Addressing Challenges in Wildlife Rehabilitation: Antimicrobial-Resistant Bacteria from Wounds and Fractures in Wild Birds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Culture and Isolation
2.3. Bacterial Identification
2.4. Antimicrobial Susceptibility Test
3. Results
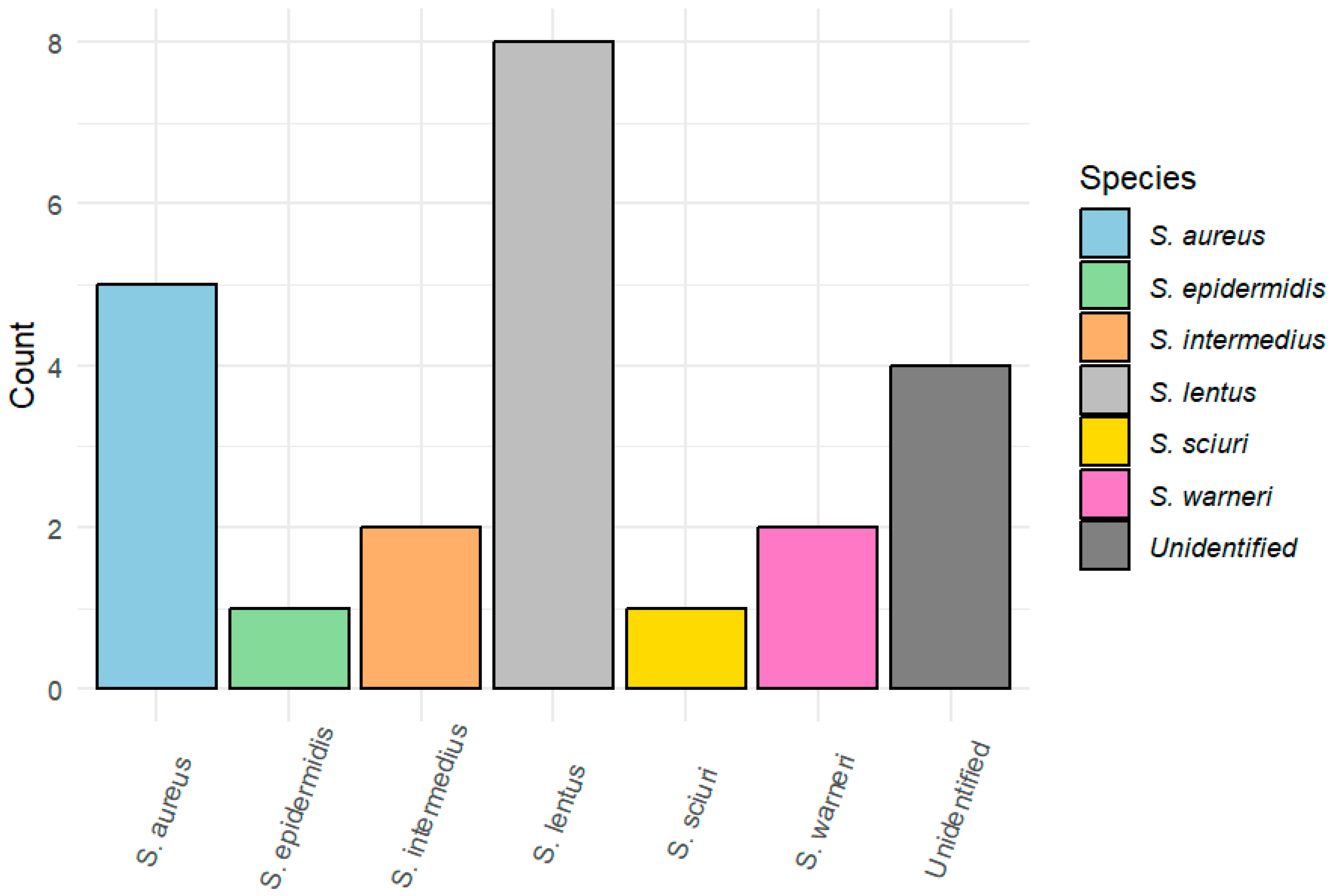
3.1. Bacterial Isolation and Identification
3.2. Antimicrobial Susceptibility Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN. The IUCN Red List of Threatened Species. Version 2023-1. 2023. Available online: https://www.iucnredlist.org/ (accessed on 16 February 2024).
- Burns, F.; Eaton, M.A.; Burfield, I.J.; Klvaňová, A.; Šilarová, E.; Staneva, A.; Gregory, R.D. Abundance decline in the avifauna of the European Union reveals cross-continental similarities in biodiversity change. Ecol. Evol. 2021, 11, 16647–16660. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Espinoza, A.; Sallaberry-Pincheira, N.; Napolitano, C. A five-year retrospective study on patterns of casuistry and insights on the current status of wildlife rescue and rehabilitation centers in Chile. Rev. Chil. Hist. Nat. 2019, 92, 6. [Google Scholar] [CrossRef]
- Punch, P. A retrospective study of the success of medical and surgical treatment of wild Australian raptors. Aust. Veter. J. 2001, 79, 747–752. [Google Scholar] [CrossRef]
- Cococcetta, C.; Coutant, T.; Collarile, T.; Vetere, A.; Di Ianni, F.; Huynh, M. Causes of Raptor Admission to the Wildlife Rehabilitation Centre in Abruzzo (Central Italy) from 2005–2016. Animals 2022, 12, 1916. [Google Scholar] [CrossRef] [PubMed]
- Vezyrakis, A.; Bontzorlos, V.; Rallis, G.; Ganoti, M. Two decades of wildlife rehabilitation in Greece: Major threats, admission trends and treatment outcomes from a prominent rehabilitation centre. J. Nat. Conserv. 2023, 73, 126372. [Google Scholar] [CrossRef]
- Molina-López, R.A.; Mañosa, S.; Torres-Riera, A.; Pomarol, M.; Darwich, L. Morbidity, outcomes and cost-benefit analysis of wildlife rehabilitation in Catalonia (Spain). PLoS ONE 2017, 12, e0181331. [Google Scholar] [CrossRef] [PubMed]
- Molina-López, R.A.; Casal, J.; Darwich, L. Causes of Morbidity in Wild Raptor Populations Admitted at a Wildlife Rehabilitation Centre in Spain from 1995–2007: A Long Term Retrospective Study. PLoS ONE 2011, 6, e24603. [Google Scholar] [CrossRef]
- Durá Alemañ, C.J.; Morales-Reyes, Z.; Ayerza, P.; Bodega, D.D.L.; Aguilera-Alcalá, N.; Botella, F.; Jiménez, J.; López-Bao, J.V.; Mateo-Tomás, P.; Moleón, M.; et al. La responsabilidad por el daño ambiental generado en el caso de la lucha contra el uso del veneno en España. Actual. Jurídica Ambient. 2020, 102/2, 564–576. Available online: http://hdl.handle.net/10261/217609 (accessed on 4 November 2023).
- Sullivan, T.N.; Wang, B.; Espinosa, H.D.; Meyers, M.A. Extreme lightweight structures: Avian feathers and bones. Mater. Today 2017, 20, 377–391. [Google Scholar] [CrossRef]
- Vigneault, A.; Fitzgerald, G.; Desmarchelier, M. A retrospective study of femoral fractures in wild birds of prey: 119 cases. J. Zoo Wildl. Med. 2021, 52, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Vergneau-Grosset, C.; Dubé, C.; Fitzgerald, G.; Lair, S. Characteristics of antebrachial fractures associated with a successful outcome among free-ranging birds of prey that received treatment in a rehabilitation program. J. Am. Veter. Med. Assoc. 2020, 256, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Tardón, A.; Bataller, E.; Llobat, L.; Jiménez-Trigos, E. Bacteria and antibiotic resistance detection in fractures of wild birds from wildlife rehabilitation centres in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101575. [Google Scholar] [CrossRef] [PubMed]
- Bertuccelli, T.; Crosta, L.; Costa, G.L.; Schnitzer, P.; Sawmy, S.; Spadola, F. Predisposing Anatomical Factors of Humeral Fractures in Birds of Prey: A Preliminary Tomographic Comparative Study. J. Avian Med. Surg. 2021, 35, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Cousquer, G. Wound Management in the Avian Wildlife Casualty. World Wide Wounds. November 2003b. 2003. Available online: http://www.worldwidewounds.com/2003/november/Cousquer/Avian-Wound-Management-Part-2.html (accessed on 4 November 2023).
- Scott, D. Natural history and medical management of raptors. In Medical Management of Wildlife Species: A Guide for Practitioners; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 215–228. [Google Scholar] [CrossRef]
- Hofstee, M.I.; Muthukrishnan, G.; Atkins, G.J.; Riool, M.; Thompson, K.; Morgenstern, M.; Stoddart, M.J.; Richards, R.G.; Zaat, S.A.; Moriarty, T.F. Current concepts of osteomyelitis: From pathologic mechanisms to advanced research methods. Am. J. Pathol. 2020, 190, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Lopes, A.F.; Soeiro, V.; Caniça, M.; Manageiro, V.; Pereira, J.E.; Maltez, L.; Capelo, J.L.; Igrejas, G.; Poeta, P. Nocturnal Birds of Prey as Carriers of Staphylococcus aureus and Other Staphylococci: Diversity, Antimicrobial Resistance and Clonal Lineages. Antibiotics 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Martín-Maldonado, B.; Montoro-Dasi, L.; Pérez-Gracia, M.T.; Jordá, J.; Vega, S.; Marco-Jiménez, F.; Marin, C. Wild Bonelli’s eagles (Aquila fasciata) as carrier of antimicrobial resistant Salmonella and Campylobacter in Eastern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101372. [Google Scholar] [CrossRef]
- Gambino, D.; Vicari, D.; Vitale, M.; Schirò, G.; Mira, F.; La Giglia, M.; Riccardi, A.; Gentile, A.; Giardina, S.; Carrozzo, A.; et al. Study on bacteria isolates and antimicrobial resistance in wildlife in Sicily, southern Italy. Microorganisms 2021, 9, 203. [Google Scholar] [CrossRef]
- Arnold, K.E.; Williams, N.J.; Bennett, M. «Disperse abroad in the land»: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef] [PubMed]
- Martín-Maldonado, B.; Rodríguez-Alcázar, P.; Fernández-Novo, A.; González, F.; Pastor, N.; López, I.; Suárez, L.; Moraleda, V.; Aranaz, A. Urban Birds as Antimicrobial Resistance Sentinels: White Storks Showed Higher Multidrug-Resistant Escherichia coli Levels than Seagulls in Central Spain. Animals 2022, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Ramey, A.M.; Ahlstrom, C.A. Antibiotic Resistant Bacteria in Wildlife: Perspectives on Trands, Acquisition and Dissemination, Data GAPS, and Future Directions. J. Wildl. Dis. 2020, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gorman, R.; Adley, C. An evaluation of five preservation techniques and conventional freezing temperatures of −20 °C and −85 °C for long-term preservation of Campylobacter jejuni. Lett. Appl. Microbiol. 2004, 38, 306–310. [Google Scholar] [CrossRef]
- BOE. Boletín Oficial del Estado, Real Decreto 53/2013, de 1 de Febrero, por el que se Establecen las Normas Básicas Aplicables para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia; BOE: Madrid, Spain, 2013; Available online: https://www.boe.es/eli/es/rd/2013/02/01/53/con (accessed on 4 November 2023).
- Markey, B.; Leonard, F.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology, 2nd ed.; Mosby Elsevier Ltd.: Dublin, Ireland, 2013; ISBN 9780723432371. [Google Scholar]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Microbiol. Infect. Dis. 2014, 20, 255–266. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing (EUCAST), Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11. 2021. Available online: http://www.eucast.org (accessed on 2 April 2024).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Chen, L.F.; Fowler, V.G., Jr. Colonization, pathogenicity, host susceptibility, and therapeutics for Staphylococcus aureus: What is the clinical relevance? Semin. Immunopathol. 2012, 34, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Kutkowska, J.; Turska-Szewczuk, A.; Kucharczyk, M.; Kucharczyk, H.; Zalewska, J.; Urbanik-Sypniewska, T. Methicillin-resistant Staphylococcus aureus and glycopeptide-resistant enterococci in fecal samples of birds from South-Eastern Poland. BMC Veter. Res. 2019, 15, 472. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; Abdullahi, I.N.; González-Azcona, C.; Ulloa, A.; Martínez, A.; García-Vela, S.; Höfle, U.; Zarazaga, M.; Lozano, C.; Torres, C. Detection of antimicrobial producing Staphylococcus from migratory birds: Potential role in nasotracheal microbiota modulation. Front. Microbiol. 2023, 14, 1144975. [Google Scholar] [CrossRef]
- Tóth, A.G.; Tóth, I.; Rózsa, B.; Dubecz, A.; Patai, Á.V.; Németh, T.; Kaplan, S.; Kovács, E.G.; Makrai, L.; Solymosi, N. Canine saliva as a possible source of antimicrobial resistance genes. Antibiotics 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Wirth, J.S.; Saravanan, V.S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 5926–5936. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Gómez, P.; Alonso, C.A.; Camacho, M.C.; Ramiro, Y.; de la Puente, J.; Fernández-Fernández, R.; Quevedo, M.; Blanco, J.M.; Báguena, G.; et al. Frequency and Characterization of Antimicrobial Resistance and Virulence Genes of Coagulase-Negative Staphylococci from Wild Birds in Spain. Detection of tst-Carrying S. sciuri Isolates. Microorganisms 2020, 8, 1317. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Wesołowska, M.; Krawiec, A.; Kosicki, J.Z. Staphylococcal species composition in the skin microbiota of domestic pigeons (Columba livia domestica). PLoS ONE 2023, 18, e0287261. [Google Scholar] [CrossRef] [PubMed]
- Mazal, C.; Sieger, B. Staphylococcus lentus: The troublemaker. Int. J. Infect. Dis. 2010, 14, e397. [Google Scholar] [CrossRef]
- Giorgio, A.; De Bonis, S.; Balestrieri, R.; Rossi, G.; Guida, M. The isolation and identification of bacteria on feathers of migratory bird species. Microorganisms 2018, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Mašlaňová, I.; Indráková, A.; Šiborová, M.; Mikulášek, K.; Bendíčková, K.; Plevka, P.; Vrbovská, V.; Zdráhal, Z.; Doškař, J.; et al. Staphylococcus sciuri bacteriophages double-convert for staphylokinase and phospholipase, mediate interspecies plasmid transduction, and package mecA gene. Sci. Rep. 2017, 7, 46319. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Both, A.; Weißelberg, S.; Heilmann, C.; Rohde, H. Emergence of coagulase-negative staphylococci. Expert Rev. Anti-infective Ther. 2020, 18, 349–366. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D.R. Virulence factors in coagulase-negative staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef]
- Sousa, M.; Silva, N.; Igrejas, G.; Silva, F.; Sargo, R.; Alegria, N.; Benito, D.; Gómez, P.; Lozano, C.; Gómez-Sanz, E.; et al. Antimicrobial resistance determinants in Staphylococcus spp. recovered from birds of prey in Portugal. Veter. Microbiol. 2014, 171, 436–440. [Google Scholar] [CrossRef]
- Atterby, C.; Ramey, A.M.; Hall, G.G.; Järhult, J.; Börjesson, S.; Bonnedahl, J. Increased prevalence of antibiotic-resistant E. coli in gulls sampled in South central Alaska is associated with urban environments. Infect. Ecol. Epidemiol. 2016, 6, 32334. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.A.R.; Pereira, I.A.; Rodrigues, D.P.; Siciliano, S. Staphylococcus spp. isolated from wild birds apprehended in the local illegal trade in Rio de Janeiro, Brazil, and relevance in public health. Lett. Appl. Microbiol. 2018, 67, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Huertas, A.; Costela-Ruiz, V.; García-Recio, E.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Reyes-Botella, C.; Manzano-Moreno, F. The Effect of Chlorhexidine, Amoxicillin, and Clindamycin on the Growth and Differentiation of Primary Human Osteoblasts. Int. J. Oral Maxillofac. Implant. 2022, 37, 283–288. [Google Scholar] [CrossRef]
- Martínez-Seijas, C.; Mascarós, P.; Lizana, V.; Martí-Marco, A.; Arnau-Bonachera, A.; Chillida-Martínez, E.; Cardells, J.; Selva, L.; Viana, D.; Corpa, J.M. Genomic Characterization of Staphylococcus aureus in Wildlife. Animals 2023, 13, 1064. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- Laborda, P.; Sanz-García, F.; Ochoa-Sánchez, L.E.; Gil-Gil, T.; Hernando-Amado, S.; Martínez, J.L. Wildlife and Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 873989. [Google Scholar] [CrossRef]
- Lhermie, G.; La Ragione, R.M.; Weese, J.S.; Olsen, J.E.; Christensen, J.P.; Guardabassi, L. Indications for the use of highest priority critically important antimicrobials in the veterinary sector. J. Antimicrob. Chemother. 2020, 75, 1671–1680. [Google Scholar] [CrossRef]

| Class of Antimicrobial | Antimicrobial | Code | Concentration (µg) | Supplier | Diameter Cut-Off (mm) |
|---|---|---|---|---|---|
| Penicillins | Ampicillin | AMP | 10 | Oxoid® | ≤18 |
| Quinolones | Ciprofloxacin | CIP | 5 | Oxoid® | ≤17 |
| Sulphonamides | Trimethoprim-sulfamethoxazole | SXT | 25 | Bio-Rad® | ≤14 |
| Macrolides | Erythromycin | ERY | 15 | Bio-Rad® | ≤21 |
| Lincosamides | Clindamycin | CMN | 2 | Bio-Rad® | ≤22 |
| Amphenicols | Chloramphenicol | CHL | 30 | Oxoid® | ≤12 |
| Tetracyclines | Tetracycline | TET | 30 | Oxoid® | ≤22 |
| Order | Family | Species | Number of Birds | Lession | Bacteria Isolated |
|---|---|---|---|---|---|
| Birds of prey | |||||
| Accipitriformes | Accipitridae | Black kite (Milvus migrans) | 6 | Open fracture (n = 5) | S. aureus, Bacillus spp. |
| Wound (n = 1) | S. aureus, S. sciuri, S. warneri | ||||
| Booted eagle (Aquila pennata) | 3 | Open fracture (n = 1) | Negative culture | ||
| Wound (n = 2) | S. lentus, Bacillus spp., E. coli | ||||
| Strigiformes | Strigidae | Eurasian eagle-owl (Bubo bubo) | 1 | Open fracture (n = 1) | S. lentus, S. intermedius, E. coli |
| Tawny owl (Strix aluco) | 2 | Open fracture (n = 1) | Negative culture | ||
| Wound (n = 1) | Negative culture | ||||
| Little owl (Athene noctua) | 2 | Open fracture (n = 2) | Negative culture | ||
| Aquatic birds | |||||
| Anseriformes | Anatidae | Mallard (Anas platyrhynchos) | 2 | Open fracture (n = 2) | Negative culture |
| Pelecaniformes | Ardeidae | Grey heron (Ardea cinerea) | 1 | Open fracture (n = 1) | S. lentus, Micrococcus spp., E. coli |
| Charadriiformes | Laridae | Lesser black-backed gull (Larus fuscus) | 2 | Open fracture (n = 1) | Negative culture |
| Wound (n = 1) | S. intermedius | ||||
| Urban birds | |||||
| Ciconiformes | Ciconidae | White stork (Ciconia ciconia) | 5 | Open fracture (n = 1) | Staphylococcus spp., S. lentus, Streptococcus spp., E. coli |
| Wound (n = 4) | S. lentus, S. epidemidis, S. warneri, Streptococcus spp., E. coli | ||||
| Columbiformes | Columbidae | Rock dove (Streptotelia decaopto) | 1 | Open fracture (n = 1) | Negative culture |
| Passeriformes | Turdidae | Common blackbird (Turdus merula) | 1 | Open fracture (n = 1) | Negative culture |
| Bird Species | Staphylococcus Species | ID | Zone Diameter a (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP | CIP | ERY | CMN | CHL | TET | SXT | |||
| Black kite (Milvus migrans) | S. aureus | 8 | 44 | 11 | 26 | 26 | 30 | 32 | 30 |
| 9 | 45 | 11 | 25 | 27 | 32 | 32 | 30 | ||
| 16 | 15 | 30 | 24 | 22 | 30 | 32 | 24 | ||
| 18 | 15 | 30 | 26 | 26 | 32 | 32 | 28 | ||
| S. sciuri | 17 | 27 | 31 | 28 | 18 | 30 | 37 | 26 | |
| S. warneri | 29 | 40 | 23 | 33 | 33 | 23 | 35 | 38 | |
| Booted eagle (Aquila pennata) | S. lentus | 11 | 37 | 25 | 36 | 34 | 19 | 25 | 22 |
| 13 | 11 | 30 | 14 | 0 | 25 | 18 | 25 | ||
| Eurasian eagle-owl (Bubo bubo) | S. intermedius | 1 | 29 | 42 | 27 | 28 | 34 | 23 | 32 |
| S. lentus | 2 | 25 | 34 | 29 | 30 | 30 | 26 | 30 | |
| Grey heron (Ardea cinerea) | S. lentus | 33 | 0 | 40 | 11 | 0 | 30 | 23 | 0 |
| 35 | 0 | 38 | 10 | 0 | 30 | 25 | 0 | ||
| Lesser black-backed gull (Larus fuscus) | S. intermedius | 7 | 37 | 30 | 24 | 1 | 19 | 25 | 29 |
| White stork (Ciconia ciconia) | S. aureus | 20 | 20 | 35 | 0 | 31 | 30 | 43 | 34 |
| S. epidermidis | 19 | 55 | 35 | 30 | 28 | 34 | 40 | 33 | |
| S. lentus | 3 | 17 | 40 | 10 | 0 | 30 | 25 | 26 | |
| 4 | 14 | 37 | 10 | 0 | 30 | 24 | 28 | ||
| 25 | 18 | 34 | 30 | 36 | 26 | 23 | 0 | ||
| S. warneri | 30 | 20 | 40 | 28 | 31 | 20 | 27 | 40 | |
| Staphylococcus spp. | 22 | 24 | 30 | 23 | 9 | 25 | 26 | 28 | |
| 24 | 22 | 22 | 24 | 7 | 22 | 28 | 32 | ||
| 31 | 0 | 29 | 22 | 0 | 22 | 22 | 20 | ||
| 32 | 25 | 25 | 21 | 0 | 27 | 22 | 26 | ||
| Prevalence | 39.1% (9/23) | 8.7% (2/23) | 30.4% (7/23) | 52.2% (12/23) | 0% (0/23) | 13% (3/23) | 13% (3/23) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ortiz, E.; Blanco Gutiérrez, M.d.M.; Calvo-Fernandez, C.; Mencía-Gutiérrez, A.; Pastor Tiburón, N.; Alvarado Piqueras, A.; Pablos-Tanarro, A.; Martín-Maldonado, B. Addressing Challenges in Wildlife Rehabilitation: Antimicrobial-Resistant Bacteria from Wounds and Fractures in Wild Birds. Animals 2024, 14, 1151. https://doi.org/10.3390/ani14081151
Sánchez-Ortiz E, Blanco Gutiérrez MdM, Calvo-Fernandez C, Mencía-Gutiérrez A, Pastor Tiburón N, Alvarado Piqueras A, Pablos-Tanarro A, Martín-Maldonado B. Addressing Challenges in Wildlife Rehabilitation: Antimicrobial-Resistant Bacteria from Wounds and Fractures in Wild Birds. Animals. 2024; 14(8):1151. https://doi.org/10.3390/ani14081151
Chicago/Turabian StyleSánchez-Ortiz, Esther, María del Mar Blanco Gutiérrez, Cristina Calvo-Fernandez, Aida Mencía-Gutiérrez, Natalia Pastor Tiburón, Alberto Alvarado Piqueras, Alba Pablos-Tanarro, and Bárbara Martín-Maldonado. 2024. "Addressing Challenges in Wildlife Rehabilitation: Antimicrobial-Resistant Bacteria from Wounds and Fractures in Wild Birds" Animals 14, no. 8: 1151. https://doi.org/10.3390/ani14081151







