Effects of β-Glucan Supplementation on LPS-Induced Endotoxemia in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
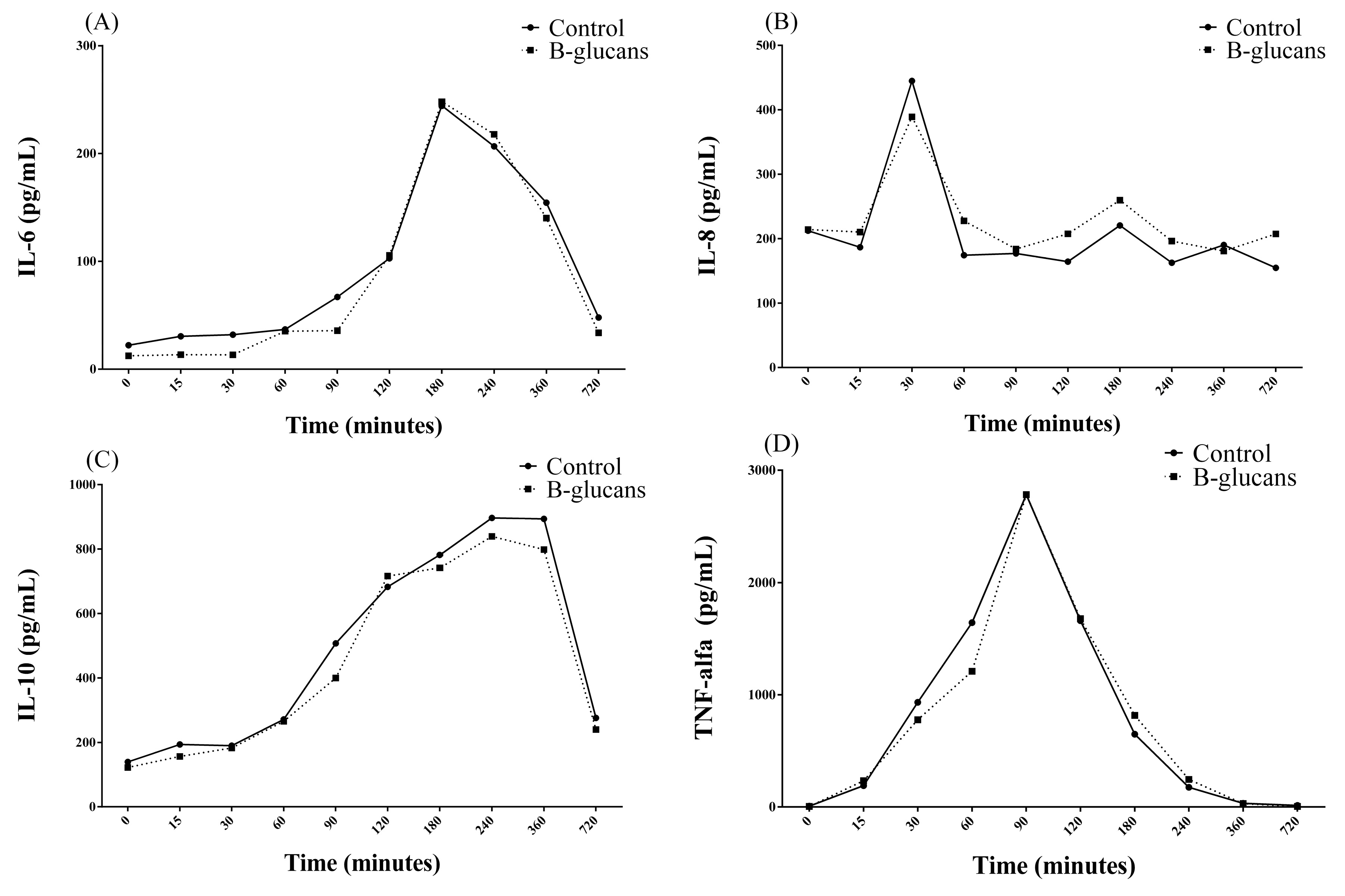
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peiró, J.R.; Campebell, R.C.; Santana, A.F.; Valadão, C.A.A. Clinical and laboratory evaluation after intraperi-toneal injection of lipopolysaccharide (LPS). J. Equine Vet. Sci. 1999, 19, 185–189. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, R.; Aittoniemi, J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J. Infect. 2011, 63, 407–419. [Google Scholar] [CrossRef]
- Chalupka, A.N.; Talmor, D. The Economics of Sepsis. Crit. Care Clin. 2012, 28, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Cloetens, L.; Ulmius, M.; Johansson-Persson, A.; Åkesson, B.; Önning, G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr. Rev. 2012, 70, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Oliveira, C. [Beta] (1-3) (1-6)-D-glucans Modulate Immune Status and Blood Glucose Levels in Dogs. Br. J. Pharm. Res. 2014, 4, 981. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S. Cell wall chemistry, morphogenesis and taxonomy of fungi. Annu. Rev. Microbiol. 1968, 22, 87–108. [Google Scholar] [CrossRef]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.D.; Jiang, Z. The Application of Fungal Beta-glucans for the Treatment of Colon Cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 725–730. [Google Scholar] [CrossRef]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble β-glucans extracted from Grifola frondosainduces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer N. Y. 2013, 133, 108–119. [Google Scholar] [CrossRef]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. Hong Kong 2011, 10, 115–119. [Google Scholar] [CrossRef]
- Santos, J.C.; Figueiredo, A.M.B.; Silva, M.V.T.; Cirovic, B.; Bree, L.C.J.; Damen, M.S.M.A.; Moorlag, S.J.C.F.M.; Gomes, R.S.; Helsen, M.M.; Oosting, M.; et al. β-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: A Crucial Role for IL-32. Cell Rep. 2019, 28, 2659–2672.e6. [Google Scholar] [CrossRef]
- Williams, D.L.; McNamee, R.B.; Jones, E.L.; Pretus, H.A.; Ensley, H.E.; Browder, I.; Di Luzio, N.R. A method for the solubilization of a (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991, 219, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Ha, T.; LI, C.; Kalbfleisch, J.H.; Laffan, J.J.; Ferguson, D.A. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1→3)-beta-D-glucan increases long-term survival in polymicrobial sepsis. Surgery 1999, 126, 54–65. [Google Scholar] [CrossRef]
- Williams, D.L.; Li, C.; Ha, T.; Ozment-Skelton, T.; Kalbfleisch, J.H.; Preiszner, J.; Brooks, L.; Breuel, K.; Schweitzer, J.B. Modulation of the Phosphoinositide 3-Kinase Pathway Alters Innate Resistance to Polymicrobial Sepsis. J. Immunol. 2004, 172, 449–456. [Google Scholar] [CrossRef]
- Luhm, J.; Langenkamp, U.; Hensel, J.; Frohn, C.; Brand, J.M.; Hennig, H.; Rink, L.; Koritke, P.; Wittkopt, N.; Williams, D.L.; et al. β-(1-3)-D-glucan modulates DNA binding of nuclear factors κB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1 β/IL-1 receptor antagonist ratio. BMC Immunol. 2006, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. New look at statistical-model identification. IEEE Trans. Autom. Control 1974, AC19, 716–723. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.K.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: San Diego, CA, USA, 2008; p. 928. [Google Scholar]
- Meyer, D.; Harvey, J.W. Hepatobiliary and skeletal muscleenzymes and liver function tests. In Veterinary Laboratory Medicine: Interpretation and Diagnosis; Meyer, D., Harvey, J.W., Eds.; W.B. Saunders Co.: St. Louis, MO, USA, 2004; Volume 21, pp. 169–192. [Google Scholar]
- Mackay, R.J. Endotoxemia. In Current Therapy in Equine Medicine, 3rd ed.; Rob1nson, N.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1992; pp. 225–232. [Google Scholar]
- Lindinger, M.I.; McCutcheon, L.J.; Geor, R.J.; Jansson, A.; Lindholm, A.; Dahlborn, K.; Ecker, G.L.; Forro, M.; Cieslar, S.; Walzak, A.; et al. Heat acclimation improves regulation of plasma volume and plasma Na+ content during exercise in horses. J. Appl. Physiol. 2000, 88, 1006–1013. [Google Scholar] [CrossRef]
- Moore, J.N. A perspective on endotoxemia. In Proceedings of the Annual Convention of the AAEP, San Diego, CA, USA, 24–28 November 2001; pp. 61–74. [Google Scholar]
- Bone, R.C.; Sibbald, W.J.; Sprung, C.L. The ACCP-SCCM Consensus Conference on Sepsis and Organ Failure. Chest 1992, 101, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Sair, M.; Etherington, P.J.; Curzen, N.P.; Winlove, C.P.; Evans, T.W. Tissue oxygenation and perfusion in endo-toxemia. Am. J. Physiol. 1996, 271, H1620–H1625. [Google Scholar]
- Furr, M.O.; Lessard, P.; Ii, N.A.W. Development of a Colic Severity Score for Predicting the Outcome of Equine Colic. Veter- Surg. 1995, 24, 97–101. [Google Scholar] [CrossRef]
- Walther, S.; Rusitzka, T.V.; Diesterbeck, U.S.; Czerny, C.P. Equine immunoglobulins and organization of immu-noglobulin genes. Dev. Comp. Immunol. 2015, 53, 303–319. [Google Scholar] [CrossRef]
- Valadao, C.A.; Peiro, J.R.; Santana, A.E.; Bechara, G.H. Evaluation of peritoneal fluid in horses with experimental endotoxemia. J. Equine Veter-Sci. 1995, 15, 124–128. [Google Scholar] [CrossRef]
- Walton, R.M.; Soothwood, L.L. Abdominocentesis and peritoneal fluid analysis. In Practical Guide to Equine Colic; Soothwood, L.L., Ed.; Willey-Blackwell: Oxford, UK, 2012; pp. 87–98. [Google Scholar]
- Beyaert, R.; Fiers, W. Tumor necrosis factor and lymphotoxin. In Citokines; Mire-Sluis, A., Thorpe, R., Eds.; Academic: San Diego, CA, USA, 1998; pp. 335–360. [Google Scholar]
- Ghiselli, R.; Giacometti, A.; Cirioni, O.; Orlando, F.; Mocchegiani, F.; Pacci, A.M.; Scalise, G.; Saba, V. Therapeutic Efficacy of the Polymyxin-like Peptide Ranalexin in an Experimental Model of Endotoxemia. J. Surg. Res. 2001, 100, 183–188. [Google Scholar] [CrossRef]
- Barton, M.H.; Collatos, C.; Moore, J.N. Endotoxin induced expression of tumour necrosis factor, tissue factor and plasminogen activator inhibitor activity by peritoneal macrophages. Equine Veter-J. 1996, 28, 382–389. [Google Scholar] [CrossRef]
- Bueno, A.C.; Seahorn, T.L.; Cornick-Seahorn, J.; Horohov, D.W.; Moore, R.M. Plasma and urine nitric oxide concentrations in horses given below a low dose of endotoxin. Am. J. Vet. Res. 1999, 60, 969–976. [Google Scholar] [CrossRef]
- Taniguchi, T.; Shibata, K.; Yamamoto, K.; Mizukoshi, Y.; Kobayashi, T. Effects of lidocaine administration on hemodynamics and cytokine responses to endotoxemia in rabbits. Crit. Care Med. 2000, 28, 755–759. [Google Scholar] [CrossRef]
- Thijs, L.G.; Hack, C.E. Time course of cytokine levels in sepsis. Intensiv. Care Med. 1995, 21, S258–S263. [Google Scholar] [CrossRef]
- Neuder, L.E.; Keener, J.M.; Eckert, R.E.; Trujillo, J.C.; Jones, S.L. Role of p38 MAPK in LPS induced pro-inflammatory cytokine and chemokine gene expression in equine leukocytes. Veter-Immunol. Immunopathol. 2009, 129, 192–199. [Google Scholar] [CrossRef]
- Mukaida, N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 284, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Choi, J.H.; Kang, Y.J.; Park, S.Y.; Choi, H.C.; Kim, H.S. Reparixin, an Inhibitor of CXCR1 and CXCR2 Receptor Activation, Attenuates Blood Pressure and Hypertension-Related Mediators Expression in Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2011, 34, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.E.; Macdonald, M.H.; Braim, A.E.P.; Aleman, M. Effect of lipopolysaccharide infusion on gene expression of inflammatory cytokines in normal horses in vivo. Equine Veter-J. 2009, 41, 717–719. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.J.C.F.M.; Khan, N.; Novakovic, B.; Kaufmann, E.; Jansen, T.; Van Crevel, R.; Divangahi, M.; Netea, M.G. β-glucan induces protective trained immunity against Mycobacterium tuberculosis infection: A key role for IL-1. Cell Rep. 2020, 31, 107634. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. The Phosphatidylinositol 3-Kinase-Akt Pathway Limits Lipopolysaccharide Activation of Signaling Pathways and Expression of Inflammatory Mediators in Human Monocytic Cells. J. Biol. Chem. 2002, 277, 32124–32132. [Google Scholar] [CrossRef]
- Stockham, S.L. Interpretation of Equine Serum Biochemical Profile Results. Veter-Clin. N. Am. Equine Pract. 1995, 11, 391–414. [Google Scholar] [CrossRef]
- Tennant, B.C. Hepatic function. In Clinical Biochemistry of Domestic Animals, 5th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: London, UK, 1997; pp. 327–352. [Google Scholar]
- Ferreira, C.S.; Vendramini, T.H.A.; Amaral, A.R.; Rentas, M.F.; Ernandes, M.C.; da Silva, F.L.; Oba, P.M.; Filho, F.D.O.R.; Brunetto, M.A. Metabolic variables of obese dogs with insulin resistance supplemented with yeast beta-glucan. BMC Veter-Res. 2022, 18, 14. [Google Scholar] [CrossRef]
- Marchi, P.H.; Vendramini, T.H.A.; Zafalon, R.V.A.; Príncipe, L.D.A.; Cesar, C.G.L.; Perini, M.P.; Putarov, T.C.; Gomes, C.O.M.S.; Balieiro, J.C.D.C.; Brunetto, M.A. Effects of increasing levels of purified beta-1,3/1,6-glucans on the fecal microbiome, digestibility and immunity variables of healthy adult dogs. Microorganisms 2024, 12, 113. [Google Scholar] [CrossRef]
| Variable | Group | SEM 4 | p | |||
|---|---|---|---|---|---|---|
| Control | β-Glucans | Treatment | Time | Treatment × Time | ||
| AST 1 (U/L) | 287.56 | 315.60 | 14.118 | 0.022 | 1.000 | 1.000 |
| GGT 2 (U/L) | 12.90 | 9.27 | 28.445 | 0.001 | 0.993 | 0.994 |
| TP 3 (g/dL) | 6.52 | 6.77 | 5.765 | 0.021 | 0.971 | 0.992 |
| Globulins (g/dL) | 3.72 | 4.01 | 9.937 | 0.010 | 0.991 | 0.988 |
| Lactate (mmol/L) | 1.40 | 1.64 | 30.451 | 0.026 | 0.001 | 0.953 |
| Variable | Group | SEM 1 | p | |||
|---|---|---|---|---|---|---|
| Control | β-Glucans | Treatment | Time | Treatment × Time | ||
| Lactate (mmol/L) | 1.05 | 1.40 | 0.413 | 0.003 | <0.001 | 0.997 |
| Neutrophils (%) | 65.87 | 55.85 | 31.130 | 0.020 | <0.001 | 0.852 |
| Macrophages (%) | 25.33 | 34.60 | 57.400 | 0.020 | 0.002 | 0.673 |
| Variable | Group | SEM 5 | p | |||
|---|---|---|---|---|---|---|
| Control | β-Glucans | Treatment | Time | Treatment × Time | ||
| IL-6 1 (pg/mL) | 99.06 | 122.39 | 156.422 | 0.549 | 0.05 | 0.499 |
| IL-8 2 (pg/mL) | 156.24 | 305.20 | 141.847 | 0.05 | 0.078 | 0.091 |
| IL-10 3 (pg/mL) | 518.81 | 526.88 | 82.869 | 0.923 | <0.001 | 0.458 |
| TNF-alfa 4 (pg/mL) | 839.45 | 778.39 | 154.134 | 0.787 | <0.001 | 0.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerenza, M.D.; Arantes, J.d.A.; Reginato, G.M.; Passarelli, D.; Balieiro, J.C.d.C.; Amaral, A.R.; Vendramini, T.H.A.; Brunetto, M.A.; Dória, R.G.S. Effects of β-Glucan Supplementation on LPS-Induced Endotoxemia in Horses. Animals 2024, 14, 474. https://doi.org/10.3390/ani14030474
Lacerenza MD, Arantes JdA, Reginato GM, Passarelli D, Balieiro JCdC, Amaral AR, Vendramini THA, Brunetto MA, Dória RGS. Effects of β-Glucan Supplementation on LPS-Induced Endotoxemia in Horses. Animals. 2024; 14(3):474. https://doi.org/10.3390/ani14030474
Chicago/Turabian StyleLacerenza, Milena Domingues, Júlia de Assis Arantes, Gustavo Morandini Reginato, Danielle Passarelli, Júlio César de Carvalho Balieiro, Andressa Rodrigues Amaral, Thiago Henrique Annibale Vendramini, Marcio Antonio Brunetto, and Renata Gebara Sampaio Dória. 2024. "Effects of β-Glucan Supplementation on LPS-Induced Endotoxemia in Horses" Animals 14, no. 3: 474. https://doi.org/10.3390/ani14030474
APA StyleLacerenza, M. D., Arantes, J. d. A., Reginato, G. M., Passarelli, D., Balieiro, J. C. d. C., Amaral, A. R., Vendramini, T. H. A., Brunetto, M. A., & Dória, R. G. S. (2024). Effects of β-Glucan Supplementation on LPS-Induced Endotoxemia in Horses. Animals, 14(3), 474. https://doi.org/10.3390/ani14030474





