Cecropin A Alleviates LPS-Induced Oxidative Stress and Apoptosis of Bovine Endometrial Epithelial Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
- The ratio of the concentrate to forage is 5:5.
- The premix contained 10,000 IU/kg of vitamin A, 5500 IU/kg of vitamin D3, 2000 IU/kg of vitamin E, and 2200 IU/kg of vitamin K3. The diet contained 61.50 mg/kg of zinc, 56.50 mg/kg of manganese, 70.50 mg/kg of iron, 10.75 mg/kg of copper, 0.75 mg/kg of iodine, 0.45 mg/kg of cobalt, and 0.50 mg/kg of selenium.
- Cows as controls were fed with a high-concentrate diet supplemented with cecropin A at a rate of 0.012% of the concentrate. The cows we sampled tissues from who were detected with SARA were fed with a high-concentrate diet with no cecropin A.
2.2. Cell Treatment
2.3. Quantitative Reverse Transcription PCR
2.4. Western Blotting
2.5. Detection of Intracellular ROS
2.6. Mitochondrial Membrane Potential Assay
2.7. Flow Cytometry
2.8. Cellular Immunofluorescence
2.9. Statistics
3. Results
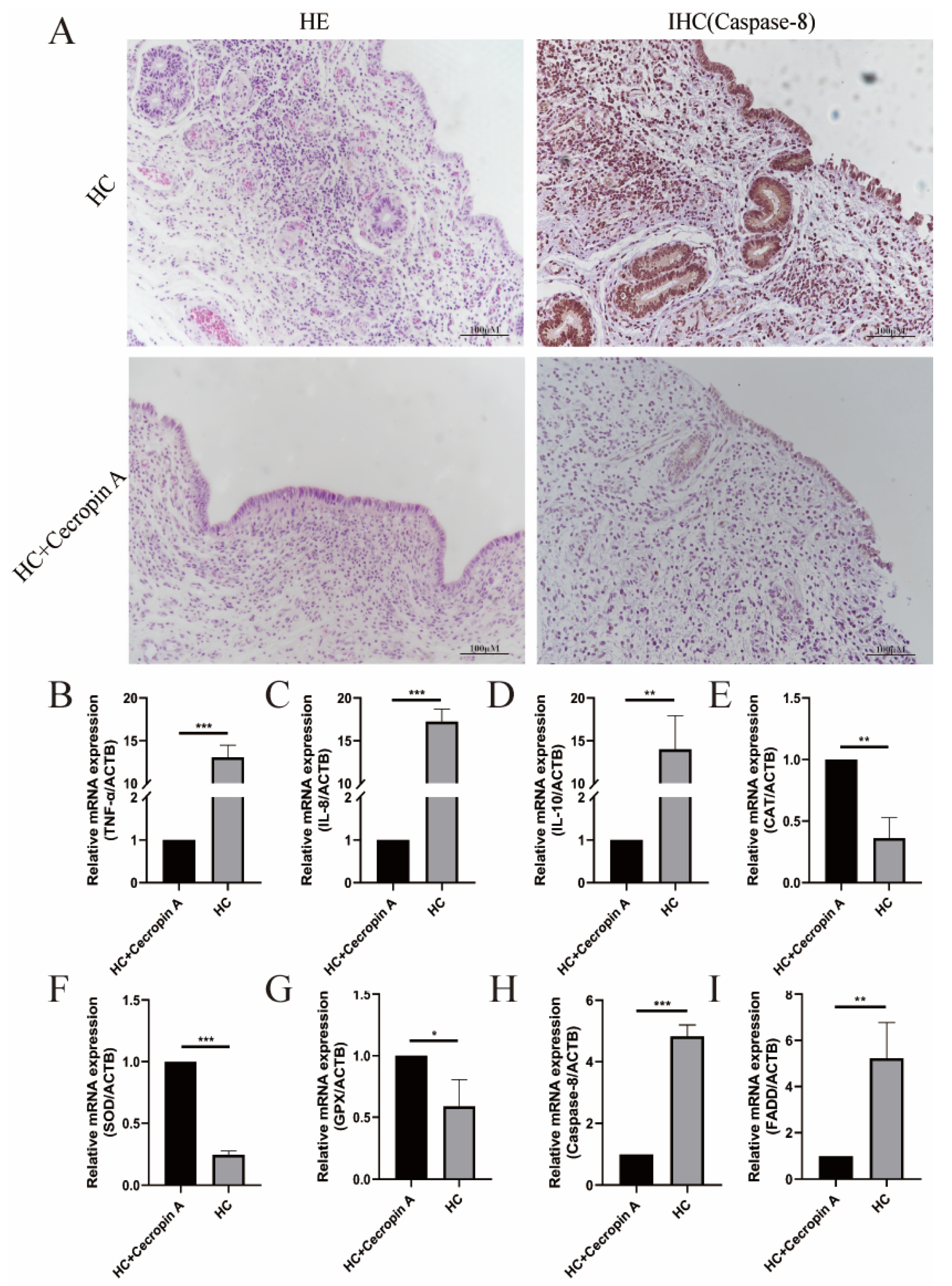
3.1. Detection of Cows’ Uterine Tissue
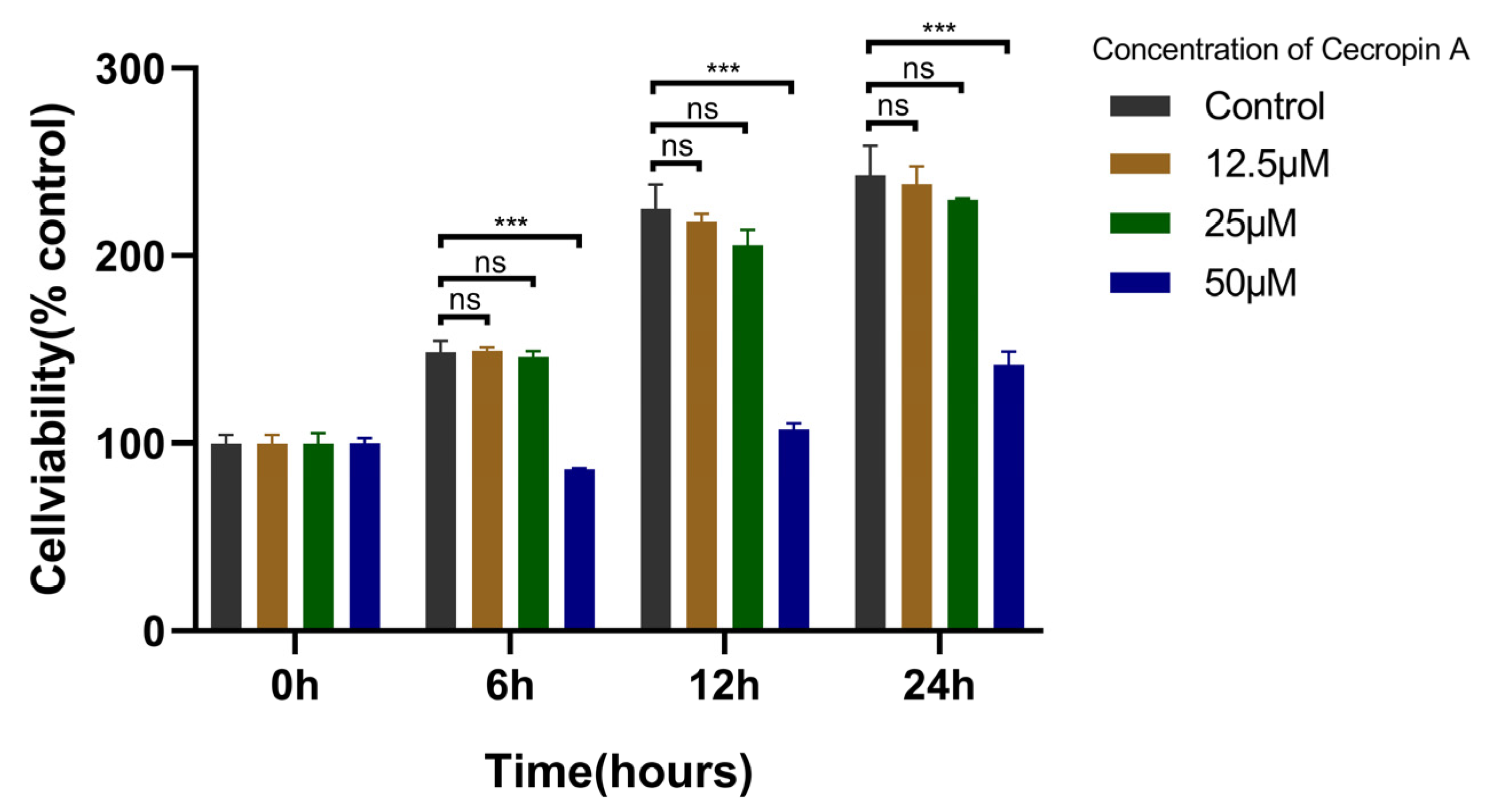
3.2. Cecropin A Toxicity Assay and Concentration Screening
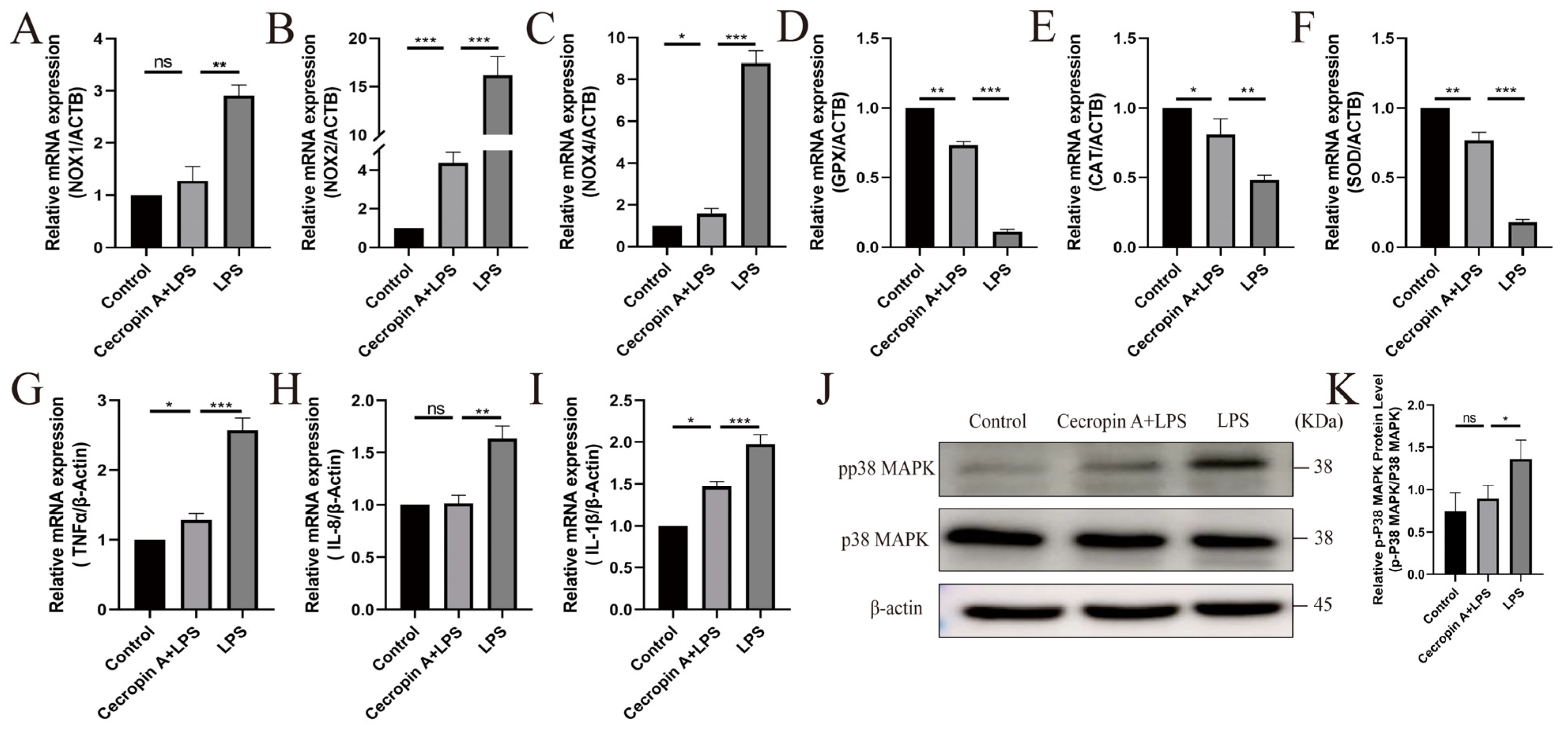
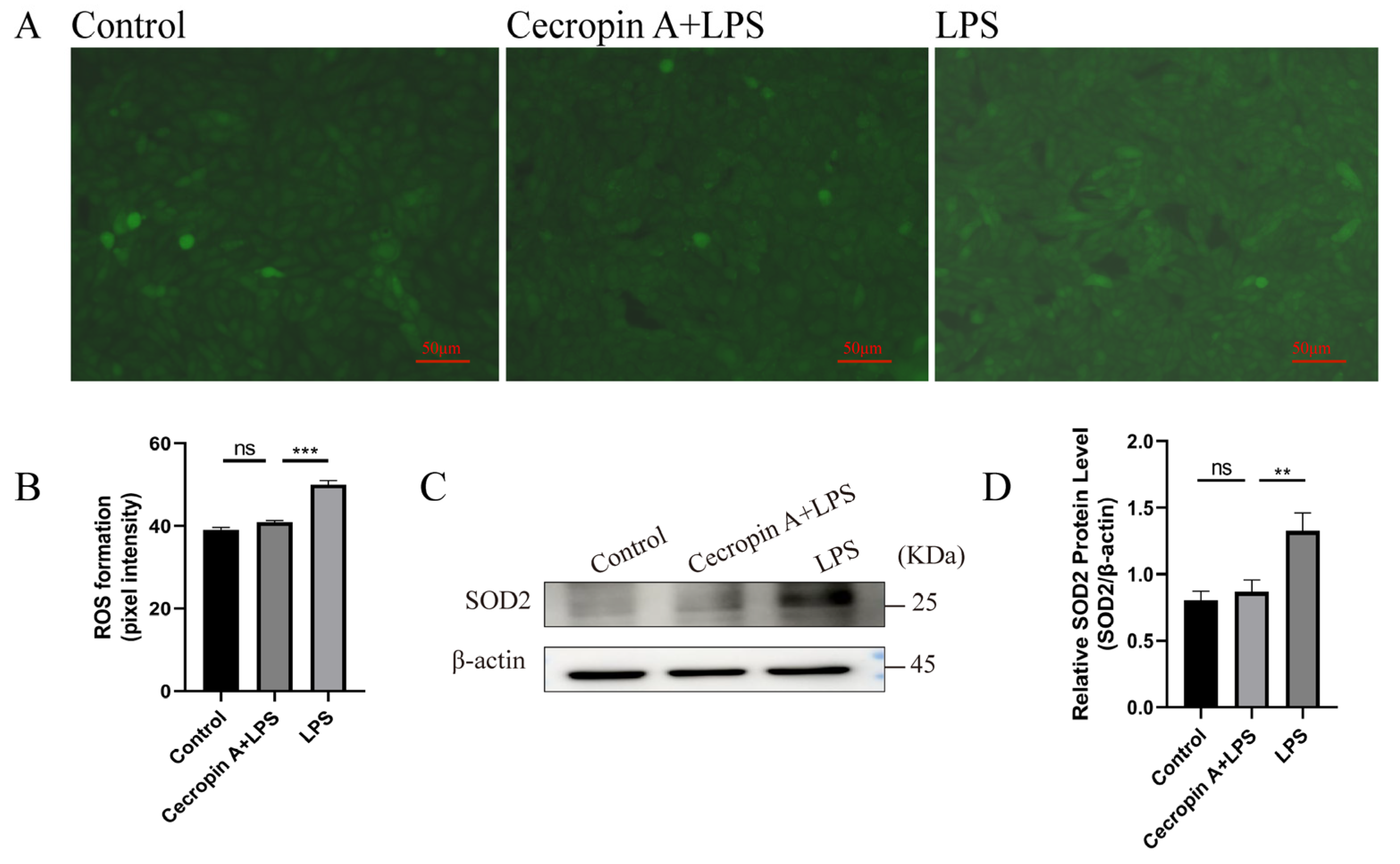
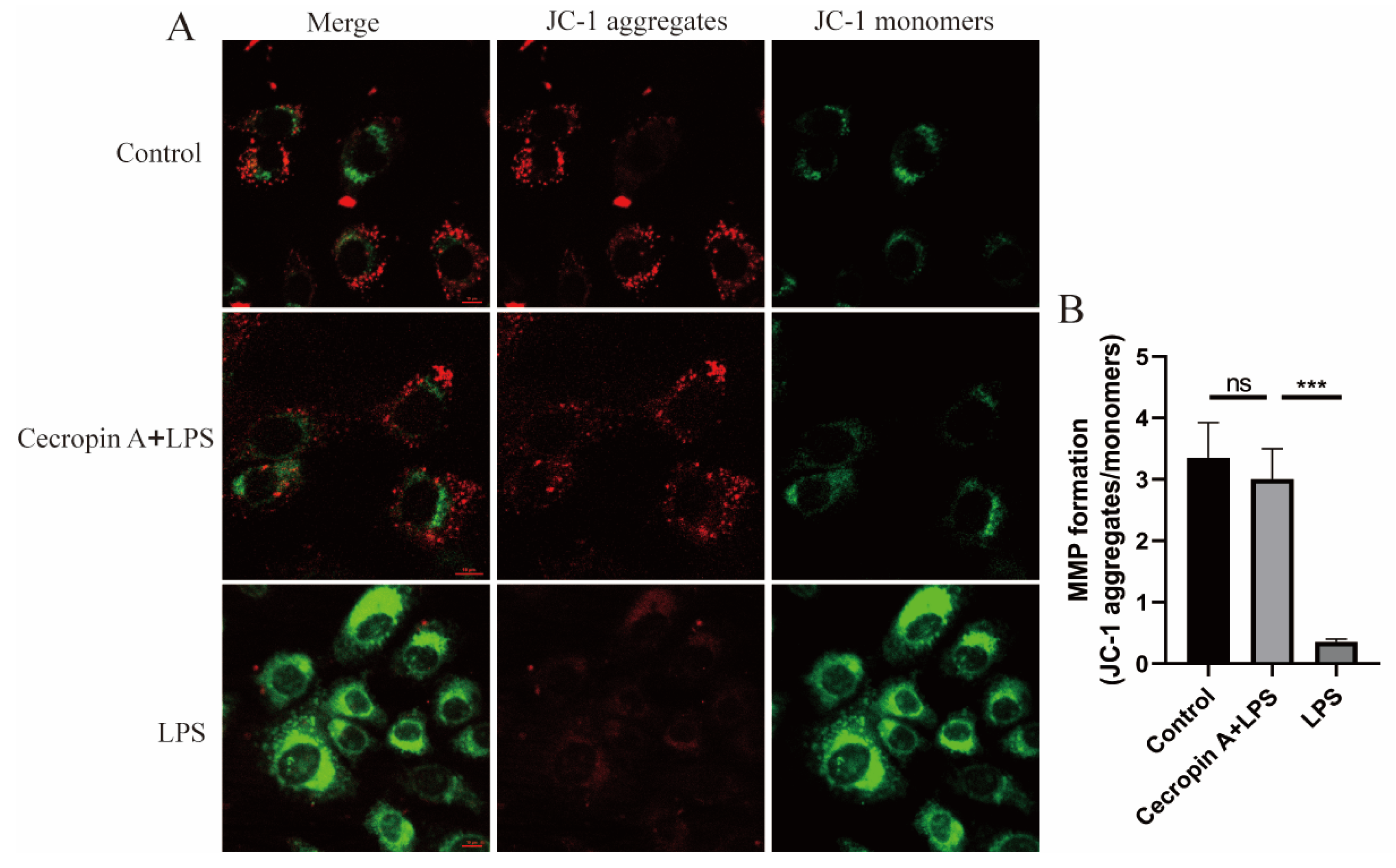
3.3. Cecropin A Prevented Mitochondrial Dysfunction in bEECs
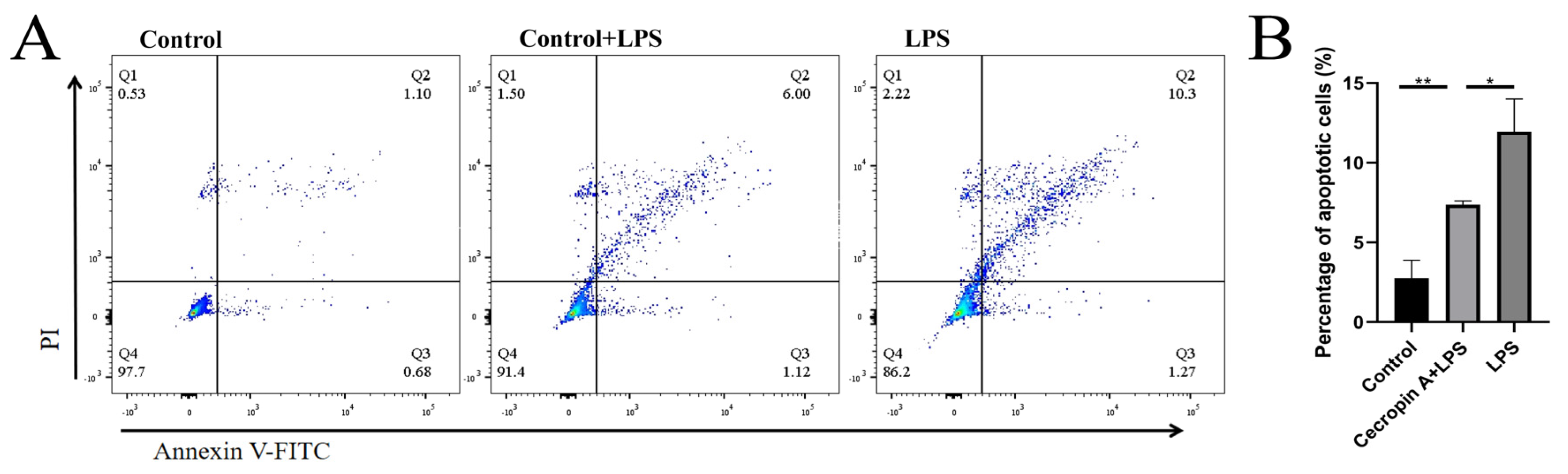
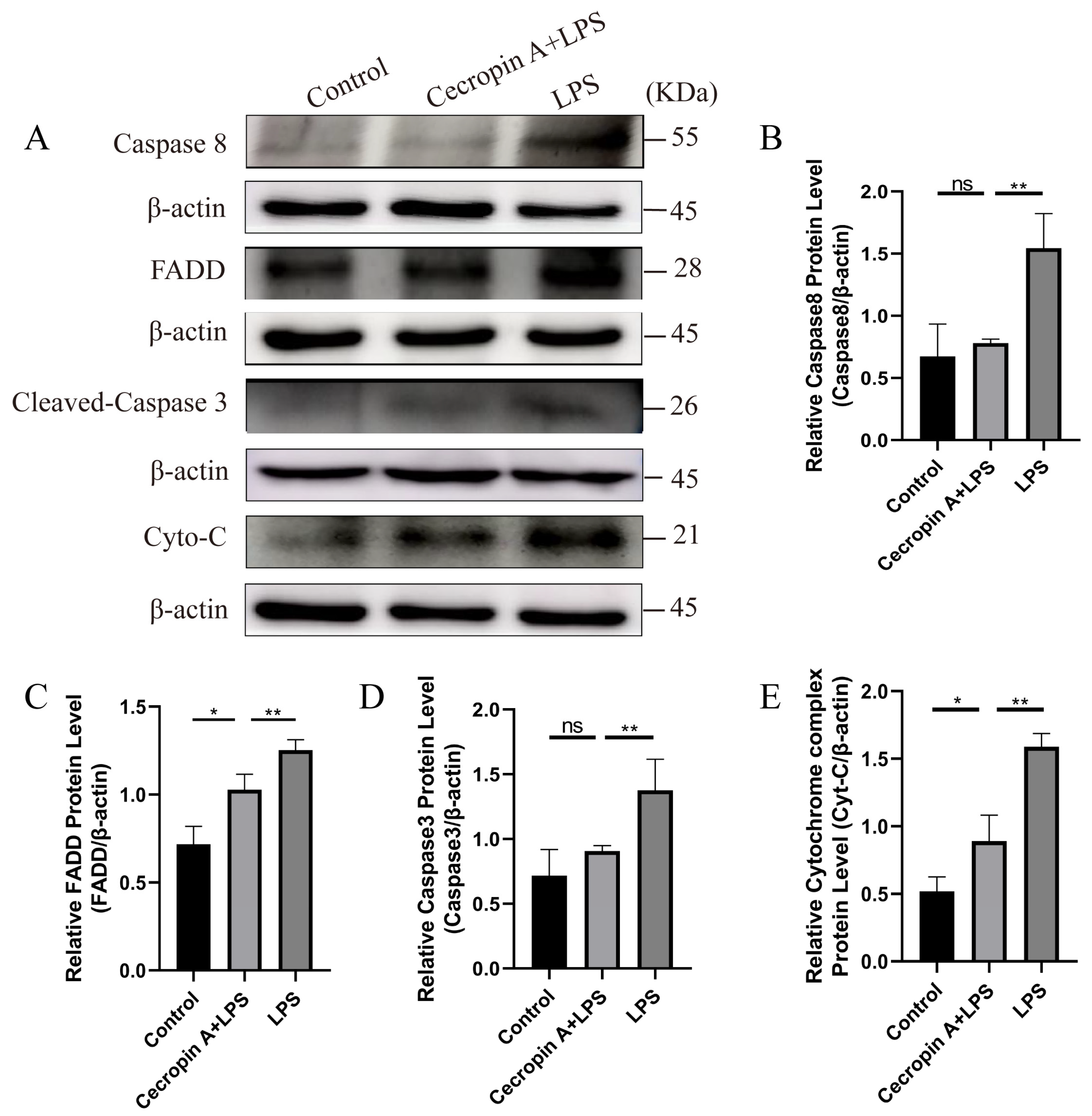
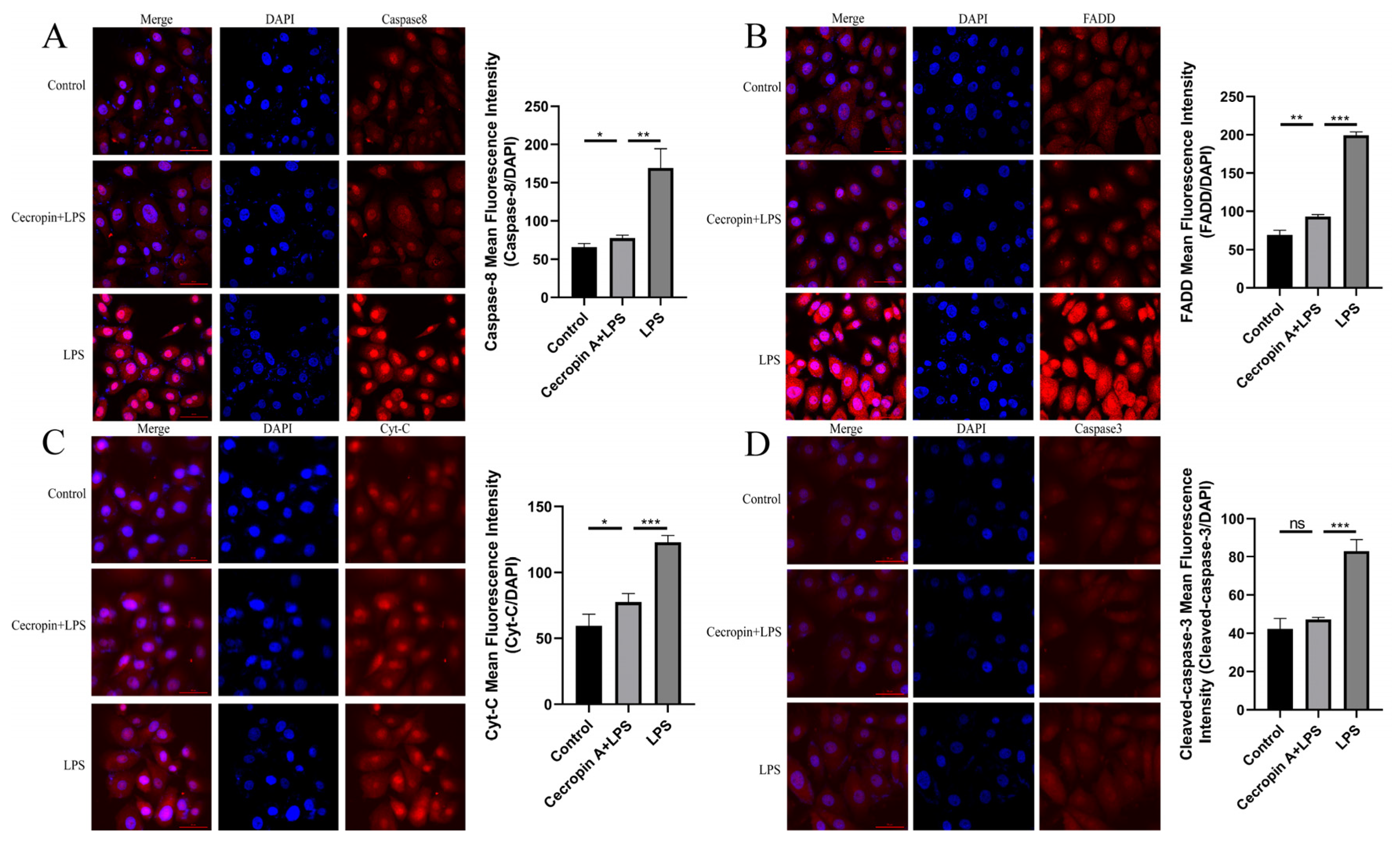
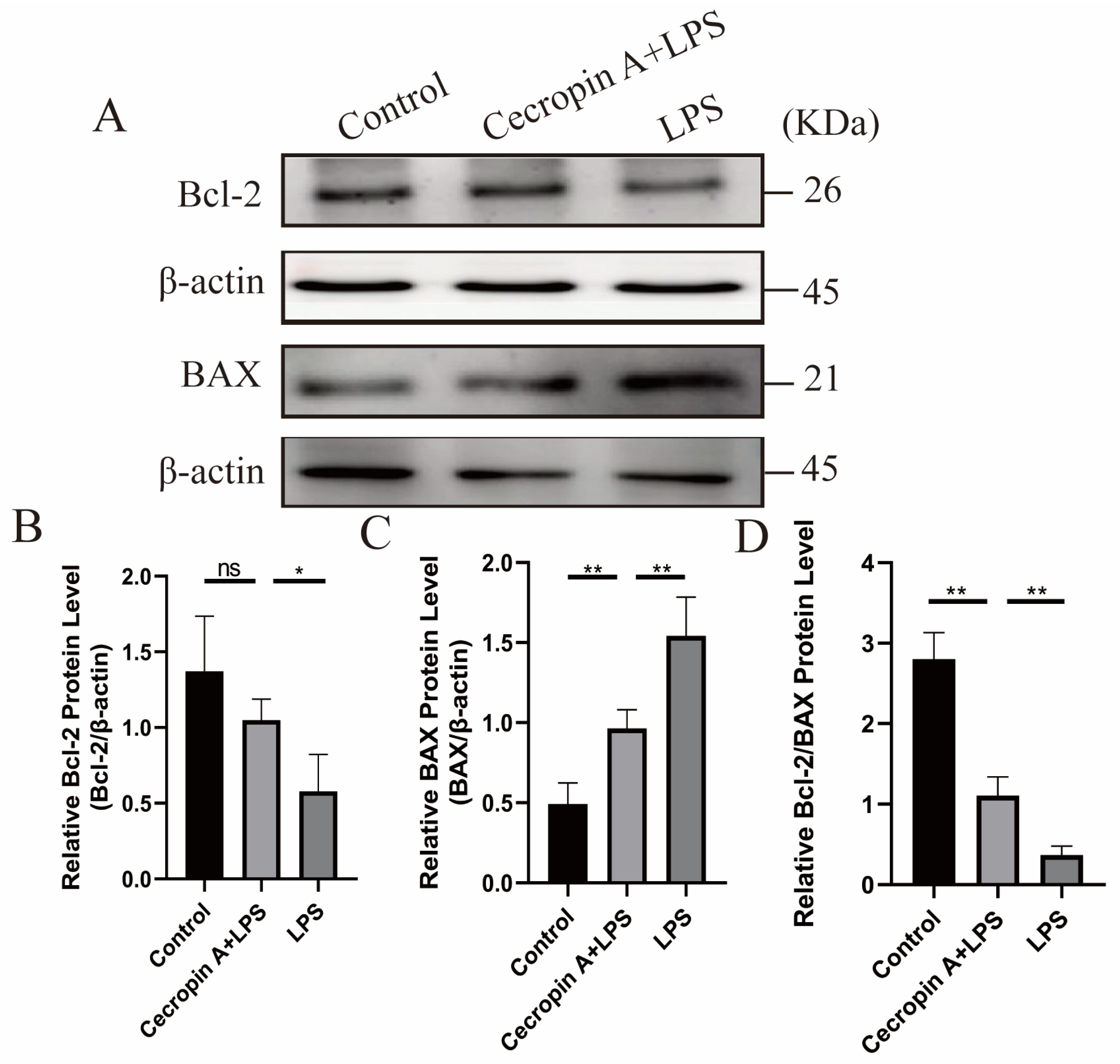
3.4. Cecropin A Inhibited LPS-Induced Apoptosis in bEECs
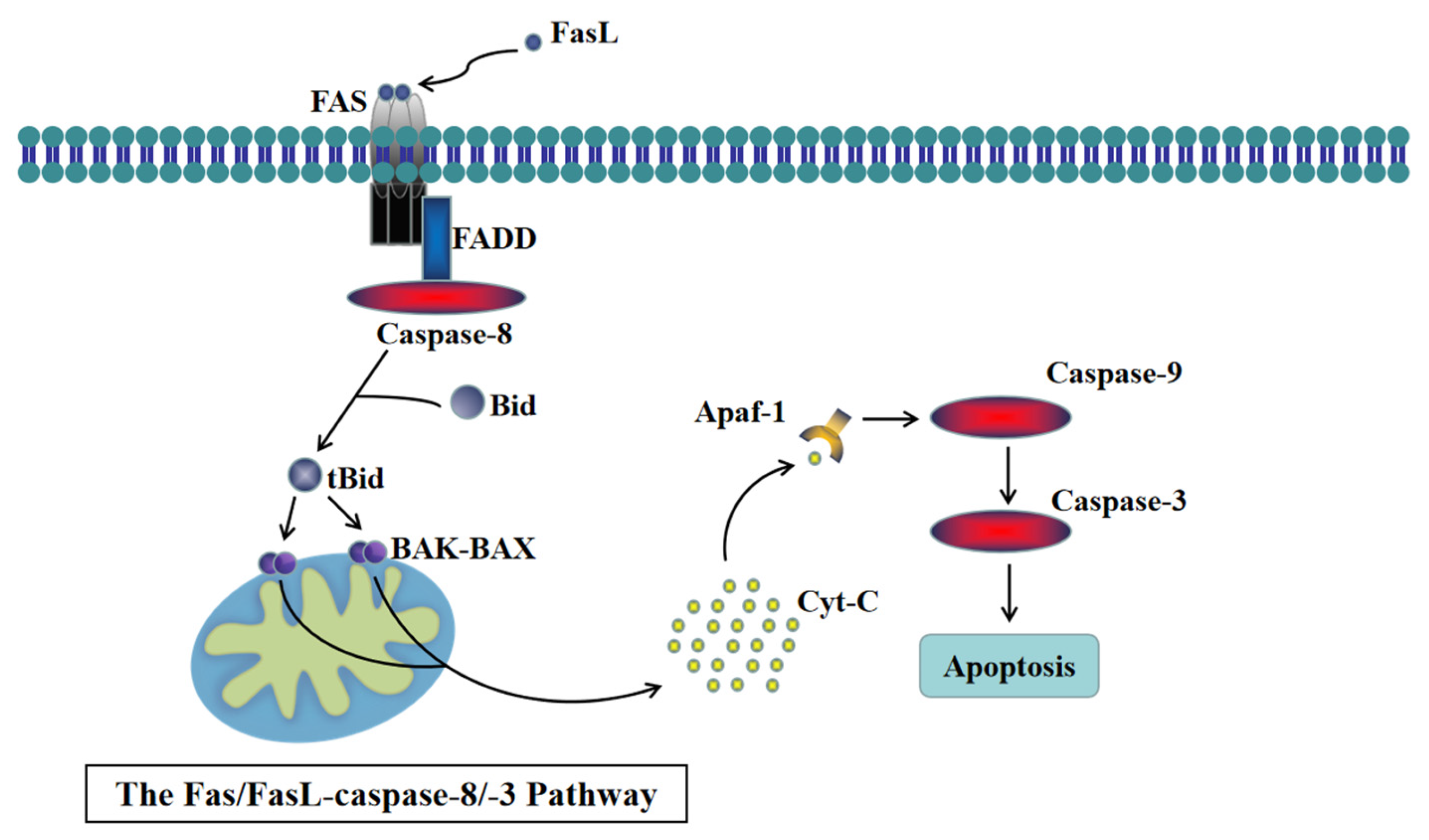
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, H.; Ma, N.; Chang, G.; Aabdin, Z.U.; Shen, X. Long-term high-concentrate diet feeding induces apoptosis of rumen epithelial cells and inflammation of rumen epithelium in dairy cows. Anim. Biotechnol. 2022, 33, 289–296. [Google Scholar] [CrossRef]
- Li, M.-Z.; Wen, X.-Y.; Liu, X.-Q.; Wang, Y.-Q.; Yan, L. LPS-Induced Activation of the cGAS-STING Pathway is Regulated by Mitochondrial Dysfunction and Mitochondrial DNA Leakage in Endometritis. J. Inflamm. Res. 2022, 15, 5707–5720. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Wang, H.; Kwon, Y.H.; Gautam, J.; Haq, S.; Grondin, J.; Steinberg, G.R.; Khan, W.I. Inhibition of NADPH Oxidase (NOX) 2 Mitigates Colitis in Mice with Impaired Macrophage AMPK Function. Biomedicines 2023, 11, 1443. [Google Scholar] [CrossRef]
- Meng, Y.; Lin, W.; Wang, N.; Wei, X.; Huang, Q.; Liao, Y. Bazedoxifene-induced ROS promote mitochondrial dysfunction and enhance osimertinib sensitivity by inhibiting the p-STAT3/SOCS3 and KEAP1/NRF2 pathways in non-small cell lung cancer. Free Radic. Biol. Med. 2023, 196, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Deng, J.S.; Huang, W.C.; Shieh, P.C.; Chung, M.I.; Huang, G.J. Salvianolic Acid C against Acetaminophen-Induced Acute Liver Injury by Attenuating Inflammation, Oxidative Stress, and Apoptosis through Inhibition of the Keap1/Nrf2/HO-1 Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 9056845. [Google Scholar] [CrossRef]
- Lutz, A.; Sanwald, J.; Thomas, M.; Feuer, R.; Sawodny, O.; Ederer, M.; Borner, C.; Humar, M.; Merfort, I. Interleukin-1β enhances FasL-induced caspase-3/-7 activity without increasing apoptosis in primary mouse hepatocytes. PLoS ONE 2014, 9, e115603. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα differentially influence NF-κB activity and FasL-induced apoptosis in primary murine hepatocytes during LPS-induced inflammation. Front. Physiol. 2019, 10, 117. [Google Scholar] [CrossRef]
- Fodor, R.-Ş.; Georgescu, A.-M.; Grigorescu, B.-L.; Cioc, A.D.; Veres, M.; Cotoi, O.-S.; Fodor, P.; Copotoiu, S.-M.; Azamfirei, L. Caspase 3 expression and plasma level of Fas ligand as apoptosis biomarkers in inflammatory endotoxemic lung injury. Rom. J. Morphol. Embryol. 2016, 57, 951–957. [Google Scholar]
- Quistad, S.; Traylor-Knowles, N. Precambrian origins of the TNFR superfamily. Cell Death Discov. 2016, 2, 16058. [Google Scholar] [CrossRef][Green Version]
- Du, Y.; Zhang, J.; Zheng, Q.; Li, M.; Liu, Y.; Zhang, B.; Liu, B.; Zhang, H.; Miao, G. Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons. Neural Regen. Res. 2014, 9, 1129–1137. [Google Scholar] [CrossRef]
- Adrain, C.; Martin, S.J. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends Biochem. Sci. 2001, 26, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Gaume, B.; Karbowski, M.; Sharpe, J.C.; Cecconi, F.; Youle, R.J. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003, 22, 4385–4399. [Google Scholar] [CrossRef] [PubMed]
- Gerner, C.; Fröhwein, U.; Gotzmann, J.; Bayer, E.; Gelbmann, D.; Bursch, W.; Schulte-Hermann, R. The Fas-induced apoptosis analyzed by high throughput proteome analysis. J. Biol. Chem. 2000, 275, 39018–39026. [Google Scholar] [CrossRef] [PubMed]
- Miramar, M.D.; Costantini, P.; Ravagnan, L.; Saraiva, L.M.; Haouzi, D.; Brothers, G.; Penninger, J.M.; Peleato, M.L.; Kroemer, G.; Susin, S.A. NADH Oxidase Activity of Mitochondrial Apoptosis-inducing Factor. J. Biol. Chem. 2001, 276, 16391–16398. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292: 246–248. 1981. J. Immunol. (Baltimore Md. 1950) 2009, 182, 6635–6637. [Google Scholar]
- Kim, J.-K.; Lee, E.; Shin, S.; Jeong, K.-w.; Lee, J.-Y.; Bae, S.-Y.; Kim, S.-H.; Lee, J.; Kim, S.R.; Lee, D.G. Structure and function of papiliocin with antimicrobial and anti-inflammatory activities isolated from the swallowtail butterfly, Papilio xuthus. J. Biol. Chem. 2011, 286, 41296–41311. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Heumann, D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells. J. Immunol. 2001, 167, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Shin, A.; Kim, Y. Anti-inflammatory activities of cecropin a and its mechanism of action. Arch. Insect Biochem. Physiol. 2015, 88, 31–44. [Google Scholar] [CrossRef]
- Chen, P.; Chen, P.; Guo, Y.; Fang, C.; Li, T. Interaction between chronic endometritis caused endometrial microbiota disorder and endometrial immune environment change in recurrent implantation failure. Front. Immunol. 2021, 12, 748447. [Google Scholar] [CrossRef]
- Zhai, Z.; Ni, X.; Jin, C.; Ren, W.; Li, J.; Deng, J.; Deng, B.; Yin, Y. Cecropin A modulates tight junction-related protein expression and enhances the barrier function of porcine intestinal epithelial cells by suppressing the MEK/ERK pathway. Int. J. Mol. Sci. 2018, 19, 1941. [Google Scholar] [CrossRef]
- Meng, M.; Zhao, X.; Huo, R.; Li, X.; Chang, G.; Shen, X. Disodium Fumarate Alleviates Endoplasmic Reticulum Stress, Mitochondrial Damage, and Oxidative Stress Induced by the High-Concentrate Diet in the Mammary Gland Tissue of Hu Sheep. Antioxidants 2023, 12, 223. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Z.; Chang, X.; Huang, C.; Qiu, R.; Wang, A.; Lin, P.; Tang, K.; Chen, H.; Zhou, D. Proteomic analysis of uterine lavage fluid of dairy cows at different time after delivery by mass spectrometry. Theriogenology 2023, 207, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Mallona, I.; Weiss, J.; Egea-Cortines, M. PcrEfficiency: A Web tool for PCR amplification efficiency prediction. BMC Bioinform. 2011, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, S.; Mahdavi, A.H.; Hajian, M.; Kowsar, R.; Varnosfaderani, S.R.; Nasr-Esfahani, M.H. The attenuation of the toxic effects of LPS on mouse pre-implantation development by alpha-lipoic acid. Theriogenology 2020, 143, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Lv, J.; Duan, H.; Yang, S.; Wu, J.; Yan, Z.; Zhang, R.; Hu, J.; Zhang, Y. Subacute Ruminal Acidosis as a Potential Factor that Induces Endometrium Injury in Sheep. Int. J. Mol. Sci. 2023, 24, 21192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Zhou, D.; Haddad, G.G. Antimicrobial peptides increase tolerance to oxidant stress in Drosophila melanogaster. J. Biol. Chem. 2011, 286, 6211–6218. [Google Scholar] [CrossRef]
- Ross, C.R.; Ricevuti, G.; Scovassi, A.I. The Antimicrobial Peptide PR-39 has a Protective Effect Against HeLa Cell Apoptosis. Chem. Biol. Drug Des. 2007, 70, 154–157. [Google Scholar] [CrossRef]
- Ryu, S.; Choi, S.-Y.; Acharya, S.; Chun, Y.-J.; Gurley, C.; Park, Y.; Armstrong, C.A.; Song, P.I.; Kim, B.-J. Antimicrobial and anti-inflammatory effects of cecropin A (1–8)–Magainin2 (1–12) hybrid peptide analog P5 against Malassezia furfur infection in human keratinocytes. J. Investig. Dermatol. 2011, 131, 1677–1683. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Margaritis, L.H. Oxidative stress and NADPH oxidase: Connecting electromagnetic fields, cation channels and biological effects. Int. J. Mol. Sci. 2021, 22, 10041. [Google Scholar] [CrossRef]
- Sul, O.-J.; Ra, S.W. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Regdon, Z.; Robaszkiewicz, A.; Kovács, K.; Rygielska, Ż.; Hegedűs, C.; Bodoor, K.; Szabó, É.; Virág, L. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic. Biol. Med. 2019, 131, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Zhang, C.; Li, R.; Guo, Z. CircPalm2 knockdown alleviates LPS-evoked pulmonary microvascular endothelial cell apoptosis and inflammation via miR-450b-5p/ROCK1 axis. Int. Immunopharmacol. 2022, 113, 109199. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Jian, Z.; Zhu, W.; Xu, J.; Fan, Y.; Xiao, F. KLF13 overexpression protects sepsis-induced myocardial injury and LPS-induced inflammation and apoptosis. Int. J. Exp. Pathol. 2023, 104, 23–32. [Google Scholar] [CrossRef]
- Song, P.; Liu, C.; Sun, M.; Liu, J.; Lin, P.; Wang, A.; Jin, Y. Oxidative stress induces bovine endometrial epithelial cell damage through mitochondria-dependent pathways. Animals 2022, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, M.S.; Sunzini, F.; Fiorucci, L.; Botti, E.; Fonti, G.L.; Conigliaro, P.; Triggianese, P.; Costa, L.; Caso, F.; Giunta, A. Potential role of cytochrome c and tryptase in psoriasis and psoriatic arthritis pathogenesis: Focus on resistance to apoptosis and oxidative stress. Front. Immunol. 2018, 9, 2363. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, Y.; Lu, Z.; Wang, H.; Feng, Z.; Wang, J.; Xiao, S.; Liu, F.; Liu, J. LL-37 attenuates inflammatory impairment via mTOR signaling-dependent mitochondrial protection. Int. J. Biochem. Cell Biol. 2014, 54, 26–35. [Google Scholar] [CrossRef][Green Version]
- Wu, H.-Y.; Lin, T.-K.; Kuo, H.-M.; Huang, Y.-L.; Liou, C.-W.; Wang, P.-W.; Chuang, J.-H.; Huang, S.-T. Phyllanthus urinaria induces apoptosis in human osteosarcoma 143B cells via activation of Fas/FasL-and mitochondria-mediated pathways. Evid.-Based Complement. Altern. Med. 2012, 2012, 925824. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Xaus, J.; Valledor, A.F.; López-López, C.; Pennington, D.J.; Celada, A. PKCϵ is involved in JNK activation that mediates LPS-induced TNF-α, which induces apoptosis in macrophages. Am. J. Physiol.-Cell Physiol. 2003, 285, C1235–C1245. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Imamura, R.; Umemura, M.; Kawabe, T.; Suda, T. Pathogen-associated molecular patterns sensitize macrophages to Fas ligand-induced apoptosis and IL-1β release. J. Immunol. 2003, 171, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
| Ingredient (% DM) | Nutrient Composition (% DM) | ||
|---|---|---|---|
| Maize | 24.92 | Crude protein | 16.21 |
| Soybean meal | 13.48 | Calcium | 1.18 |
| Barley | 12.00 | Phosphorus | 0.51 |
| Distiller-dried grains with solubility | 5.91 | Neutral detergent fiber (NDF) | 29.92 |
| Silage corn | 6.00 | Non-fibrous carbohydrate (NFC) | 42.34 |
| Alfalfa | 17.00 | Crude ash | 4.87 |
| Oat grass | 17.00 | Crude fat | 3.05 |
| Limestone | 1.48 | Starch | 27.82 |
| CaHPO4 | 0.92 | ||
| NaCl | 0.37 | NDF/NFC | 0.71 |
| Premix | 0.92 | Dry matter | 48.32 |
| Gene Name | ID | Primer Sequences (from 50′ to 30′) | Size (bp) | Primer Efficiencies |
|---|---|---|---|---|
| CAT | NM_001035386.2 | F: AGAGGAAACGCCTGTGTGAG R: ATGCGGGAGCCATATTCAGG | 115 | 97.91% |
| SOD | NM_174615.2 | F: CTCTACTTGGTTGGGGCGTC R: TCGAAGTGGATGGTGCCTTG | 122 | 95.3% |
| GPx | NM_174076.3 | F: AACGTAGCATCGCTCTGAGG R: GATGCCCAAACTGGTTGCAG | 121 | 105.23% |
| NOX1 | NM_001191340.1 | F: TGTCTTTCCTGAGAGGCACC R: TTTGTGGAAGGCGAGGTTGT | 80 | 93.22% |
| NOX2 | NM_174035.4 | F: CAAGATGGAGGTGGGCCAAT R: GAGGTCAGGGTGAAAGGGTG | 81 | 95.44% |
| NOX4 | NM_001304775.1 | F: TCTGGACCTTTGTGCCT R: GACGGATGACTTGTGACTG | 95 | 96.82% |
| TNF-α | NM_173966 | F: GCTCTTACCGGAACACTTCG R: GGACACCTTGACCTCCTGAA | 238 | 108.1% |
| IL-1β | NM_174093 | F: AACCGAGAAGTGGTGTTCTGC R: TTGGGGTAGACTTTGGGGTCT | 167 | 102.62% |
| IL-8 | NM_173925.2 | F: CATTCCACACCTTTCCACCC R: AGGCAGACCTCGTTTCCATT | 116 | 93.88% |
| IL-10 | NM_174088.1 | F: CACAGGCTGAGAACCACG R: AGGGCAGAAAGCGATGA | 108 | 95.43% |
| FADD | NM_001007816.1 | F: CCGGAGGACCGAGACCTG R: CGTCAGATACTCCGAGGTGC | 97 | 98.43% |
| Caspase-8 | XM_005202615.5 | F: AGCATAGCACGGAAGCAGG R: GGTCTTATCCAAAGCGTCTGC | 87 | 96.32% |
| ACTB (β-Actin) | NM_173979.3 | F: GGCACCCAGCACAATGAAGA R: GCCAATCCACACGGAGTACTT | 67 | 99.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Y.; Sun, M.; Li, B.; Li, Y.; Hua, S. Cecropin A Alleviates LPS-Induced Oxidative Stress and Apoptosis of Bovine Endometrial Epithelial Cells. Animals 2024, 14, 768. https://doi.org/10.3390/ani14050768
Zhao Y, Zhang Y, Sun M, Li B, Li Y, Hua S. Cecropin A Alleviates LPS-Induced Oxidative Stress and Apoptosis of Bovine Endometrial Epithelial Cells. Animals. 2024; 14(5):768. https://doi.org/10.3390/ani14050768
Chicago/Turabian StyleZhao, Yu, Yang Zhang, Mingkun Sun, Bowen Li, Yuqiong Li, and Song Hua. 2024. "Cecropin A Alleviates LPS-Induced Oxidative Stress and Apoptosis of Bovine Endometrial Epithelial Cells" Animals 14, no. 5: 768. https://doi.org/10.3390/ani14050768
APA StyleZhao, Y., Zhang, Y., Sun, M., Li, B., Li, Y., & Hua, S. (2024). Cecropin A Alleviates LPS-Induced Oxidative Stress and Apoptosis of Bovine Endometrial Epithelial Cells. Animals, 14(5), 768. https://doi.org/10.3390/ani14050768






