Predicting Risk of Ammonia Exposure in Broiler Housing: Correlation with Incidence of Health Issues
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment
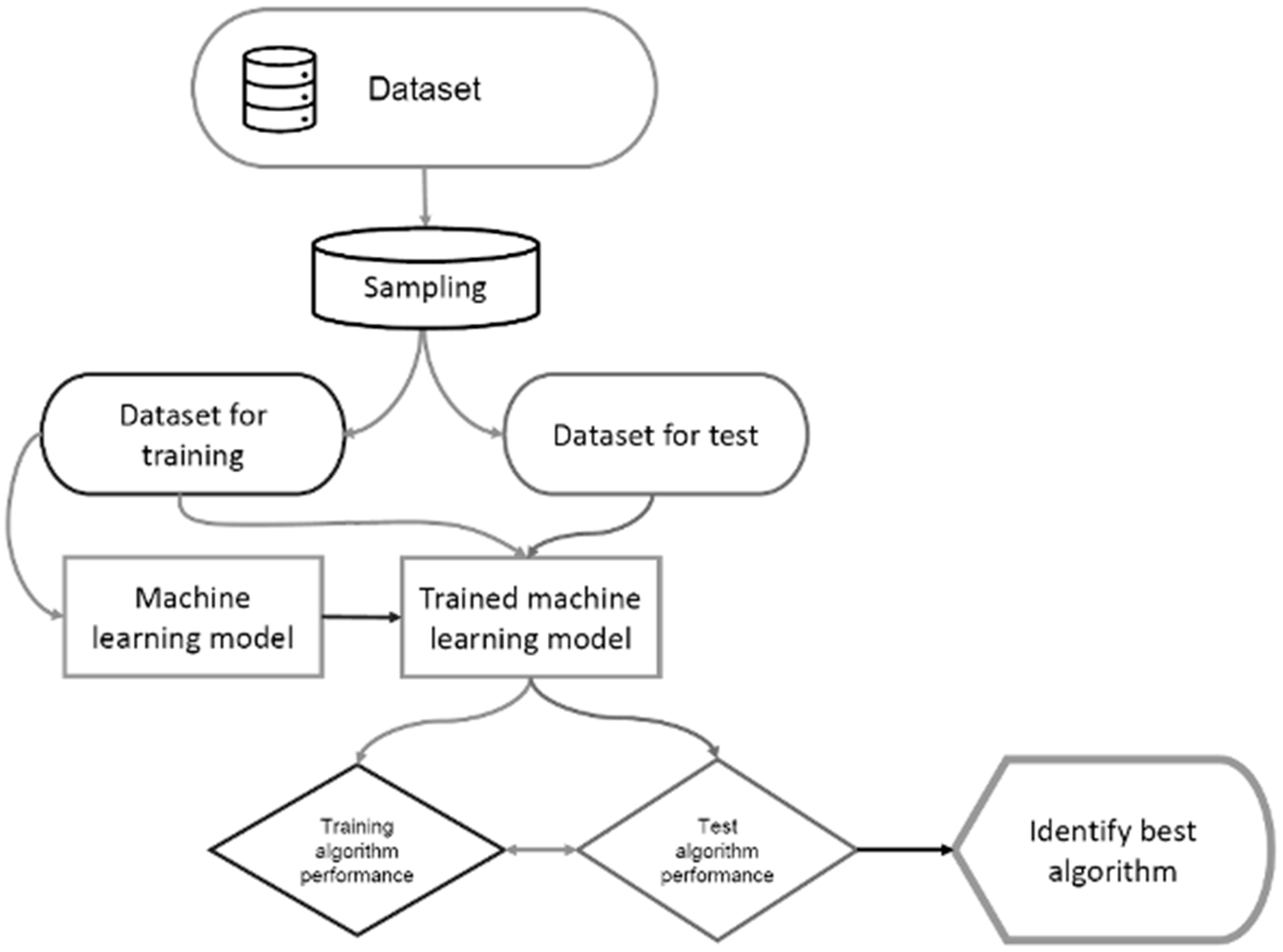
2.2. Data Mining Approach
2.3. Performance Measures of Classification Models
2.4. Spearman Correlation Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ti, C.; Xia, L.; Chang, S.X.; Yan, X. Potential for Mitigating Global Agricultural Ammonia Emission: A Meta-Analysis. Environ. Pollut. 2019, 245, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Chambers, B.J.; Walker, A.W. Ammonia Emissions from Broiler Litter and Laying Hen Manure Management Systems. Biosyst. Eng. 2004, 89, 175–185. [Google Scholar] [CrossRef]
- Pescatore, A.J.; Casey, K.D.; Gates, R.S. Ammonia Emissions from Broiler Houses. J. Appl. Poult. Res. 2005, 14, 635–637. [Google Scholar] [CrossRef]
- Zhao, L.; Hadlocon, L.J.S.; Manuzon, R.B.; Darr, M.J.; Keener, H.M.; Heber, A.J.; Ni, J. Ammonia Concentrations and Emission Rates at a Commercial Poultry Manure Composting Facility. Biosyst. Eng. 2016, 150, 69–78. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Sousa, F.C.; Saraz, J.O.; Calderano, A.A.; Tinôco, I.F.F.; Carneiro, A.P.S. Ammonia Emission in Poultry Facilities: A Review for Tropical Climate Areas. Atmosphere 2021, 12, 1091. [Google Scholar] [CrossRef]
- Tasistro, A.S.; Ritz, C.W.; Kissel, D.E. Ammonia Emissions from Broiler Litter: Response to Bedding Materials and Acidifiers. Br. Poult. Sci. 2007, 48, 399–405. [Google Scholar] [CrossRef]
- Kearney, G.D.; Shaw, R.; Prentice, M.; Tutor-Marcom, R. Evaluation of Respiratory Symptoms and Respiratory Protection Behavior Among Poultry Workers in Small Farming Operations. J. Agromed. 2014, 19, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Nemer, M.; Sikkeland, L.I.B.; Kasem, M.; Kristensen, P.; Nijem, K.; Bjertness, E.; Skare, Ø.; Bakke, B.; Kongerud, J.; Skogstad, M. Airway Inflammation and Ammonia Exposure among Female Palestinian Hairdressers: A Cross-Sectional Study. Occup. Environ. Med. 2015, 72, 428–434. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Wathes, C.M. Ammonia and Poultry Welfare: A Review. World’s Poult. Sci. J. 2000, 56, 235–245. [Google Scholar] [CrossRef]
- Barbosa, L.V.S.; De Moura, D.J.; Estellés, F.; Ramón-Moragues, A.; Calvet, S.; Villagrá, A. Assessment of Husbandry Practices That Can Reduce the Negative Effects of Exposure to Low Ammonia Concentrations in Broiler Houses. Animals 2022, 12, 1096. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA Data Mining Software: An Update. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Larose, D.T. Data Mining and Predictive Analytics; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9781118868706. [Google Scholar]
- Uçar, M.K.; Nour, M.; Sindi, H.; Polat, K. The Effect of Training and Testing Process on Machine Learning in Biomedical Datasets. Math. Probl. Eng. 2020, 2020, e2836236. [Google Scholar] [CrossRef]
- Carlile, F.S. Ammonia in Poultry Houses: A Literature Review. Worlds Poult. Sci. J. 1984, 40, 99–113. [Google Scholar] [CrossRef]
- Commissione Europea. Commission directive 2009/161/EU of 17 December 2009 establishing a third list of indicative values of occupational exposure. Med. Lav. 2010, 101, 145. [Google Scholar]
- Bowes, D.; Hall, T.; Gray, D. Comparing the Performance of Fault Prediction Models Which Report Multiple Performance Measures: Recomputing the Confusion Matrix. In Proceedings of the 8th International Conference on Predictive Models in Software Engineering, Lund, Sweden, 21–22 September 2012; pp. 109–118. [Google Scholar]
- Han, J.; Kamber, M.; Pei, J. Data Mining: Concepts and Techniques, 3rd ed.; Morgan Kaufmann: Waltham, MA, USA, 2012; ISBN 9780123814791. [Google Scholar]
- Ojo, R.O.; Ajayi, A.O.; Owolabi, H.A.; Oyedele, L.O.; Akanbi, L.A. Internet of Things and Machine Learning Techniques in Poultry Health and Welfare Management: A Systematic Literature Review. Comput. Electron. Agric. 2022, 200, 107266. [Google Scholar] [CrossRef]
- Debauche, O.; Mahmoudi, S.; Mahmoudi, S.A.; Manneback, P.; Bindelle, J.; Lebeau, F. Edge Computing and Artificial Intelligence for Real-Time Poultry Monitoring. Procedia Comput. Sci. 2020, 175, 534–541. [Google Scholar] [CrossRef]
- Lashari, M.H.; Memon, A.A.; Shah, S.A.A.; Nenwani, K.; Shafqat, F. IoT Based Poultry Environment Monitoring System. In Proceedings of the 2018 IEEE International Conference on Internet of Things and Intelligence System (IOTAIS), Bali, Indonesia, 1–3 November 2018; pp. 1–5. [Google Scholar]
- Choukidar, G.A.; Dawande, N.A. Smart Poultry Farm Automation and Monitoring System. In Proceedings of the 2017 International Conference on Computing, Communication, Control and Automation (ICCUBEA), Pune, India, 17–18 August 2017; pp. 1–5. [Google Scholar]
- Gunawan, T.S.; Sabar, M.F.; Nasir, H.; Kartiwi, M.; Motakabber, S.M.A. Development of Smart Chicken Poultry Farm Using RTOS on Arduino. In Proceedings of the 2019 IEEE International Conference on Smart Instrumentation, Measurement and Application (ICSIMA), Kuala Lumpur, Malaysia, 27–29 August 2019; pp. 1–5. [Google Scholar]
- Lahlouh, I.; Rerhrhaye, F.; Elakkary, A.; Sefiani, N. Experimental Implementation of a New Multi Input Multi Output Fuzzy-PID Controller in a Poultry House System. Heliyon 2020, 6, e04645. [Google Scholar] [CrossRef]
- Li, G.; Ji, B.; Li, B.; Shi, Z.; Zhao, Y.; Dou, Y.; Brocato, J. Assessment of Layer Pullet Drinking Behaviors under Selectable Light Colors Using Convolutional Neural Network. Comput. Electron. Agric. 2020, 172, 105333. [Google Scholar] [CrossRef]
- Lorencena, M.C.; Southier, L.F.P.; Casanova, D.; Ribeiro, R.; Teixeira, M. A Framework for Modelling, Control and Supervision of Poultry Farming. Int. J. Prod. Res. 2020, 58, 3164–3179. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C. Design of Sick Chicken Automatic Detection System Based on Improved Residual Network. In Proceedings of the 2020 IEEE 4th Information Technology, Networking, Electronic and Automation Control Conference (ITNEC), Chongqing, China, 12–14 June 2020; Volume 1, pp. 2480–2485. [Google Scholar]
- So-In, C.; Poolsanguan, S.; Rujirakul, K. A Hybrid Mobile Environmental and Population Density Management System for Smart Poultry Farms. Comput. Electron. Agric. 2014, 109, 287–301. [Google Scholar] [CrossRef]
- Pereira, W.F.; da Silva Fonseca, L.; Putti, F.F.; Góes, B.C.; de Paula Naves, L. Environmental Monitoring in a Poultry Farm Using an Instrument Developed with the Internet of Things Concept. Comput. Electron. Agric. 2020, 170, 105257. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, T. Detection of Sick Broilers by Digital Image Processing and Deep Learning. Biosyst. Eng. 2019, 179, 106–116. [Google Scholar] [CrossRef]
- Banerjee, D.; Biswas, S.; Daigle, C.; Siegford, J.M. Remote Activity Classification of Hens Using Wireless Body Mounted Sensors. In Proceedings of the 2012 9th International Conference on Wearable and Implantable Body Sensor Networks, London, UK, 9–12 May 2012; pp. 107–112. [Google Scholar]
- Küçüktopcu, E.; Cemek, B. Comparison of Neuro-Fuzzy and Neural Networks Techniques for Estimating Ammonia Concentration in Poultry Farms. J. Environ. Chem. Eng. 2021, 9, 105699. [Google Scholar] [CrossRef]


| Day | SHD_Weight (g/bird) | FHD_Weight (g/bird) | FLD_Weight (g/bird) | SHD_Feed Consumption (g/animal/week) | FHD_Feed Consumption (g/animal/week) | FLD_Feed Consumption (g/animal/week) | SHD_Feed Conversion Ratio | FHD_Feed Conversion Ratio | FLD_Feed Conversion Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 7 | 144.8 | 157.75 | 160.45 | 0.11 | 0.134 | 0.13 | 1.14 | 1.2 | 1.12 |
| 14 | 323.1 | 413.2 | 428.6 | 0.26 | 0.371 | 0.36 | 1.58 | 1.35 | 1.38 |
| 21 | 544.55 | 788.25 | 789.15 | 0.5 | 0.713 | 0.665 | 2.08 | 2.02 | 1.89 |
| 28 | 907.3 | 1234.6 | 1233.55 | 0.67 | 1169.5 | 1086.5 | 1.88 | 2.66 | 2.46 |
| 35 | 1298.15 | 1810.05 | 1788.3 | 0.68 | 1448.5 | 1272.0 | 1.83 | 2.54 | 2.27 |
| 42 | 1785.05 | 2471.15 | 2461.1 | 0.9 | 1506.0 | 1362.5 | 1.89 | 2.25 | 2.09 |
| 49 | 2234.15 | 516.49 | 2.37 | ||||||
| 56 | 2726.4 | 1198.5 | 2.45 | ||||||
| 63 | 3201.45 | 1284.5 | 2.73 |
| Ammonia Concentration Risk | |
|---|---|
| Target Attributes | Risk Level |
| No risk | 0–1 pm |
| Low risk | 2–9 pm |
| Moderate risk | 10–14 pm |
| High risk | 15–20 pm |
| Very high risk | >21 pm |
| Classifier Models | J48 Tree | SMO | Naive Bayes | Multilayer Perceptron |
|---|---|---|---|---|
| Correctly classified instances (%) | 100 | 91.58 | 92.44 | 99.05 |
| Incorrectly classified instances (%) | 00 | 8.42 | 7.56 | 0.95 |
| Kappa statistic (%) | 100 | 89.12 | 90.28 | 98.77 |
| J48 Tree Model | ||||||
| Accuracy by Class | TP Rate (%) | FP Rate (%) | Precision (%) | Recall (%) | F-Measure (%) | MCC (%) |
| No risk | 100 | 100 | 100 | 100 | 100 | 100 |
| Low risk | 100 | 100 | 100 | 100 | 100 | 100 |
| Moderate risk | 100 | 100 | 100 | 100 | 100 | 100 |
| High risk | 100 | 100 | 100 | 100 | 100 | 100 |
| Very high risk | 100 | 100 | 100 | 100 | 100 | 100 |
| SMO Model | ||||||
| Accuracy by Class | TP Rate (%) | FP Rate (%) | Precision (%) | Recall (%) | F-Measure (%) | MCC (%) |
| No risk | 97 | 1.0 | 98 | 97 | 97 | 96 |
| Low risk | 77 | 1.0 | 93 | 77 | 84 | 81 |
| Moderate risk | 94 | 6.0 | 83 | 94 | 89 | 85 |
| High risk | 95 | 2.0 | 87 | 95 | 91 | 90 |
| Very high risk | 92 | 0.01 | 99 | 92 | 95 | 95 |
| Naive Bayes Model | ||||||
| Accuracy by Class | TP Rate (%) | FP Rate (%) | Precision (%) | Recall (%) | F-Measure (%) | MCC (%) |
| No risk | 96 | 00 | 100 | 96 | 98 | 97 |
| Low risk | 88 | 2.0 | 93 | 88 | 90 | 88 |
| Moderate risk | 89 | 3.0 | 89 | 89 | 89 | 86 |
| High risk | 95 | 2.0 | 87 | 95 | 91 | 89 |
| Very high risk | 94 | 2.0 | 86 | 94 | 90 | 89 |
| Multilayer Perceptron Model | ||||||
| Accuracy by Class | TP Rate (%) | FP Rate (%) | Precision (%) | Recall (%) | F-Measure (%) | MCC (%) |
| No risk | 99 | 1.0 | 99 | 99 | 99 | 98 |
| Low risk | 98 | 1.0 | 98 | 98 | 98 | 98 |
| Moderate risk | 100 | 1.0 | 99 | 100 | 99 | 99 |
| High risk | 99 | 00 | 100 | 99 | 99 | 99 |
| Very high risk | 100 | 00 | 100 | 100 | 100 | 100 |
| J48 Tree Model | ||||||
|---|---|---|---|---|---|---|
| No Risk | Low Risk | Moderate Risk | High Risk | Very High Risk | Total | Classified as |
| 1750 | 0 | 0 | 0 | 0 | 1750 | No risk |
| 0 | 1189 | 0 | 0 | 0 | 1189 | Low risk |
| 0 | 0 | 1359 | 0 | 0 | 1359 | Moderate risk |
| 0 | 0 | 0 | 740 | 0 | 740 | High risk |
| 0 | 0 | 0 | 0 | 650 | 650 | Very high risk |
| 1750 | 1189 | 1359 | 740 | 650 | 5688 | |
| SMO Model | ||||||
| No Risk | Low Risk | Moderate Risk | High Risk | Very High Risk | Total | Classified as |
| 1702 | 48 | 0 | 0 | 0 | 1750 | No risk |
| 41 | 918 | 230 | 0 | 0 | 1189 | Low risk |
| 0 | 20 | 1280 | 55 | 0 | 1355 | Moderate risk |
| 0 | 0 | 27 | 706 | 7 | 2095 | High risk |
| 0 | 0 | 0 | 51 | 599 | 650 | Very high risk |
| 1743 | 986 | 1537 | 812 | 606 | 5688 | |
| Naive Bayes model | ||||||
| No Risk | Low Risk | Moderate Risk | High Risk | Very High Risk | Total | Classified as |
| 1685 | 59 | 0 | 0 | 6 | 1750 | No risk |
| 0 | 1046 | 135 | 0 | 8 | 1189 | Low risk |
| 0 | 20 | 1211 | 67 | 61 | 1359 | Moderate risk |
| 0 | 0 | 15 | 702 | 23 | 740 | High risk |
| 0 | 0 | 0 | 36 | 614 | 650 | Very high risk |
| 1685 | 1125 | 1361 | 805 | 712 | 5688 | |
| Multilayer Perceptron model | ||||||
| No Risk | Low Risk | Moderate Risk | High Risk | Very High Risk | Total | Classified as |
| 1731 | 19 | 0 | 0 | 0 | 1750 | No risk |
| 22 | 1164 | 2 | 1 | 0 | 1189 | Low risk |
| 2 | 0 | 1357 | 0 | 0 | 1359 | Moderate risk |
| 0 | 0 | 8 | 732 | 0 | 2099 | High risk |
| 0 | 0 | 0 | 0 | 650 | 650 | Very high risk |
| 1755 | 1183 | 1359 | 733 | 650 | 5688 | |
| Sample 1 | Sample 2 | Correlation | 95% CI ρ | p-Value |
|---|---|---|---|---|
| Risk NH3 (ppm) 10–14 pmm | bronchi | 0.189 | −0.600; 0.792 | 0.654 |
| Risk NH3 (ppm) 10–14 pmm | lungs | 0.549 | −0.313; 0.915 | 0.159 |
| Risk NH3 (ppm) 10–14 pmm | eyes | 0.378 | −0.470; 0.863 | 0.356 |
| Risk NH3 (ppm) 10–14 pmm | paws | 0.375 | −0.472; 0.862 | 0.360 |
| Risk NH3 (ppm) 10–14 pmm | other injuries | 0.750 | −0.019; 0.961 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, L.V.S.; Lima, N.D.d.S.; Barros, J.d.S.G.; de Moura, D.J.; Estellés, F.; Ramón-Moragues, A.; Calvet-Sanz, S.; García, A.V. Predicting Risk of Ammonia Exposure in Broiler Housing: Correlation with Incidence of Health Issues. Animals 2024, 14, 615. https://doi.org/10.3390/ani14040615
Barbosa LVS, Lima NDdS, Barros JdSG, de Moura DJ, Estellés F, Ramón-Moragues A, Calvet-Sanz S, García AV. Predicting Risk of Ammonia Exposure in Broiler Housing: Correlation with Incidence of Health Issues. Animals. 2024; 14(4):615. https://doi.org/10.3390/ani14040615
Chicago/Turabian StyleBarbosa, Leonardo V. S., Nilsa Duarte da Silva Lima, Juliana de Souza Granja Barros, Daniella Jorge de Moura, Fernando Estellés, Adrian Ramón-Moragues, Salvador Calvet-Sanz, and Arantxa Villagrá García. 2024. "Predicting Risk of Ammonia Exposure in Broiler Housing: Correlation with Incidence of Health Issues" Animals 14, no. 4: 615. https://doi.org/10.3390/ani14040615
APA StyleBarbosa, L. V. S., Lima, N. D. d. S., Barros, J. d. S. G., de Moura, D. J., Estellés, F., Ramón-Moragues, A., Calvet-Sanz, S., & García, A. V. (2024). Predicting Risk of Ammonia Exposure in Broiler Housing: Correlation with Incidence of Health Issues. Animals, 14(4), 615. https://doi.org/10.3390/ani14040615






