [Google Scholar] [CrossRef]
- Xue, Y.; Su, X.; Jiang, M.; Yu, Z.; Yang, H.; Qin, L.; Giannoudis, P.V.; Guo, J.J. Pure Platelet-Rich Plasma Facilitates the Repair of Damaged Cartilage and Synovium in a Rabbit Hemorrhagic Arthritis Knee Model. Arthritis Res. Ther. 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Di Martino, A.; Marcacci, M. Platelet-Rich Plasma (PRP) to Treat Sports Injuries: Evidence to Support Its Use. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 516–527. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, P.; Liao, B.; You, H.; Cai, Y. Effects and Action Mechanisms of Individual Cytokines Contained in PRP on Osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 713. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.S.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.J.M.A.; Lenz, M.E.; Sah, R.L.; Masuda, K. Platelet-Rich Plasma Stimulates Porcine Articular Chondrocyte Proliferation and Matrix Biosynthesis. Osteoarthr. Cartil. 2006, 14, 1272–1280. [Google Scholar] [CrossRef]
- Lippross, S.; Moeller, B.; Haas, H.; Tohidnezhad, M.; Steubesand, N.; Wruck, C.J.; Kurz, B.; Seekamp, A.; Pufe, T.; Varoga, D. Intraarticular Injection of Platelet-Rich Plasma Reduces Inflammation in a Pig Model of Rheumatoid Arthritis of the Knee Joint. Arthritis Rheum. 2011, 63, 3344–3353. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- van Weeren, P.R.; de Grauw, J.C. Pain in Osteoarthritis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 619–642. [Google Scholar] [CrossRef]
- McIlwraith, C.W. From Arthroscopy to Gene Therapy-30 Years of Looking in Joints. In Proceedings of the American Association of Equine Practitioners, Seattle, WA, USA, 3–7 December 2005. [Google Scholar]
- Laufer, S.; Greim, C.; Bertsche, T. An In-Vitro Screening Assay for the Detection of Inhibitors of Proinflammatory Cytokine Synthesis: A Useful Tool for the Development of New Antiarthritic and Disease Modifying Drugs. Osteoarthr. Cartil. 2002, 10, 961–967. [Google Scholar] [CrossRef]
- Verschooten, F.; Verbeeck, J. Flexion Test of the Metacarpophalangeal and Interphalangeal Joints and Flexion Angle of the Metacarpophalangeal Joint in Sound Horses. Equine Vet. J. 1997, 29, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, R.; Jüni, P.; Nüesch, E.; Dieppe, P.A.; Reichenbach, S. Association of Radiographic Osteoarthritis, Pain on Passive Movement and Knee Range of Motion: A Cross-Sectional Study. Man. Ther. 2015, 20, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Bertone, A.L.; Ishihara, A.; Zekas, L.J.; Wellman, M.L.; Lewis, K.B.; Schwarze, R.A.; Barnaba, A.R.; Schmall, M.L.; Kanter, P.M.; Genovese, R.L. Evaluation of a Single Intra-Articular Injection of Autologous Protein Solution for Treatment of Osteoarthritis in Horses. Am. J. Vet. Res. 2014, 75, 141–151. [Google Scholar] [CrossRef]
- Tyrnenopoulou, P.; Diakakis, N.; Karayannopoulou, M.; Savvas, I.; Koliakos, G. Evaluation of Intra-Articular Injection of Autologous Platelet Lysate (PL) in Horses with Osteoarthritis of the Distal Interphalangeal Joint. Vet. Q. 2016, 36, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Camurcu, Y.; Sofu, H.; Ucpunar, H.; Kockara, N.; Cobden, A.; Duman, S. Single-Dose Intra-Articular Corticosteroid Injection Prior to Platelet-Rich Plasma Injection Resulted in Better Clinical Outcomes in Patients with Knee Osteoarthritis: A Pilot Study. J. Back. Musculoskelet. Rehabil. 2018, 31, 603–610. [Google Scholar] [CrossRef]
- Smith, L.C.R.; Wylie, C.E.; Palmer, L.; Ramzan, P.H.L. A Longitudinal Study of Fractures in 1488 Thoroughbred Racehorses Receiving Intrasynovial Medication: 2006–2011. Equine Vet. J. 2018, 50, 774–780. [Google Scholar] [CrossRef]
- Ferris, D.J.; Frisbie, D.D.; McIlwraith, C.W.; Kawcak, C.E. Current Joint Therapy Usage in Equine Practice: A Survey of Veterinarians 2009. Equine Vet. J. 2011, 43, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Kawcak, C.E.; Norrdin, R.W.; Frisbie, D.D.; Trotter, G.W.; Mcilwraith, C.W. Effects of Osteochondral Fragmentation and Intra-Articular Triamcinolone Acetonide Treatment on Subchondral Bone in the Equine Carpus. Equine Vet. J. 1998, 30, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Pieter, H.L. Ramzan The Racehorse: A Veterinary Manual, 2nd ed.; Taylor & Francis Ltd.: London, UK, 2023. [Google Scholar]
- Lindner, A.; Dingerkus, A. Incidence of Training Failure among Thoroughbred Horses at Cologne, Germany. Prev. Vet. Med. 1993, 16, 85–94. [Google Scholar] [CrossRef]
- Khoury, M.A.; Chamari, K.; Tabben, M.; Alkhelaifi, K.; Papacostas, E.; Marín Fermín, T.; Laupheimer, M.; D Hooghe, P. Knee Osteoarthritis: Clinical and MRI Outcomes After Multiple Intra-Articular Injections With Expanded Autologous Adipose-Derived Stromal Cells or Platelet-Rich Plasma. Cartilage 2023, 14, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Elksniņš-Finogejevs, A.; Vidal, L.; Peredistijs, A. Intra-Articular Platelet-Rich Plasma vs Corticosteroids in the Treatment of Moderate Knee Osteoarthritis: A Single-Center Prospective Randomized Controlled Study with a 1-Year Follow Up. J. Orthop. Surg. Res. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCarrel, T.M.; Minas, T.; Fortier, L.A. Optimization of Leukocyte Concentration in Platelet-Rich Plasma for the Treatment of Tendinopathy. J. Bone Jt. Surg. Am. 2012, 94, e143. [Google Scholar] [CrossRef]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The Effect of Platelet-Rich Plasma Formulations and Blood Products on Human Synoviocytes: Implications for Intra-Articular Injury and Therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef]
- Meijer, M.C.; Busschers, E.; Van Weeren, P.R. Which Joint Is Most Important for the Positive Outcome of a Flexion Test of the Distal Forelimb of a Sound Horse? Equine Vet. Educ. 2001, 13, 319–323. [Google Scholar] [CrossRef]
- Kearney, C.M.; Van Weeren, P.R.; Cornelissen, B.P.M.; Den Boon, P.; Brama, P.A.J. Which Anatomical Region Determines a Positive Flexion Test of the Distal Aspect of a Forelimb in a Nonlame Horse? Equine Vet. J. 2010, 42, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Uslu Güvendi, E.; Aşkin, A.; Güvendi, G.; Koçyiğit, H. Comparison of Efficiency between Corticosteroid and Platelet Rich Plasma Injection Therapies in Patients with Knee Osteoarthritis. Arch. Rheumatol. 2018, 33, 273–281. [Google Scholar] [CrossRef]
- Görmeli, G.; Görmeli, C.A.; Ataoglu, B.; Çolak, C.; Aslantürk, O.; Ertem, K. Multiple PRP Injections Are More Effective than Single Injections and Hyaluronic Acid in Knees with Early Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; Saltzman, B.M.; Mascarenhas, R.; Khair, M.M.; Verma, N.N.; Bach, B.R.; Cole, B.J. Does Intra-Articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared With Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-Analyses. Arthroscopy 2015, 31, 2213–2221. [Google Scholar] [CrossRef]
| Groups | Baseline | T1 | T2 | T3 | p-Value * | |
|---|---|---|---|---|---|---|
| Effusion score (median; range) | ||||||
| Group TA (n = 31) | 1; 0–3 | 0; 0–1 a | 0; 0–2 | na | <0.001 | |
| Group TA+PRP (n = 22) | 2; 0–3 | 0; 0–1 a | 0; 0–1 a | 1; 0–1 a | <0.001 | |
| p-value # | 1.00 | 1.00 | 0.87 | |||
| Passive flexion score (median; range) | ||||||
| Group TA (n = 31) | 2; 0–3 | 0; 0–2 a | 1; 0–2 a,b | na | <0.03 | |
| Group TA+PRP (n = 22) | 2; 0–3 | 0; 0–2 a | 0; 0–2 a | 0; 0–1 a,c | <0.001 | |
| p-value # | 1.00 | 1.00 | 0.007 | <0.001 | ||
| Lameness score (median; range) | ||||||
| Group TA (n = 31) | 0; 0–2 | 0; 0–1 | 0; 0–1 | na | 1.00 | |
| Group TA+PRP (n = 22) | 0; 0–3 | 0; 0–1 | 0; 0–1 a | 0; 0–1 a | 0.002 | |
| p-value # | 0.36 | 1.00 | 1.00 | |||
| Weeks of follow-up (median; range) | ||||||
| Group TA (n = 31) | 4; 4–5 | na | ||||
| Group TA+PRP (n = 22) | 7; 6–8 | na | ||||
| p-value # | <0.001 | |||||
| Adverse Effects (prevalence; %) | ||||||
| No | Yes | |||||
| Group TA (n = 31) | 31 (100%) | 0 (0%) | ||||
| Group TA+PRP (n = 22) | 22 (100%) | 0 (0%) | ||||
| p-value # | na | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidoni, K.; Chiaradia, E.; Pepe, M.; Di Meo, A.; Tognoloni, A.; Seccaroni, M.; Beccati, F. The Combined Use of Triamcinolone and Platelet-Rich Plasma in Equine Metacarpophalangeal Joint Osteoarthritis Treatments: An In Vivo and In Vitro Study. Animals 2024, 14, 3645. https://doi.org/10.3390/ani14243645
Guidoni K, Chiaradia E, Pepe M, Di Meo A, Tognoloni A, Seccaroni M, Beccati F. The Combined Use of Triamcinolone and Platelet-Rich Plasma in Equine Metacarpophalangeal Joint Osteoarthritis Treatments: An In Vivo and In Vitro Study. Animals. 2024; 14(24):3645. https://doi.org/10.3390/ani14243645
Chicago/Turabian StyleGuidoni, Kübra, Elisabetta Chiaradia, Marco Pepe, Antonio Di Meo, Alessia Tognoloni, Matteo Seccaroni, and Francesca Beccati. 2024. "The Combined Use of Triamcinolone and Platelet-Rich Plasma in Equine Metacarpophalangeal Joint Osteoarthritis Treatments: An In Vivo and In Vitro Study" Animals 14, no. 24: 3645. https://doi.org/10.3390/ani14243645
APA StyleGuidoni, K., Chiaradia, E., Pepe, M., Di Meo, A., Tognoloni, A., Seccaroni, M., & Beccati, F. (2024). The Combined Use of Triamcinolone and Platelet-Rich Plasma in Equine Metacarpophalangeal Joint Osteoarthritis Treatments: An In Vivo and In Vitro Study. Animals, 14(24), 3645. https://doi.org/10.3390/ani14243645








