Spatiotemporal Niche Separation among Passeriformes in the Halla Mountain Wetland of Jeju, Republic of Korea: Insights from Camera Trap Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Filed Sampling
2.3. Activity Pattern
2.4. Analysis of Habitat Usage
2.5. Analysis of Observation Frequency and Population Dynamics
3. Results
3.1. Monitoring and Circadian Rhythms of Passeriformes
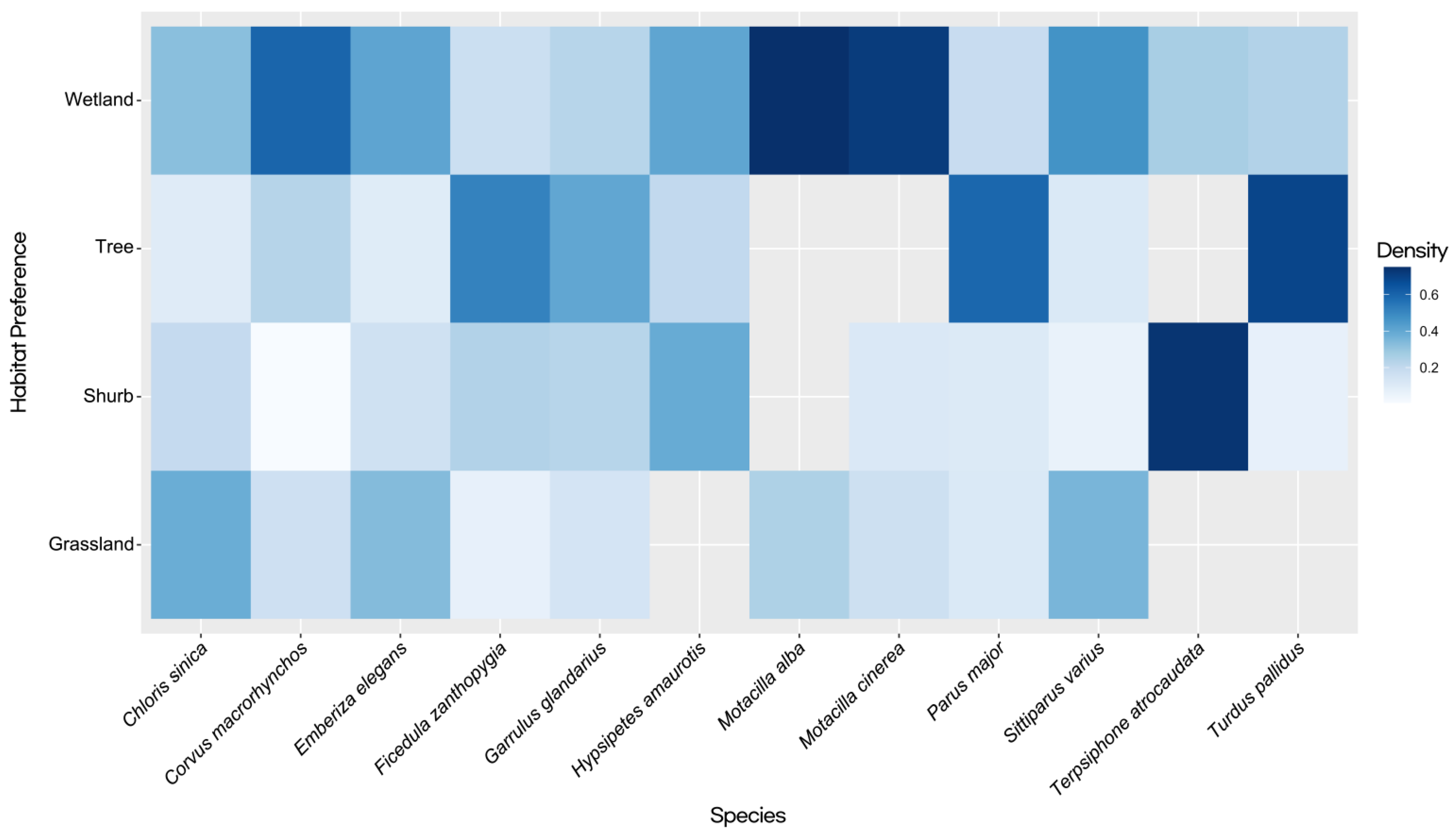
3.2. Habitat Usage Patterns and Population Density Influences
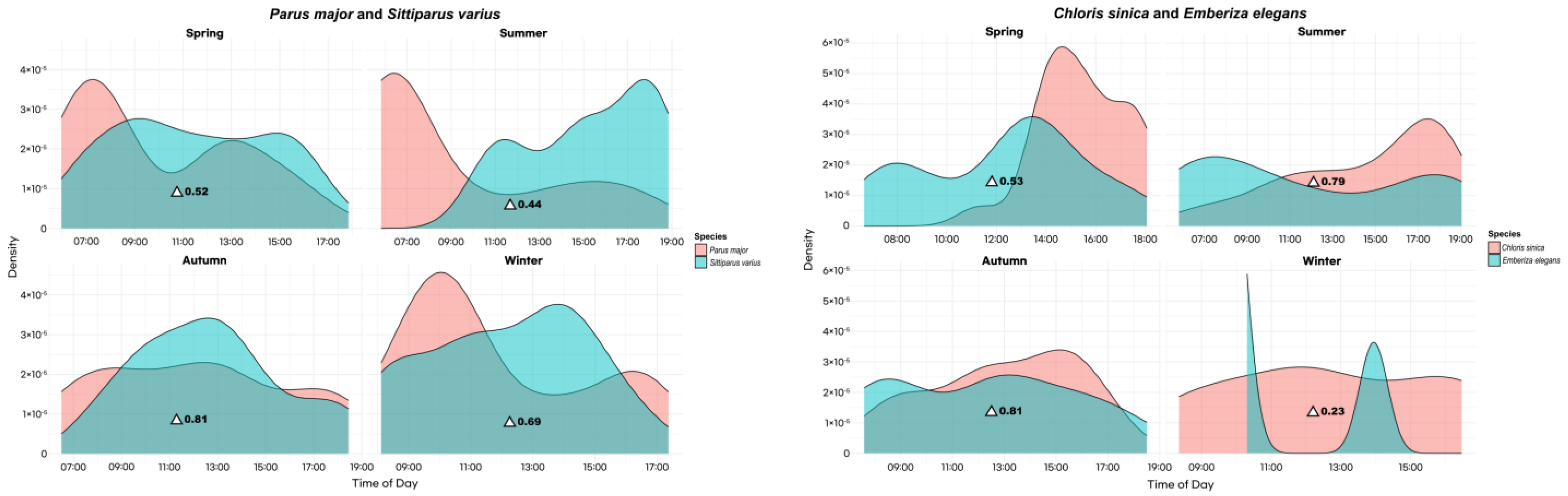
3.3. Seasonal Variations in Activity Patterns and Temporal Overlap of Bird Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Bateman, P.; Fleming, P.; Wolfe, A. A different kind of ecological modelling: The use of clay model organisms to explore predator–prey interactions in vertebrates. J. Zool. 2017, 301, 251–262. [Google Scholar] [CrossRef]
- Sellers, L. Rhythms of Life: The Biological Clocks That Control the Daily Lives of Every Living Thing. J. Coll. Sci. Teach. 2006, 35, 57. [Google Scholar]
- Strauss, S.Y.; Irwin, R.E. Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 435–466. [Google Scholar] [CrossRef]
- Gonzalez, A.; Rayfield, B.; Lindo, Z. The disentangled bank: How loss of habitat fragments and disassembles ecological networks. Am. J. Bot. 2011, 98, 503–516. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Harsch, M.A.; Ettinger, A.K.; Ford, K.R.; Theobald, E.J. How will biotic interactions influence climate change–induced range shifts? Ann. N. Y. Acad. Sci. 2013, 1297, 112–125. [Google Scholar] [CrossRef]
- Marra, P.P.; Studds, C.E.; Wilson, S.; Sillett, T.S.; Sherry, T.W.; Holmes, R.T. Non-breeding season habitat quality mediates the strength of density-dependence for a migratory bird. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150624. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities: Positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. Bioscience 2001, 51, 235–246. [Google Scholar]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Sillett, T.S.; Holmes, R.T. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 2002, 71, 296–308. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Ye, Y.; Hu, C.; Jiang, Y.; Davison, G.W.; Ding, C. Three-dimensional niche partitioning between two colonially nesting ardeid species in central China. Avian Res. 2021, 12, 33. [Google Scholar] [CrossRef]
- Catchpole, C.K.; Slater, P.J. Bird Song: Biological Themes and Variations; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Marzluff, J.M.; Bowman, R.; Donnelly, R. A historical perspective on urban bird research: Trends, terms, and approaches. In Avian Ecology and Conservation in an Urbanizing World; Springer: Boston, MA, USA, 2001; pp. 1–17. [Google Scholar]
- Ogurtsov, S.S.; Zheltukhin, A.S.; Kotlov, I.P. Daily activity patterns of large and medium-sized mammals based on camera traps data in the Central Forest Nature Reserve, Valdai Upland, Russia. Nat. Conserv. Res. Зanoвeднaя Hayкa 2018, 3, 68–88. [Google Scholar] [CrossRef]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. camtrapR: An R package for efficient camera trap data management. Methods Ecol. Evol. 2016, 7, 1457–1462. [Google Scholar] [CrossRef]
- Imbeau, L.; Savard, J.-P.L.; Gagnon, R. Comparing bird assemblages in successional black spruce stands originating from fire and logging. Can. J. Zool. 2000, 77, 1850–1860. [Google Scholar] [CrossRef]
- Martin, K.; Eadie, J.M. Nest webs: A community-wide approach to the management and conservation of cavity-nesting forest birds. For. Ecol. Manag. 1999, 115, 243–257. [Google Scholar] [CrossRef]
- Klaassen, R.H.; Hake, M.; Strandberg, R.; Alerstam, T. Geographical and temporal flexibility in the response to crosswinds by migrating raptors. Proc. R. Soc. B Biol. Sci. 2011, 278, 1339–1346. [Google Scholar] [CrossRef]
- Dokter, A.M.; Shamoun-Baranes, J.; Kemp, M.U.; Tijm, S.; Holleman, I. High altitude bird migration at temperate latitudes: A synoptic perspective on wind assistance. PLoS ONE 2013, 8, e52300. [Google Scholar] [CrossRef]
- Newton, I. The Migration Ecology of Birds; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kim, E.-S.; Oh, C.H.; Park, H.C.; Lee, S.-H.; Choi, J.; Lee, S.-H.; Cho, H.-B.; Lim, W.; Kim, H.; Yoon, Y.-K. Disturbed regeneration of saplings of Korean fir (Abies koreana Wilson), an endemic tree species, in Hallasan National Park, a UNESCO Biosphere Reserve, Jeju Island, Korea. J. Mar. Isl. Cult. 2016, 5, 68–78. [Google Scholar] [CrossRef]
- Jeong, Y.H. The Relationship of the Habitat Environment in Noro Oream Wetland and Mulchat Oream Wetland on the Bird Population in Mt. Halla. Master’s Thesis, Jeju National University, Jeju, Republic of Korea, 2020. [Google Scholar]
- NIFS. National Institute of Forest Science: Hourly Weather Data. Available online: http://mw.nifos.go.kr/SiteData/Site_01Hour.aspx?SearchType=Hour (accessed on 20 March 2023).
- Hutto, R.L.; Young, J.S. Regional landbird monitoring: Perspectives from the northern Rocky Mountains. Wildl. Soc. Bull. 2002, 30, 738–750. [Google Scholar]
- Buckley, L.B.; Urban, M.C.; Angilletta, M.J.; Crozier, L.G.; Rissler, L.J.; Sears, M.W. Can mechanism inform species’ distribution models? Ecol. Lett. 2010, 13, 1041–1054. [Google Scholar] [CrossRef]
- Choi, C.-Y.; Lee, E.-J.; Nam, H.-Y.; Lee, W.-S. Effects of postfire logging on bird populations and communities in burned forests. J. Korean Soc. For. Sci. 2007, 96, 115–123. [Google Scholar]
- Grimm, V.; Augusiak, J.; Focks, A.; Frank, B.M.; Gabsi, F.; Johnston, A.S.; Liu, C.; Martin, B.T.; Meli, M.; Radchuk, V. Towards better modelling and decision support: Documenting model development, testing, and analysis using TRACE. Ecol. Model. 2014, 280, 129–139. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Efford, M.G. Estimation of population density by spatially explicit capture–recapture analysis of data from area searches. Ecology 2011, 92, 2202–2207. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Knudsen, E.; Lind, J.; Both, C. Characterizing bird migration phenology using data from standardized monitoring at bird observatories. Clim. Res. 2007, 35, 59–77. [Google Scholar] [CrossRef]
- Neu, C.W.; Byers, C.R.; Peek, J.M. A technique for analysis of utilization-availability data. J. Wildl. Manag. 1974, 38, 541–545. [Google Scholar] [CrossRef]
- Byers, C.R.; Steinhorst, R.K.; Krausman, P.R. Clarification of a technique for analysis of utilization-availability data. J. Wildl. Manag. 1984, 48, 1050–1053. [Google Scholar] [CrossRef]
- Tenan, S.; Tavecchia, G.; Oro, D.; Pradel, R. Assessing the effect of density on population growth when modeling individual encounter data. Ecology 2019, 100, e02595. [Google Scholar] [CrossRef] [PubMed]
- Stoklosa, J.; Blakey, R.V.; Hui, F.K. An overview of modern applications of negative binomial modelling in ecology and biodiversity. Diversity 2022, 14, 320. [Google Scholar] [CrossRef]
- Mowers, R.P.; Bucciarelli, B.; Cao, Y.; Samac, D.A.; Xu, Z. Good Statistical Practices in Agronomy Using Categorical Data Analysis, with Alfalfa Examples Having Poisson and Binomial Underlying Distributions. Crops 2022, 2, 154–171. [Google Scholar] [CrossRef]
- Brumm, H.; Zollinger, S.A. The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 2011, 148, 1173–1198. [Google Scholar] [CrossRef]
- Marler, P.R.; Slabbekoorn, H. Nature’s Music: The Science of Birdsong; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Murakami, M.; Nakano, S. Species-specific foraging behavior of birds in a riparian forest. Ecol. Res. 2001, 16, 913–923. [Google Scholar] [CrossRef]
- Gill, F.B. Species taxonomy of birds: Which null hypothesis? Auk Ornithol. Adv. 2014, 131, 150–161. [Google Scholar] [CrossRef]
- Pérez-Irineo, G.; Santos-Moreno, A. Bird activity patterns in the understorey of an evergreen forest in Oaxaca, Mexico. Neotrop. Biol. Conserv. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Olff, H. Spatial scaling laws yield a synthetic theory of biodiversity. Nature 1999, 400, 557–560. [Google Scholar] [CrossRef]
- Lima, S.L. Predators and the breeding bird: Behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 2009, 84, 485–513. [Google Scholar] [CrossRef]
- Møller, A.P. Flight distance and population trends in European breeding birds. Behav. Ecol. 2008, 19, 1095–1102. [Google Scholar] [CrossRef]
- Smetzer, J.R.; Paxton, K.L.; Hart, P.J.; Paxton, E.H. Activity patterns of Hawaiian forest birds in a fragmented and continuous landscape. J. Avian Biol. 2022, 2022, e02905. [Google Scholar] [CrossRef]
- Robbins, C.S. Effect of time of day on bird activity. Stud. Avian Biol. 1981, 6, 275–286. [Google Scholar]
- Holt, R.D. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2009, 106, 19659–19665. [Google Scholar] [CrossRef]
- Ceia, F.R.; Ramos, J.A. Individual specialization in the foraging and feeding strategies of seabirds: A review. Mar. Biol. 2015, 162, 1923–1938. [Google Scholar] [CrossRef]
- Powell, L.L.; Ames, E.M.; Wright, J.R.; Matthiopoulos, J.; Marra, P.P. Interspecific competition between resident and wintering birds: Experimental evidence and consequences of coexistence. Ecology 2021, 102, e03208. [Google Scholar] [CrossRef]
- Youngflesh, C.; Socolar, J.; Amaral, B.R.; Arab, A.; Guralnick, R.P.; Hurlbert, A.H.; LaFrance, R.; Mayor, S.J.; Miller, D.A.; Tingley, M.W. Migratory strategy drives species-level variation in bird sensitivity to vegetation green-up. Nat. Ecol. Evol. 2021, 5, 987–994. [Google Scholar] [CrossRef]
- Seward, A.M.; Beale, C.M.; Gilbert, L.; Jones, T.H.; Thomas, R.J. The impact of increased food availability on reproduction in a long-distance migratory songbird: Implications for environmental change? PLoS ONE 2014, 9, e111180. [Google Scholar] [CrossRef]
- Wolf, B.O.; Walsberg, G.E. Thermal effects of radiation and wind on a small bird and implications for microsite selection. Ecology 1996, 77, 2228–2236. [Google Scholar] [CrossRef]
- Ferretti, F.; Fattorini, N. Competitor densities, habitat, and weather: Effects on interspecific interactions between wild deer species. Integr. Zool. 2021, 16, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Ryeland, J.; Weston, M.A.; Symonds, M.R. The importance of wetland margin microhabitat mosaics; the case of shorebirds and thermoregulation. J. Appl. Ecol. 2021, 58, 382–391. [Google Scholar] [CrossRef]
- Bicego, K.C.; Barros, R.C.; Branco, L.G. Physiology of temperature regulation: Comparative aspects. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 616–639. [Google Scholar] [CrossRef] [PubMed]
- Belovsky, G.E. Generalist herbivore foraging and its role in competitive interactions. Am. Zool. 1986, 26, 51–69. [Google Scholar] [CrossRef]
- Goodale, E.; Beauchamp, G.; Magrath, R.D.; Nieh, J.C.; Ruxton, G.D. Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 2010, 25, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Holdridge, E.M.; Cuellar-Gempeler, C.; terHorst, C.P. A shift from exploitation to interference competition with increasing density affects population and community dynamics. Ecol. Evol. 2016, 6, 5333–5341. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.D.; Karanth, K.U. Camera Traps in Animal Ecology: Methods and Analyses; O’Connell, A.F., Ed.; Springer: New York, NY, USA, 2011; Volume 271. [Google Scholar]
- Marra, P.P.; Hunter, D.; Perrault, A.M. Migratory connectivity and the conservation of migratory animals. Environ. Law 2011, 41, 317. [Google Scholar]


| Family | Scientific Name | Time Categorization a | Migration Pattern b |
|---|---|---|---|
| Zosteropidae | Zosterops japonicus | C | Res |
| Fringillidae | Chloris sinica | C | Res |
| Tudidae | Turdus pallidus | C | Res |
| Turdus hortulorum | E | SV | |
| Phylloscopidae | Phylloscopus xanthodryas | C | SV |
| Cettidae | Horornis diphone | M | Res |
| Pycnonotidae | Hypsipetes amaurotis | C | Res |
| Paridae | Sittiparus varius | C | Res |
| Parus major | C | Res | |
| Musicapidae | Phoenicurus auroreus | E | Res |
| Muscicapa dauurica | E | PM | |
| Tarsiger cyanurus | C | PM | |
| Ficedula zanthopygia | C | SV | |
| Cyanoptila cyanomelana | M | SV | |
| Motacillidae | Motacilla cinerea | C | Res |
| Motacilla alba | C | Res | |
| Monarchidae | Terpsiphone atrocaudata | C | SV |
| Emberizidae | Emberiza elegans | C | Res |
| Emberiza cioides | E | Res | |
| Corvidae | Garrulus glandarius | C | Res |
| Corvus macrorhynchos | C | Res | |
| Aegithalidae | Aegithalos caudatus | E | Res |
| Habitat | Total Area (ha) | Po a (ha) | Observed Usage b | Expected Usage c | P_i d | Bonferroni CI for P_i e |
|---|---|---|---|---|---|---|
| Wetland | 0.1748 | 0.03927 | 452 | 44.61 | 0.398 | 0.362 ≤ P_i ≤ 0.434 |
| Grassland | 5.4825 | 0.03927 | 206 | 44.61 | 0.181 | 0.153 ≤ P_i ≤ 0.210 |
| Shrub | 5.2049 | 0.04712 | 211 | 55.53 | 0.186 | 0.157 ≤ P_i ≤ 0.215 |
| Tree | 62.8258 | 0.06283 | 267 | 71.37 | 0.235 | 0.204 ≤ P_i ≤ 0.267 |
| Total | 73.688 | 1136 | 216.12 |
| Variable | Estimate | Z Value a | Pr(>|z|) b |
|---|---|---|---|
| (Intercept) | 4.768 | 55.356 | <2 × 10−16 *** |
| Body size | 0.001 | 0.529 | 0.596 |
| Low density | −1.154 | −10.491 | <2 × 10−16 *** |
| Medium density | −0.099 | −1.453 | 0.146 |
| Residual deviance | 356.92 | ||
| AIC | 438.24 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.-H.; Choi, S.-H.; Banjade, M.; Jin, S.-D.; Park, S.-M.; Kunwar, B.; Oh, H.-S. Spatiotemporal Niche Separation among Passeriformes in the Halla Mountain Wetland of Jeju, Republic of Korea: Insights from Camera Trap Data. Animals 2024, 14, 724. https://doi.org/10.3390/ani14050724
Jeong Y-H, Choi S-H, Banjade M, Jin S-D, Park S-M, Kunwar B, Oh H-S. Spatiotemporal Niche Separation among Passeriformes in the Halla Mountain Wetland of Jeju, Republic of Korea: Insights from Camera Trap Data. Animals. 2024; 14(5):724. https://doi.org/10.3390/ani14050724
Chicago/Turabian StyleJeong, Young-Hun, Sung-Hwan Choi, Maniram Banjade, Seon-Deok Jin, Seon-Mi Park, Binod Kunwar, and Hong-Shik Oh. 2024. "Spatiotemporal Niche Separation among Passeriformes in the Halla Mountain Wetland of Jeju, Republic of Korea: Insights from Camera Trap Data" Animals 14, no. 5: 724. https://doi.org/10.3390/ani14050724







