High Exposure to Livestock Pathogens in Southern Pudu (Pudu puda) from Chile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
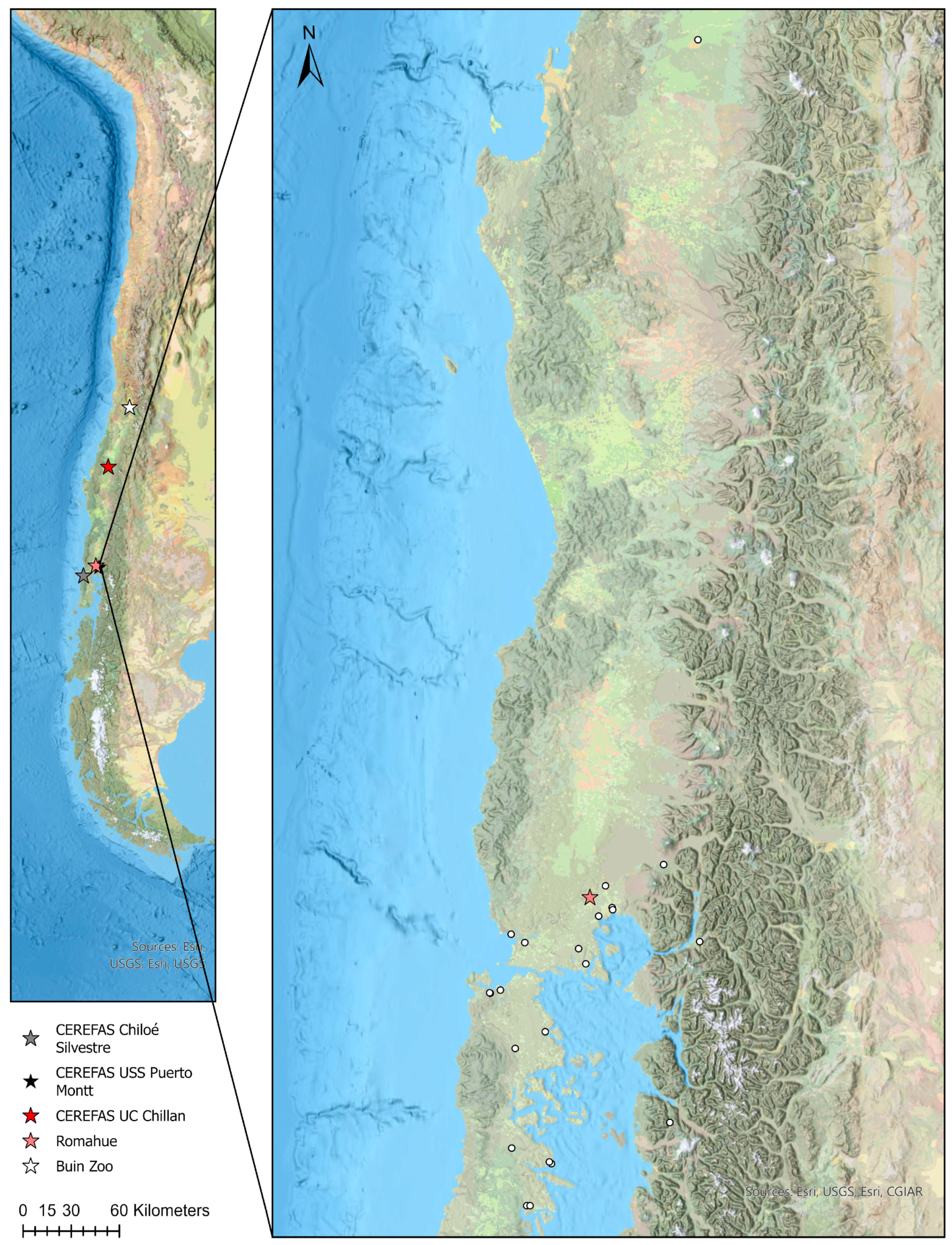
2.1. Serum Samples
2.2. Laboratory Analyses
2.3. Data Analysis
3. Results
3.1. Overall Exposure
3.2. Leptospira interrogans Serovars
3.3. Data Analysis
4. Discussion
4.1. Wild Animals
4.1.1. Leptospira interrogans
4.1.2. Pestivirus
4.1.3. Toxoplasma gondii
4.1.4. Chlamydia abortus
4.2. Under-Human-Care Pudus
4.2.1. Neospora caninum
4.2.2. Toxoplasma gondii
4.2.3. Leptospira interrogans
4.2.4. Pestivirus
4.2.5. Chlamydia abortus
4.3. Other Pathogens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavez-Fox, M.; Estay, S.A. Correspondence between the habitat of the threatened pudú (Cervidae) and the national protected-area system of Chile. BMC Ecol. 2016, 16, 1. [Google Scholar] [CrossRef]
- Jimenez, J.E. Southern Pudu Pudu puda (Molina 1782). Neotropical Cervidology: Biology and Medicine of Latin American Deer; Funep and IUCN: Jaboticabal, Brazil; Gland, Switzerland, 2010. [Google Scholar]
- Silva-Rodríguez, E.; Pastore, H.; Jiménez, J. Pudu puda. The IUCN Red List of Threatened Species. 2016. Available online: https://www.iucnredlist.org/es/species/18848/22164089 (accessed on 20 July 2022).
- Biblioteca del Congreso Nacional de Chile. Supreme Decree no. 151 of the Ministerio Secretaria General de la Presidencia de Chile. [First Species Classification Process, MMA]. 2007. Available online: https://www.bcn.cl/leychile/navegar?idNorma=259402 (accessed on 25 July 2022). (In Spanish).
- Salgado, R.; Hidalgo-Hermoso, E.; Pizarro-Lucero, J. Detection of persistent pestivirus infection in pudú (Pudu puda) in a captive population of artiodactyls in Chile. BMC Vet. Res. 2018, 14, 37. [Google Scholar] [CrossRef]
- Hidalgo-Hermoso, E.; Celis, S.; Cabello, J.; Kemec, I.; Ortiz, C.; Lagos, R.; Verasay, J.; Moreira-Arce, D.; Vergara, P.M.; Vera, F.; et al. Molecular survey of selected viruses in Pudus (Pudu puda) in Chile revealing first identification of caprine herpesvirus—2 (CpHV-2) in South American ungulates. Vet. Q. 2023, 43, 1–7. [Google Scholar] [CrossRef]
- Besser, T.E.; Cassirer, E.F.; Lisk, A.; Nelson, D.; Manlove, K.R.; Cross, P.C.; Hogg, J.T. Natural history of a bighorn sheep pneumonia epizootic: Source of infection, course of disease, and pathogen clearance. Ecol. Evol. 2021, 11, 14366–14382. [Google Scholar] [CrossRef]
- Ruder, M.G.; Lysyk, T.J.; Stallknecht, D.E.; Foil, L.D.; Johnson, D.J.; Chase, C.C.; Dargatz, D.A.; Gibbs, E.P.J. Transmission and Epidemiology of Bluetongue and Epizootic Hemorrhagic Disease in North America: Current Perspectives, Research Gaps, and Future Directions. Vector-Borne Zoonotic Dis. 2015, 15, 348–363. [Google Scholar] [CrossRef]
- Fereidouni, S.; Freimanis, G.L.; Orynbayev, M.; Ribeca, P.; Flannery, J.; King, D.P.; Zuther, S.; Beer, M.; Höper, D.; Kydyrmanov, A.; et al. Mass Die-Off of Saiga Antelopes, Kazakhstan, 2015. Emerg. Infect. Dis. 2019, 25, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Pruvot, M.; Fine, A.E.; Hollinger, C.; Strindberg, S.; Damdinjav, B.; Buuveibaatar, B.; Chimeddorj, B.; Bayandonoi, G.; Khishgee, B.; Sandag, B.; et al. Outbreak of Peste des Petits Ruminants among Critically Endangered Mongolian Saiga and Other Wild Ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 2020, 26, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.A.; Wambua, J.M.; Mwanzia, J.; Wamwayi, H.; Ndungu, E.K.; Barrett, T.; Kock, N.D.; Rossiter, P.B. Rinderpest epidemic in wild ruminants in Kenya 1993–1997. Vet. Rec. 1999, 145, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillamón, F.; Caballero-Gómez, J.; Agüero, M.; Camacho-Sillero, L.; Risalde, M.A.; Zorrilla, I.; Villalba, R.; Rivero-Juárez, A.; García-Bocanegra, I. Re-emergence of bluetongue virus serotype 4 in Iberian ibex (Capra pyrenaica) and sympatric livestock in Spain, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Gauss, C.; Dubey, J.; Vidal, D.; Cabezón, O.; Ruiz-Fons, F.; Vicente, J.; Marco, I.; Lavin, S.; Gortazar, C.; Almería, S. Prevalence of Toxoplasma gondii antibodies in red deer (Cervus elaphus) and other wild ruminants from Spain. Vet. Parasitol. 2006, 136, 193–200. [Google Scholar] [CrossRef]
- Kuiken, T.; Cromie, R. Protect wildlife from livestock diseases. Science 2022, 378, 6615. [Google Scholar] [CrossRef] [PubMed]
- Rojas, H.; Romero, J. Where to next with animal health in Latin America? The transition from endemic to disease-free status. Rev. Sci. Tech. 2017, 36, 331–348. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, M.J.; Hidalgo-Hermoso, E.; Cacho-Zanette, L.; de Campos Binder, L.; Rivera, A.M.; Molina-Flores, B.; Maia-Elkhoury, A.N.S.; Schneider Vianna, R.; Valadas, S.Y.O.B.; Natal Vigilato, M.A.; et al. Characteristics and perspectives of disease at the wildlife-livestock interface in Central and South America. In Diseases at the Wildlife-Livestock Interface: Research and Perspectives in a Changing World, Wildlife Research Monographs 3; Vicente, J., Vercauteren, K.C., Gortazar, C., Eds.; Springer: Cham, Switzerland, 2021; pp. 271–304. [Google Scholar]
- Peel, A.J.; McKinley, T.J.; Baker, K.S.; Barr, J.A.; Crameri, G.; Hayman, D.T.; Feng, Y.-R.; Broder, C.C.; Wang, L.-F.; Cunningham, A.A.; et al. Use of cross-reactive serological assays for detecting novel pathogens in wildlife: Assessing an appropriate cutoff for henipavirus assays in African bats. J. Virol. Methods 2013, 193, 295–303. [Google Scholar] [CrossRef]
- Martins, M.; Boggiatto, P.M.; Buckley, A.; Cassmann, E.D.; Falkenberg, S.; Caserta, L.C.; Fernandes, M.H.V.; Kanipe, C.; Lager, K.; Palmer, M.V.; et al. From Deer-to-Deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer. PLoS Pathog. 2022, 18, e1010197. [Google Scholar] [CrossRef] [PubMed]
- Caserta, L.C.; Martins, M.; Butt, S.L.; Hollingshead, N.A.; Covaleda, L.M.; Ahmed, S.; Everts, M.R.R.; Schuler, K.L.; Diel, D.G. White-tailed deer (Odocoileus virginianus) may serve as a wildlife reservoir for nearly extinct SARS-CoV-2 variants of concern. Proc. Natl. Acad. Sci. USA 2023, 120, e2215067120. [Google Scholar] [CrossRef]
- Santos, N.; Colino, E.F.; Arnal, M.C.; de Luco, D.F.; Sevilla, I.; Garrido, J.M.; Fonseca, E.; Valente, A.M.; Balseiro, A.; Queirós, J.; et al. Complementary roles of wild boar and red deer to animal tuberculosis maintenance in multi-host communities. Epidemics 2022, 41, 100633. [Google Scholar] [CrossRef]
- González-Barrio, D.; Ruiz-Fons, F. Coxiella burnetii in wild mammals: A systematic review. Transbound. Emerg. Dis. 2019, 66, 662–671. [Google Scholar] [CrossRef]
- Di Francesco, A.; Donati, M.; Nicoloso, S.; Orlandi, L.; Baldelli, R.; Salvatore, D.; Sarli, G.; Cevenini, R.; Morandi, F. Chlamydiosis: Seroepidemiologic survey in a red deer (Cervus elaphus) population in Italy. J. Wildl. Dis. 2012, 48, 488–491. [Google Scholar] [CrossRef]
- Ndengu, M.; Matope, G.; Tivapasi, M.; Scacchia, M.; Bonfini, B.; Pfukenyi, D.M.; de Garine-Wichatitsky, M. Sero-prevalence of chlamydiosis in cattle and selected wildlife species at a wildlife/livestock interface area of Zimbabwe. Trop. Anim. Health Prod. 2018, 50, 1107–1117. [Google Scholar] [CrossRef]
- Hidalgo-Hermoso, E.; Cabello, J.; Novoa-Lozano, I.; Celis, S.; Ortiz, C.; Kemec, I.; Lagos, R.; Verasay, J.; Mansell-Venegas, M.; Moreira-Arce, D.; et al. Molecular detection and characterization of hemoplasmas in the Pudu (Pudu puda), a native cervid from Chile. J. Wildl. Dis. 2022, 58, 8–14. [Google Scholar] [CrossRef]
- Santodomingo, A.; Robbiano, S.; Thomas, R.; Parragué-Migone, C.; Cabello-Stom, J.; Vera-Otarola, F.; Valencia-Soto, C.; Moreira-Arce, D.; Moreno, L.; Hidalgo-Hermoso, E.; et al. A search for piroplasmids and spirochetes in threatened pudu (Pudu puda) and associated ticks from Southern Chile unveils a novel Babesia sp. and a variant of Borrelia chilensis. Transbound. Emerg. Dis. 2022, 69, 3737–3748. [Google Scholar] [CrossRef]
- Santodomingo, A.; Thomas, R.; Robbiano, S.; Uribe, J.E.; Parragué-Migone, C.; Cabello-Stom, J.; Vera-Otarola, F.; Valencia-Soto, C.; Moreira-Arce, D.; Hidalgo-Hermoso, E.; et al. Wild deer (Pudu puda) from Chile harbor a novel ecotype of Anaplasma phagocytophilum. Parasites Vectors 2023, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Lorca-Oró, C.; López-Olvera, J.R.; Fernández-Sirera, L.; Solanes, D.; Navarro, N.; García-Bocanegra, I.; Lavín, S.; Domingo, M.; Pujols, J. Evaluation of the efficacy of commercial vaccines against bluetongue virus serotypes 1 and 8 in experimentally infected red deer (Cervus elaphus). Vet. Microbiol. 2012, 154, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Mainar-Jaime, R.; Berzal-Herranz, B.; Arias, P.; Rojo-Vázquez, F. Epidemiological pattern and risk factors associated with bovine viral-diarrhoea virus (BVDV) infection in a non-vaccinated dairy-cattle population from the Asturias region of Spain. Prev. Vet. Med. 2001, 52, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Schubert, E.; Schnee, C. Ring trial of the German Reference Laboratory for chlamydiosis. In Proceedings of the AVID Meeting, Kloster Banz, Germany, 15 January 2015. [Google Scholar]
- González-Barrio, D.; Ortiz, J.A.; Ruiz-Fons, F. Estimating the Efficacy of a Commercial Phase I Inactivated Vaccine in Decreasing the Prevalence of Coxiella burnetii Infection and Shedding in Red Deer (Cervus elaphus). Front. Vet. Sci. 2017, 4, 208. [Google Scholar] [CrossRef] [PubMed]
- OIE. Q Fever. In Manual of Diagnostic Test and Vaccines for Terrestrial Animals; OIE: Paris, France, 2018; Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.01.17_Q-FEVER.pdf (accessed on 23 July 2021).
- Witkowski, L.; Czopowicz, M.; Nagy, D.A.; Potarniche, A.V.; Aoanei, M.A.; Imomov, N.; Mickiewicz, M.; Welz, M.; Szaluś-Jordanow, O.; Kaba, J. Seroprevalence of Toxoplasma gondii in wild boars, red deer and roe deer in Poland. Parasite 2015, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Sailleau, C.; Breard, E.; Viarouge, C.; Belbism, G.; Lilin, T.; Vitour, D.; Zientara, A. Experimental infection of calves with seven serotypes of epizootic hemorrhagic disease virus: Production and characterization of reference sera. Vet. Ital. 2019, 55, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bréard, E.; Viarouge, C.; Donnet, F.; Sailleau, C.; Rossi, S.; Pourquier, P.; Vitour, D.; Comtet, L.; Zientara, S. Evaluation of a commercial ELISA for detection of epizootic haemorrhagic disease antibodies in domestic and wild ruminant sera. Transbound. Emerg. Dis. 2020, 67, 2475–2481. [Google Scholar] [CrossRef]
- OIE. Brucellosis (infection with B. abortus, B. melitensis and B. suis. In Manual of Diagnostic Test and Vaccines for Terrestrial Animals; OIE: Paris, France, 2018; Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.01.04_BRUCELL.pdf (accessed on 10 April 2020).
- Hidalgo-Hermoso, E.; Cabello, J.; Verasay, J.; Moreira-Arce, D.; Hidalgo, M.; Abalos, P.; Borie, C.; Galarce, N.; Napolitano, C.; Sacristán, I.; et al. Serosurvey for selected parasitic and bacterial pathogens in Darwin’s fox (Lycalopex fulvipes): Not only dog diseases are a threat. J. Wildl. Dis. 2022, 58, 76–85. [Google Scholar] [CrossRef]
- Moreno-Beas, E.; Abalos, P.; Hidalgo-Hermoso, E. Seroprevalence of nine Leptospira interrogans serovars in wild carnivores, ungulates, and primates from a zoo population in a Metropolitan region of Chile. J. Zoo Wildl. Med. 2015, 46, 774–778. [Google Scholar] [CrossRef]
- OIE. Leptospirosis. In Manual of Diagnostic Test and Vaccines for Terrestrial Animals; OIE: Paris, France, 2021; Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.01.12_Leptospirosis.pdf (accessed on 26 July 2020).
- Thomas, J.; Infantes-Lorenzo, J.; Moreno, I.; Romero, B.; Garrido, J.; Juste, R.; Domínguez, M.; Domínguez, L.; Gortazar, C.; Risalde, M. A new test to detect antibodies against Mycobacterium tuberculosis complex in red deer serum. Vet. J. 2019, 244, 98–103. [Google Scholar] [CrossRef]
- OIE. Infectious bovine rhinotracheitis/ infectious pustular vulvovaginitis. In Manual of Diagnostic Test and Vaccines for Terrestrial Animals; OIE: Paris, France, 2018; Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.04.11_IBR_IPV.pdf (accessed on 8 March 2020).
- Wernike, K.; Fischer, L.; Holsteg, M.; Aebischer, A.; Petrov, A.; Marquart, K.; Schotte, U.; Schön, J.; Hoffmann, D.; Hechinger, S.; et al. Serological screening in wild ruminants in Germany, 2021/2022: No evidence of SARS-CoV-2, bluetongue virus or pestivirus spread but high seroprevalences against Schmallenberg virus. Transbound. Emerg. Dis. 2022, 69, E3289–E3296. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Krzysiak, M.K.; Jabłoński, A.; Kęsik, J.; Bednarski, M.; Rola, J. Hepatitis E Virus Antibody Prevalence in Wildlife in Poland. Zoonoses Public Health 2015, 62, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.-F.; Li, Y.; Zhou, Y.; Bai, Y.D.; Wang, W.-L.; Cong, W. Seroprevalence of Neospora caninum infection in farmed sika deer (Cervus nippon) in China. Vet. Parasitol. 2015, 211, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. Epidemiology: An Introduction; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Muñoz, R.; Hidalgo-Hermoso, E.; Fredes, F.; Alegría-Morán, R.; Celis, S.; Ortiz-Tacci, C.; Kemec, I.; Mansell, M.; Verasay, J.; Ramírez-Toloza, G. Serological prevalence and risk factors of Toxoplasma gondii in Zoo Mammals in Chile. Prev. Vet. Med. 2021, 194, 105445. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, R.; Martin, W.; Stryhn, H. Methods in Epidemiologic Research, 1st ed.; VER Inc.: Charlottetown, PE, Canada, 2012; p. 890. [Google Scholar]
- Kleinbaum, D.G.; Klein, M. Introduction to Logistic Regression; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–39. [Google Scholar]
- Harlow, L.L. The Essence of Multivariate Thinking: Basic Themes and Methods, 3rd ed.; Routledge: Oxfordshire, UK, 2023. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Nakazawa, M.; Nakazawa, M.M. “Fmsb”: Functions for Medical Statistics Book with Some Demographic Data. R Package Version 0.7.0. 2019. Available online: https://CRAN.R-project.org/package=fmsb (accessed on 20 July 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2021. Available online: http://CRAN.R-project.org/package=nlme (accessed on 20 July 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Wickham, H. ggplot2. WIREs Comp. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Lele, S.; Keim, J.; Solymos, P. Resource selection: Resource Selection (Probability) Functions for Use-Availability. Data. 2019. Available online: https://github.com/psolymos/ResourceSelection (accessed on 20 July 2022).
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.E.; Meredith, A.L.; Knobel, D.L.; Shaw, D.J.; Bronsvoort, B.M.d.C.; Cleaveland, S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R. Soc. Interface 2007, 4, 973–984. [Google Scholar] [CrossRef]
- Meredith, A.L.; Cleaveland, S.C.; Denwood, M.J.; Brown, J.K.; Shaw, D.J. Coxiella burnetii(Q-Fever) Seroprevalence in Prey and Predators in the United Kingdom: Evaluation of Infection in Wild Rodents, Foxes and Domestic Cats Using a Modified ELISA. Transbound. Emerg. Dis. 2015, 62, 639–649. [Google Scholar] [CrossRef]
- Barroso, P.; Acevedo, P.; Vicente, J. The importance of long-term studies on wildlife diseases and their interfaces with humans and domestic animals: A review. Transbound. Emerg. Dis. 2021, 68, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Vada, R.; Zanet, S.; Ferroglio, E. Fifty Years of Wildlife Diseases in Europe: A Citation Database Meta-Analysis. Vet. Sci. 2022, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Uhart, M.M.; Vila, A.R.; Beade, M.S.; Balcarce, A.; Karesh, W.B. Health Evaluation of Pampas Deer (Ozotoceros bezoarticus celer) at Campos del Tuyú Wildlife Reserve, Argentina. J. Wildl. Dis. 2003, 39, 887–893. [Google Scholar] [CrossRef][Green Version]
- Deem, S.L.; Noss, A.J.; Villarroel, R.; Uhart, M.M.; Karesh, W.B. Disease Survey of Free-ranging Grey Brocket Deer (Mazama gouazoubira) in the Gran Chaco, Bolivia. J. Wildl. Dis. 2004, 40, 92–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corti, P.; Saucedo, C.; Herrera, P. Evidence of Bovine Viral Diarrhea, but Absence of Infectious Bovine Rhinotracheitis and Bovine Brucellosis in the Endangered Huemul Deer (Hippocamelus bisulcus) in Chilean Patagonia. J. Wildl. Dis. 2013, 49, 744–746. [Google Scholar] [CrossRef]
- Paz, L.N.; Hamond, C.; Pinna, M.H. Detection of Leptospira interrogans in Wild Sambar Deer (Rusa unicolor), Brazil. Ecohealth 2022, 19, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gardner, I.; Hietala, S.; Boyce, W. Validity of using serological tests for diagnosis of diseases in wild animals. Rev. Sci. Tech. 1996, 15, 323–335. [Google Scholar] [CrossRef]
- Gilbert, A.T.; Fooks, A.R.; Hayman, D.T.S.; Horton, D.L.; Müller, T.; Plowright, R.; Peel, A.J.; Bowen, R.; Wood, J.L.N.; Mills, J.; et al. Deciphering Serology to Understand the Ecology of Infectious Diseases in Wildlife. Ecohealth 2013, 10, 298–313. [Google Scholar] [CrossRef]
- Jia, B.; Colling, A.; Stallknecht, D.E.; Blehert, D.; Bingham, J.; Crossley, B.; Eagles, D.; Gardner, I.A. Validation of laboratory tests for infectious diseases in wild mammals: Review and recommendations. J. Vet. Diagn. Investig. 2020, 32, 776–792. [Google Scholar] [CrossRef]
- Hidalgo-Hermoso, E.; Ruiz-Fons, F.; Cabello-Stom, J.; Ramírez, N.; López, R.; Sánchez, F.; Mansell, M.; Sánchez, C.; Simonetti, J.A.; Peñaranda, D.; et al. Lack of Exposure to Mycobacterium bovis and Mycobacterium avium subsp. paratuberculosis in Chilean Cervids, and Evidence of a New Mycobacterium-Like Sequence. J. Wildl. Dis. 2022, 58, 680–684. [Google Scholar] [CrossRef]
- Messam, L.L.M.; Branscum, A.J.; Collins, M.T.; Gardner, I.A. Frequentist and Bayesian approaches to prevalence estimation using examples from Johne’s disease. Anim. Health Res. Rev. 2008, 9, 1–23. [Google Scholar] [CrossRef]
- Martínez-Mesa, J.; González-Chica, D.A.; Bastos, J.L.; Bonamigo, R.R.; Duquia, R.P. Sample size: How many participants do I need in my research? An. Bras. Dermatol. 2014, 89, 609–615. [Google Scholar] [CrossRef]
- Sykes, J.E.; Haake, D.A.; Gamage, C.D.; Mills, W.Z.; Nally, J.E. A global one health perspective on leptospirosis in humans and animals. J. Am. Vet. Med. Assoc. 2022, 260, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Bellinati, L.; Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Martignago, F.; Leopardi, S.; Natale, A. Synanthropic and Wild Animals as Sentinels of Zoonotic Agents: A Study of Leptospira Genotypes Circulating in Northeastern Italy. Int. J. Environ. Res. Public Health 2023, 20, 3783. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.S.; Pinto, P.S.; Lilenbaum, W. A systematic review of leptospirosis on wild animals in Latin America. Trop. Anim. Health Prod. 2018, 50, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, E.; Radaelli, E.; Bertoletti, I.; Bianchi, A.; Scanziani, E.; Tagliabue, S.; Mattiello, S. Leptospira spp. infection in wild ruminants: A survey in Central Italian Alps. Vet. Ital. 2014, 50, 285–291. [Google Scholar] [PubMed]
- Cilia, G.; Bertelloni, F.; Fratini, F. Leptospira Infections in Domestic and Wild Animals. Pathogens 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Espí, A.; Prieto, J.M.; Alzaga, V. Leptospiral antibodies in Iberian red deer (Cervus elaphus hispanicus), fallow deer (Dama dama) and European wild boar (Sus scrofa) in Asturias, Northern Spain. Vet. J. 2010, 183, 226–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roug, A.; Swift, P.; Torres, S.; Jones, K.; Johnson, C.K. Serosurveillance for Livestock Pathogens in Free-Ranging Mule Deer (Odocoileus hemionus). PLoS ONE 2012, 7, e50600. [Google Scholar] [CrossRef] [PubMed]
- Cantu, A.; Ortega-S, J.A.; Mosqueda, J.; Garcia-Vazquez, Z.; Henke, S.E.; George, J.E. Prevalence of Infectious Agents in Free-ranging White-tailed Deer in Northeastern Mexico. J. Wildl. Dis. 2008, 44, 1002–1007. [Google Scholar] [CrossRef]
- Mathias, L.A.; Girio, R.J.S.; Duarte, J.M.B. Serosurvey for Antibodies against Brucella abortus and Leptospira interrogans in Pampas Deer from Brazil. J. Wildl. Dis. 1999, 35, 112–114. [Google Scholar] [CrossRef]
- Vieira, A.S.; Rosinha, G.M.S.; de Oliveira, C.E.; Vasconcellos, S.A.; Lima-Borges, P.A.; Tomás, W.M.; Mourão, G.M.; Lacerda, A.C.R.; Soares, C.O.; de Araújo, F.R.; et al. Survey of Leptospira spp in pampas deer (Ozotoceros bezoarticus) in the Pantanal wetlands of the state of Mato Grosso do Sul, Brazil by serology and polymerase chain reaction. Mem. Inst. Oswaldo Cruz 2011, 106, 763–768. [Google Scholar] [CrossRef]
- Zimpel, C.K.; Grazziotin, A.L.; Filho, I.R.d.B.; Guimaraes, A.M.d.S.; dos Santos, L.C.; de Moraes, W.; Cubas, Z.S.; de Oliveira, M.J.; Pituco, E.M.; Lara, M.D.C.C.d.S.H.; et al. Occurrence of antibodies anti-Toxoplasma gondii, Neospora caninum and Leptospira interrogans in a captive deer herd in Southern Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 482–487. [Google Scholar] [CrossRef]
- Aguirre, A.; Hansen, D.E.; Starkey, E.E.; McLean, R.G. Serologic survey of wild cervids for potential disease agents in selected national parks in the United States. Prev. Vet. Med. 1995, 21, 313–322. [Google Scholar] [CrossRef]
- Bahnson, C.S.; Grove, D.M.; Maskey, J.J.J.; Smith, J.R. Exposure to Select Pathogens in an Expanding Moose (Alces alces) Population in North Dakota, USA. J. Wildl. Dis. 2021, 57, 648–651. [Google Scholar] [CrossRef] [PubMed]
- New, J.C.; Wathen, W.G.; Dlutkowski, S. Prevalence of leptospira antibodies in white-tailed deer, cades cove, great smoky mountains national park, Tennessee, USA. J. Wildl. Dis. 1993, 29, 561–567. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedersen, K.; Anderson, T.D.; Maison, R.M.; Wiscomb, G.W.; Pipas, M.J.; Sinnett, D.R.; Baroch, J.A.; Gidlewski, T. Leptospira antibodies detected in wildlife in the USA and the US Virgin Islands. J. Wildl. Dis. 2018, 54, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.M.; Mech, L.D.; Nelson, M.E. Prevalence of Antibody Titers to Leptospira Spp. in Minnesota White-tailed Deer. J. Wildl. Dis. 1992, 28, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.N.; DePerno, C.S.; Jenks, J.A.; Stoskopf, M.K.; Kennedy-Stoskopf, S.; Swanson, C.C.; Brinkman, T.J.; Osborn, R.G.; Tardiff, J.A. Selenium Status and Antibodies to Selected Pathogens in White-tailed Deer (Odocoileus virginianus) in Southern Minnesota. J. Wildl. Dis. 2008, 44, 181–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zmudzki, J.; Jablonski, A.; Arent, Z.; Zebek, S.; Nowak, A.; Stolarek, A.; Parzeniecka-Jaworska, M. First report of Leptospira infections in red deer, roe deer, and fallow deer in Poland. J. Vet. Res. 2016, 60, 257–260. [Google Scholar] [CrossRef]
- Žele-Vengušt, D.; LindtnerKnific, R.; Mlakar-Hrženjak, N.; Jerina, K.; Vengust, G. Exposure of free-ranging wild animals to zoonotic Leptospira interrogans sensu stricto in Slovenia. Animals 2021, 11, 2722. [Google Scholar] [CrossRef]
- Slavica, A.; Cvetnić, Ž.; Milas, Z.; Janicki, Z.; Turk, N.; Konjevic, D.; Severin, K.; Toncic, J.; Lipej, Z. Incidence of leptospiral antibodies in different game species over a 10-year period (1996–2005) in Croatia. Eur. J. Wildl. Res. 2008, 54, 305–311. [Google Scholar] [CrossRef]
- Grégoire, F.; Bakinahe, R.; Petitjean, T.; Boarbi, S.; Delooz, L.; Fretin, D.; Saulmont, M.; Mori, M. Laboratory Diagnosis of Bovine Abortions Caused by Non-Maintenance Pathogenic Leptospira spp.: Necropsy, Serology and Molecular Study Out of a Belgian Experience. Pathogens 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Montes, V.; Monti, G. Pathogenic Leptospira spp. Seroprevalence and Herd-Level Risk Factors Associated with Chilean Dairy Cattle. Animals 2021, 11, 3148. [Google Scholar] [CrossRef] [PubMed]
- Ayanegui-Alcerreca, M.; Wilson, P.; Mackintosh, C.; Collins-Emerson, J.; Heuer, C.; Midwinter, A.; Castillo-Alcala, F. Leptospirosis in farmed deer in New Zealand: A review. N. Z. Vet. J. 2007, 55, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Vilcek, S.; Nettleton, P.F. Pestiviruses in wild animals. Vet. Microbiol. 2006, 116, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marco, I.; Cabezón, O.; Velarde, R.; Fernandez-Sirera, L.; Colom-Cadena, A.; Serrano, E.; Rosell, R.; Cases-Diaz, E. The two sides of border disease in pyrenean chamois (Rupicapra pyrenaica): Silent persistence and population collapse. Anim. Health Res. Rev. 2015, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wang, Q.; Liu, H.-Y.; Li, L.-M.; Tian, T.; Yin, J.-Y.; Zheng, W.; Ma, Q.-X.; Wang, T.-T.; Li, T.; et al. Prevalence of bovine viral diarrhea virus in cattle between 2010 and 2021: A global systematic review and meta-analysis. Front. Vet. Sci. 2023, 9, 1086180. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Neill, J.D. Challenges in Identifying and Determining the Impacts of Infection with Pestiviruses on the Herd Health of Free Ranging Cervid Populations. Front. Microbiol. 2016, 7, 921. [Google Scholar] [CrossRef]
- Kirchgessner, M.S.; Dubovi, E.J.; Whipps, C.M. Spatial point pattern analyses of Bovine viral diarrhea virus infection in domestic livestock herds and concomitant seroprevalence in wild white-tailed deer (Odocoileus virginianus) in New York State, USA. J. Vet. Diagn. Investig. 2013, 25, 226–233. [Google Scholar] [CrossRef]
- Huaman, J.L.; Pacioni, C.; Forsyth, D.M.; Pople, A.; Hampton, J.O.; Carvalho, T.G.; Helbig, K.J. Serosurveillance and Molecular Investigation of Wild Deer in Australia Reveals Seroprevalence of Pestivirus Infection. Viruses 2020, 12, 752. [Google Scholar] [CrossRef]
- Frölich, K. Bovine Virus Diarrhea and Mucosal Disease in Free-ranging and aptive Deer (Cervidae) in Germany. J. Wildl. Dis. 1995, 31, 247–250. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, V.; Kukielka, D.; Rivera-Arroyo, B.; Martínez-López, B.; Heras, A.I.d.L.; Sánchez-Vizcaíno, J.M.; Vicente, J. Evidence of shared bovine viral diarrhea infections between red deer and extensively raised cattle in south-central Spain. BMC Vet. Res. 2016, 12, 11. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; López-Olvera, J.R.; Marco, I.; Rosell, R.; Colom-Cadena, A.; Soto-Heras, S.; Lavín, S.; Cabezón, O. Pestivirus in alpine wild ruminants and sympatric livestock from the Cantabrian Mountains, Spain. Vet. Rec. 2016, 178, 586. [Google Scholar] [CrossRef]
- Lillehaug, A.; Vikøren, T.; Larsen, I.-L.; Åkerstedt, J.; Tharaldsen, J.; Handeland, K. Antibodies to ruminant alpha-herpesviruses and pestiviruses in Norwegian cervids. J. Wildl. Dis. 2003, 39, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, V.; Nebel, L.; Schüpbach-Regula, G.; Zanoni, R.G.; Schweizer, M. Influence of border disease virus (BDV) on serological surveillance within the bovine virus diarrhea (BVD) eradication program in Switzerland. BMC Vet. Res. 2017, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Lucero, J.; Celedón, M.-O.; Aguilera, M.; Decalisto, A. Molecular characterization of pestiviruses isolated from bovines in Chile. Vet. Microbiol. 2006, 115, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Donoso, A.; Inostroza, F.; Celedón, M.; Pizarro-Lucero, J. Genetic diversity of Bovine Viral Diarrhea Virus from cattle in Chile between 2003 and 2007. BMC Vet. Res. 2018, 14, 314. [Google Scholar] [CrossRef]
- Pizarro-Lucero, J.; Celedón, M.O.; Navarro, C.; Ortega, R.; González, D. Identification of a pestivirus isolated from a free-ranging pudu (Pudu puda) in Chile. Vet. Rec. 2005, 157, 292–294. [Google Scholar] [CrossRef]
- Righi, C.; Petrini, S.; Pierini, I.; Giammarioli, M.; De Mia, G.M. Global Distribution and Genetic Heterogeneity of Border Disease Virus. Viruses 2021, 13, 950. [Google Scholar] [CrossRef]
- Alocilla, O.A.; Monti, G. Bovine Viral Diarrhea Virus within and herd prevalence on pasture-based dairy systems, in southern Chile dairy farms. Prev. Vet. Med. 2022, 198, 105533. [Google Scholar] [CrossRef] [PubMed]
- Passler, T.; Ditchkoff, S.S.; Walz, P.H. Bovine Viral Diarrhea Virus (BVDV) in White-Tailed Deer (Odocoileus virginianus). Front. Microbiol. 2016, 7, 945. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Dubey, J.P. Toxoplasmosis in wild and domestic animals. In Toxoplasma gondii, 3rd ed.; Weiss, L.M., Kim, K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 293–320. [Google Scholar] [CrossRef]
- Fisk, E.A.; Cassirer, E.F.; Huggler, K.S.; Pessier, A.P.; White, L.A.; Ramsay, J.D.; Goldsmith, E.W.; Drankhan, H.R.; Wolking, R.M.; Manlove, K.R.; et al. Abortion and neonatal mortality due to Toxoplasma gondii in bighorn sheep (Ovis canadensis). J. Wildl. Dis. 2023, 59, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Sharif, M.; Sarvi, S.; Mirzaei, N.; Abediankenari, S.; Arefkhah, N.; Amouei, A.; Gholami, S.; Anvari, D.; Ahmadpour, E.; et al. Identification and multilocus genotyping of Toxoplasma gondii isolates from congenital infection in north of Iran. Parasitol. Res. 2023, 122, 177–184. [Google Scholar] [CrossRef] [PubMed]
- de Barros, R.A.M.; Torrecilhas, A.C.; Marciano, M.A.M.; Mazuz, M.L.; Pereira-Chioccola, V.L.; Fux, B. Toxoplasmosis in Human and Animals Around the World. Diagnosis and Perspectives in the One Health Approach. Acta Trop. 2022, 231, 106432. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H. Epidemiologic and Public Health Significance of Toxoplasma gondii Infections in Venison: 2009–2020. J. Parasitol. 2021, 107, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Verma, S.K.; Kwok, O.C.H.; Pedersen, K.; Rosenthal, B.M.; Su, C. White-tailed deer (Odocoileus virginianus) are a reservoir of a diversity of Toxoplasma gondii strains in the USA and pose a risk to consumers of undercooked venison. Parasitology 2020, 147, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Gong, Q.-L.; Wang, Q.; Wang, C.-R.; Zhang, X.-X. The global seroprevalence of Toxoplasma gondii in deer from 1978 to 2019: A systematic review and meta-analysis. Acta Trop. 2020, 208, 105529. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Battisti, E.; Zanet, S.; Trisciuoglio, A.; Ferroglio, E. A systematic review and meta-analysis of Toxoplasma gondii in roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Europe. Zoonoses Public Health 2021, 68, 182–193. [Google Scholar] [CrossRef]
- Sepúlveda, M.A.; Muñoz-Zanzi, C.; Rosenfeld, C.; Jara, R.; Pelican, K.M.; Hill, D. Toxoplasma gondii in feral American minks at the Maullín river, Chile. Vet. Parasitol. 2011, 175, 60–65. [Google Scholar] [CrossRef]
- Munoz-Zanzi, C.; Campbell, C.; Berg, S. Seroepidemiology of toxoplasmosis in rural and urban communities from Los Rios Region, Chile. Infect. Ecol. Epidemiol. 2016, 6, 30597. [Google Scholar] [CrossRef]
- Turin, L.; Surini, S.; Wheelhouse, N.; Rocchi, M.S. Recent advances and public health implications for environmental exposure to Chlamydia abortus: From enzootic to zoonotic disease. Vet. Res. 2022, 53, 37. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.; Ding, H.; Zeng, H. Septic shock with Chlamydia abortus infection. Lancet Infect. Dis. 2022, 22, 912. [Google Scholar] [CrossRef] [PubMed]
- Burnard, D.; Polkinghorne, A. Chlamydial infections in wildlife–conservation threats and/or reservoirs of ‘spill-over’ infections? Vet. Microbiol. 2016, 196, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.; Caro, M.; Vicente, J.; Cuello, F.; Reyes-Garcia, A.; Buendía, A.; Rodolakis, A.; Gortázar, C. High prevalence of antibodies against Chlamydiaceae and Chlamydophila abortus in wild ungulates using two “in house” blocking-ELISA tests. Vet. Microbiol. 2009, 135, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, L.A.; Pinedo, M.F.A.; Origlia, J.; Fernández, G.; Uzal, F.A.; Travería, G.E. First report of caprine abortions due to Chlamydia abortus in Argentina. Vet. Med. Sci. 2019, 5, 162–167. [Google Scholar] [CrossRef]
- Baldini, M.H.M.; Rosa, J.C.C.; Matos, A.C.D.; Cubas, Z.S.; Guedes, M.I.M.C.; de Moraes, W.; de Oliveira, M.J.; Felippi, D.A.; Lobato, Z.I.P.; de Moraes, A.N. Multiple bluetongue virus serotypes causing death in Brazilian dwarf brocket deer (Mazama nana) in Brazil, 2015–2016. Vet. Microbiol. 2018, 227, 143–147. [Google Scholar] [CrossRef]
- Favero, C.M.; Matos, A.C.D.; Campos, F.S.; Candino, M.V.; Costa, E.A.; Heinemann, M.B.; Barbosa-Stancioli, E.F.; Lobato, Z.I.P. Epizootic hemorrhagic disease in brocket deer, Brazil. Emerg. Infect. Dis. 2013, 19, 346–348. [Google Scholar] [CrossRef]
- Lima, D.A.R.; Zimpel, C.K.; Patané, J.S.; Silva-Pereira, T.T.; Etges, R.N.; Rodrigues, R.A.; Davila, A.M.R.; Ikuta, C.Y.; Neto, J.S.F.; Guimaraes, A.M.S.; et al. Genomic analysis of an outbreak of bovine tuberculosis in a man-made multi-host species system: A call for action on wildlife in Brazil. Transbound. Emerg. Dis. 2022, 69, 580–591. [Google Scholar] [CrossRef]
- Munson, L.; Cook, R.A. Monitoring, investigation, and surveillance of diseases in captive wildlife. J. Zoo Wildl. Med. 1993, 24, 281–290. [Google Scholar]
- Donahoe, S.L.; Lindsay, S.A.; Krockenberger, M.; Phalen, D.; Šlapeta, J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 2015, 4, 216–238. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.; Shams, F.; Rahmanian, V.; Farhoodi, M.; Nadali, B.; Raziee, Y. The global seroprevalence of Neospora caninum infection in deer: A systematic review and meta-analysis study. Small Rumin. Res. 2022, 214, 106745. [Google Scholar] [CrossRef]
- Moore, D. Neosporosis in South America. Vet. Parasitol. 2005, 127, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.P.; Moré, G.; Urtizbiría, F.; Hecker, Y.P.; Cirone, K.M.; Scioli, M.V.; Paolicchi, F.A.; Fiorentino, M.A.; Uriarte, E.L.L.; Cantón, G.J.; et al. Epidemic abortions due to Neospora caninum infection in farmed red deer (Cervus elaphus). Parasitol. Res. 2022, 121, 1475–1485. [Google Scholar] [CrossRef]
- Basso, W.; Moré, G.; Quiroga, M.A.; Balducchi, D.; Schares, G.; Venturini, M.C. Neospora caninum is a cause of perinatal mortality in axis deer (Axis axis). Vet. Parasitol. 2014, 199, 255–258. [Google Scholar] [CrossRef]
- Formenti, N.; Trogu, T.; Pedrotti, L.; Gaffuri, A.; Lanfranchi, P.; Ferrari, N. Toxoplasma gondii Infection in Alpine Red Deer (Cervus elaphus): Its Spread and Effects on Fertility. PLoS ONE 2015, 10, e0138472. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lewis, B.; Beam, K.; Abbitt, B. Transplacental toxoplasmosis in a reindeer (Rangifer tarandus) fetus. Vet. Parasitol. 2002, 110, 131–135. [Google Scholar] [CrossRef]
- Denk, D.; De Neck, S.; Khaliq, S.; Stidworthy, M.F. Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases. Animals 2022, 12, 619. [Google Scholar] [CrossRef]
- Dubey, J.P. Clinical toxoplasmosis in zoo animals and its management. Emerg. Anim. Species 2022, 2, 100002. [Google Scholar] [CrossRef]
- Tonin, A.A.; Azevedo, M.I.; Silva, A.S.; dos Santos, L.G.; de Moura, J., Jr.; Rodrigues Martins, J.L.; Schaefer, P.C.; Telles Badke, M.R. Infection in the pampas deer (Ozotoceros bezoarticus) by four serotypes of Leptospira interrogans. Comp. Clin. Pathol. 2011, 20, 267–268. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Neill, J.D.; Chase, C.C.L. Impact of Bvdv infection of white-tailed deer during second and third trimesters of pregnancy. J. Wildl. Dis. 2012, 48, 758–762. [Google Scholar] [CrossRef]
- Rola, J.; Larska, M.; Socha, W.; Rola, J.G.; Materniak, M.; Urban-Chmiel, R.; Thiry, E.; Żmudziński, J.F. Seroprevalence of bovine herpesvirus 1 related alphaherpesvirus infections in free-living and captive cervids in Poland. Vet. Microbiol. 2017, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Roundy, C.M.; Nunez, C.M.; Thomas, L.F.; Auckland, L.D.; Tang, W.; Richison, J.J.; Green, B.R.; Hilton, C.D.; Cherry, M.J.; Pauvolid-Corrêa, A.; et al. High Seroprevalence of SARS-CoV-2 in White-Tailed Deer (Odocoileus virginianus) at One of Three Captive Cervid Facilities in Texas. Microbiol. Spectr. 2022, 10, e0057622. [Google Scholar] [CrossRef] [PubMed]
- Holding, M.; Otter, A.D.; Dowall, S.; Takumi, K.; Hicks, B.; Coleman, T.; Hemingway, G.; Royds, M.; Findlay-Wilson, S.; Curran-French, M.; et al. Screening of wild deer populations for exposure to SARS-CoV-2 in the United Kingdom, 2020–2021. Transbound. Emerg. Dis. 2022, 69, e3244–e3249. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, A.C.; Vieira-Pinto, M. 15 years overview of European zoonotic surveys in wild boar and red deer: A systematic review. One Health 2023, 16, 100519. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Hermoso, E.; Sepúlveda-García, P.; Cabello, J.; Celis, S.; Valencia, C.; Ortiz, C.; Kemec, I.; Moreira-Arce, D.; Orsola, M.; Canales, N.; et al. Molecular survey and phylogenetic analysis of Bartonella sp., Coxiella sp., and hemoplamas in pudu (Pudu puda) from Chile: First report of Bartonella henselae in a wild ungulate species. Front. Vet. Sci. 2023, 10, 1161093. [Google Scholar] [CrossRef]
- Nava, M.; Corlatti, L.; Formenti, N.; Trogu, T.; Pedrotti, L.; Gugiatti, A.; Lanfranchi, P.; Luzzago, C.; Ferrari, N. Parasite-mediated manipulation? Toxoplasma gondii infection increases risk behaviour towards culling in red deer. Biol. Lett. 2023, 19, 20230292. [Google Scholar] [CrossRef]
- Böhm, M.; White, P.C.; Chambers, J.; Smith, L.; Hutchings, M. Wild deer as a source of infection for livestock and humans in the UK. Vet. J. 2007, 174, 260–276. [Google Scholar] [CrossRef]
| Pathogen | Test | References |
|---|---|---|
| Bluetongue virus | INgezim BTV DR (Gold Standard Diagnostics, Madrid, Spain) | [27] |
| Pestivirus | VNT and INgezim Pestivirus Compac (Gold Standard Diagnostics, Madrid, Spain) | [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] |
| Chlamydia abortus | ID Screen® Chlamydophila abortus Indirect Multi-species (IDvet, Grabels, France) and ELISA CHEKIT Chlamydophila abortus Antibody Test Kit, IDEXX Laboratories, Bern, Switzerland | [29] |
| Coxiella burnetii | PrioCHECK™ Ruminant Q Fever Ab Plate Kit (ThermoFisher Scientific, Waltham, MA, USA) and ELISA CHEKIT Q-Fever (Coxiella burnetii) Antibody Test Kit, IDEXX Laboratories, Bern, Switzerland | [30,31] |
| Toxoplasma gondii | ID Screen® Toxoplasmosis Indirect Multi-species (IDvet, Grabels, France) | [32] |
| Epizootic Hemorrhagic Disease Virus | ID Screen® EHDV Competition (IDvet, Grabels, France) | [33,34] |
| Brucella abortus | Rose Bengal test (Bengatestt, Parsippany, NJ, USA) and C ELISA (SVANOVIRt Brucella Antibody Test, SVANOVA, Uppsala, Sweden) | [35,36] |
| Leptospira interrogans | MAT (Pomona, Grippotyphosa, Copenhageni, Hardjo, Canicola) | [37,38] |
| Mycobacterium bovis | in-house P22 ELISA | [39] |
| Bovine Herpesvirus-1 | VNT | [40] |
| SARS-CoV-2 | VNT | [41] |
| Hepatitis E | ELISA | [42] |
| Neospora caninum | ELISA (CHEKIT Neospora caninum Antibody Test Kit, IDEXX Laboratories, Bern, Switzerland) | [43] |
| Pathogen | n | Captive * | Free-Ranging * | Differences 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Pestivirus | 145 | 3/45 (6.67%) | 8/100 (8.00%) | −0.0984 | 0.0889 | 0.384 |
| Leptospira spp. | 93 | 3/28 (10.71%) | 10/65 (15.38%) | −0.2165 | 0.1231 | 0.787 |
| Toxoplasma gondii | 67 | 12/32 (37.50%) | 3/35 (8.57%) | 0.0677 | 0.5109 | <0.001 |
| Neospora caninum | 32 | 2/21 (9.52%) | 0/11 (0.00%) | −0.0996 | 0.2901 | 0.7731 |
| Chlamydia abortus | 72 | 5/39 (12.82%) | 1/33 (3.03%) | −0.0502 | 0.2460 | 0.2847 |
| Bluetongue virus | 60 | 0/26 (0.00%) | 0/34 (0.00%) | - | - | |
| SARS-CoV-2 | 17 | - | 0/17 (0.00%) | - | - | |
| Hepatitis E virus | 20 | - | 0/20 (0.00%) | - | - | |
| Coxiella burneti | 74 | 0/35 (0.00%) | 0/39 (0.00%) | - | - | |
| Brucella abortus | 73 | 0/31 (0.00%) | 0/42 (0.00%) | - | - | |
| BoHV-1 | 86 | 0/47 (0.00%) | 0/39 (0.00%) | - | - | |
| EHDV | 60 | 0/26 (0.00%) | 0/34 (0.00%) | - | - | |
| Model | Variable | Categories | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Leptospira spp. | (Intercept) | 0 | 0.138 | 0.066 | 0.289 | |
| Age | Adult | Reference | ||||
| Fawn | 0.028 | 7.25 | 1.244 | 42.257 | ||
| Juvenile | 0.649 | 0.604 | 0.069 | 5.29 | ||
| Indeterminate | 0.176 | 7.25 | 0.412 | 127.7 | ||
| Toxoplasma gondii | (Intercept) | 0.213 | 0.632 | 0.307 | 1.301 | |
| Condition | Under human care | Reference | ||||
| Free-range | 0.007 | 0.148 | 0.037 | 0.594 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Hermoso, E.; Verasay Caviedes, S.; Pizarro-Lucero, J.; Cabello, J.; Vicencio, R.; Celis, S.; Ortiz, C.; Kemec, I.; Abuhadba-Mediano, N.; Asencio, R.; et al. High Exposure to Livestock Pathogens in Southern Pudu (Pudu puda) from Chile. Animals 2024, 14, 526. https://doi.org/10.3390/ani14040526
Hidalgo-Hermoso E, Verasay Caviedes S, Pizarro-Lucero J, Cabello J, Vicencio R, Celis S, Ortiz C, Kemec I, Abuhadba-Mediano N, Asencio R, et al. High Exposure to Livestock Pathogens in Southern Pudu (Pudu puda) from Chile. Animals. 2024; 14(4):526. https://doi.org/10.3390/ani14040526
Chicago/Turabian StyleHidalgo-Hermoso, Ezequiel, Sebastián Verasay Caviedes, Jose Pizarro-Lucero, Javier Cabello, Rocio Vicencio, Sebastián Celis, Carolina Ortiz, Ignacio Kemec, Nour Abuhadba-Mediano, Ronie Asencio, and et al. 2024. "High Exposure to Livestock Pathogens in Southern Pudu (Pudu puda) from Chile" Animals 14, no. 4: 526. https://doi.org/10.3390/ani14040526
APA StyleHidalgo-Hermoso, E., Verasay Caviedes, S., Pizarro-Lucero, J., Cabello, J., Vicencio, R., Celis, S., Ortiz, C., Kemec, I., Abuhadba-Mediano, N., Asencio, R., Vera, F., Valencia, C., Lagos, R., Moreira-Arce, D., Salinas, F., Ramirez-Toloza, G., Muñoz-Quijano, R., Neira, V., Salgado, R., ... Ruiz-Fons, F. (2024). High Exposure to Livestock Pathogens in Southern Pudu (Pudu puda) from Chile. Animals, 14(4), 526. https://doi.org/10.3390/ani14040526







