Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feeding Diets
2.2. Fish and Feeding Trial
2.3. Sample Collection and Measurement of Growth Indices
2.4. Assessment of Proximate Composition
2.5. Assessment of Hematological and Serum Biochemical Parameters
2.6. Measurement of Digestive Enzyme Activities
2.7. Measurement and Analysis of Muscle Quality
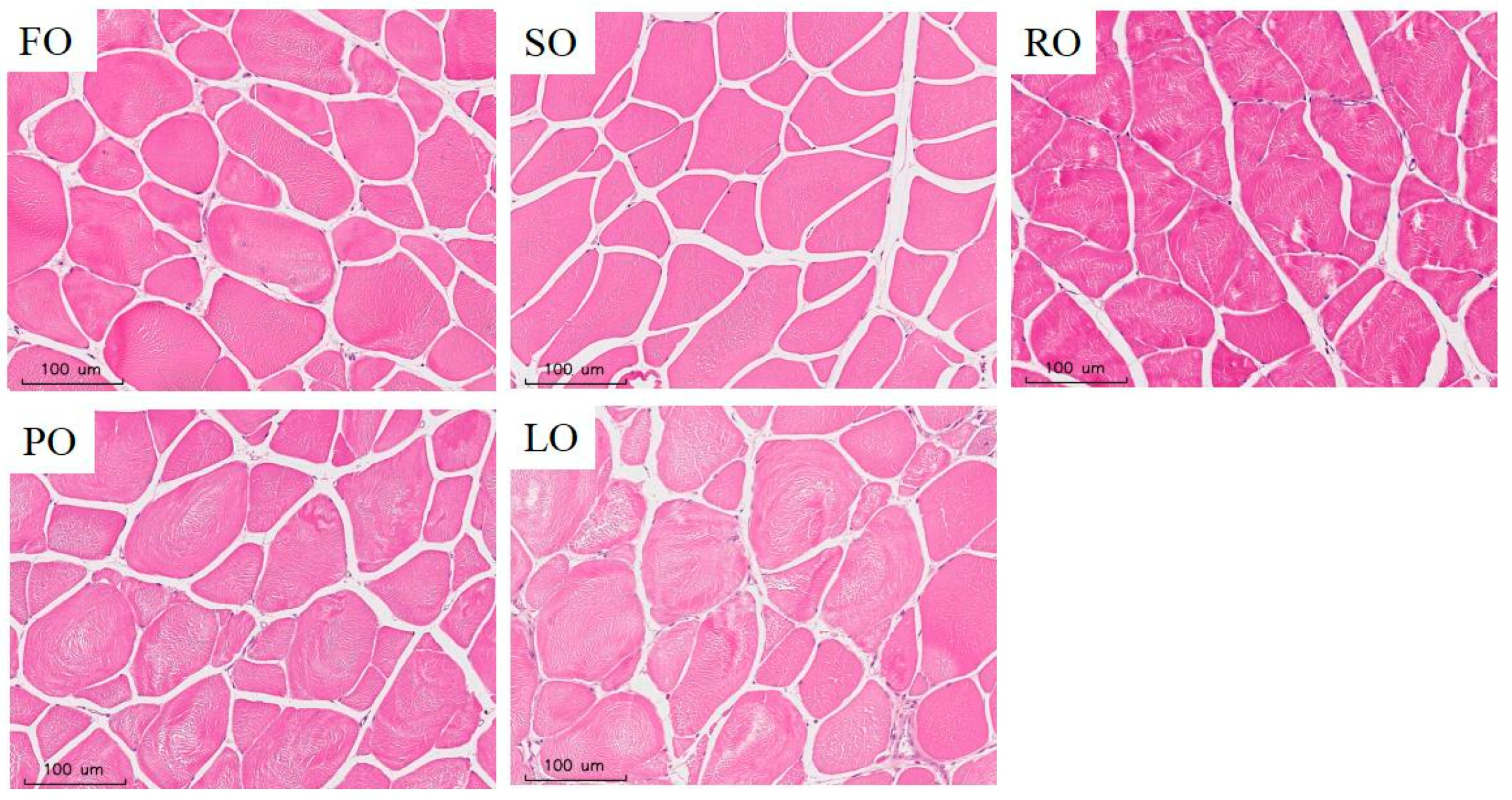
2.7.1. Muscle Texture, Odor and Histomorphometry
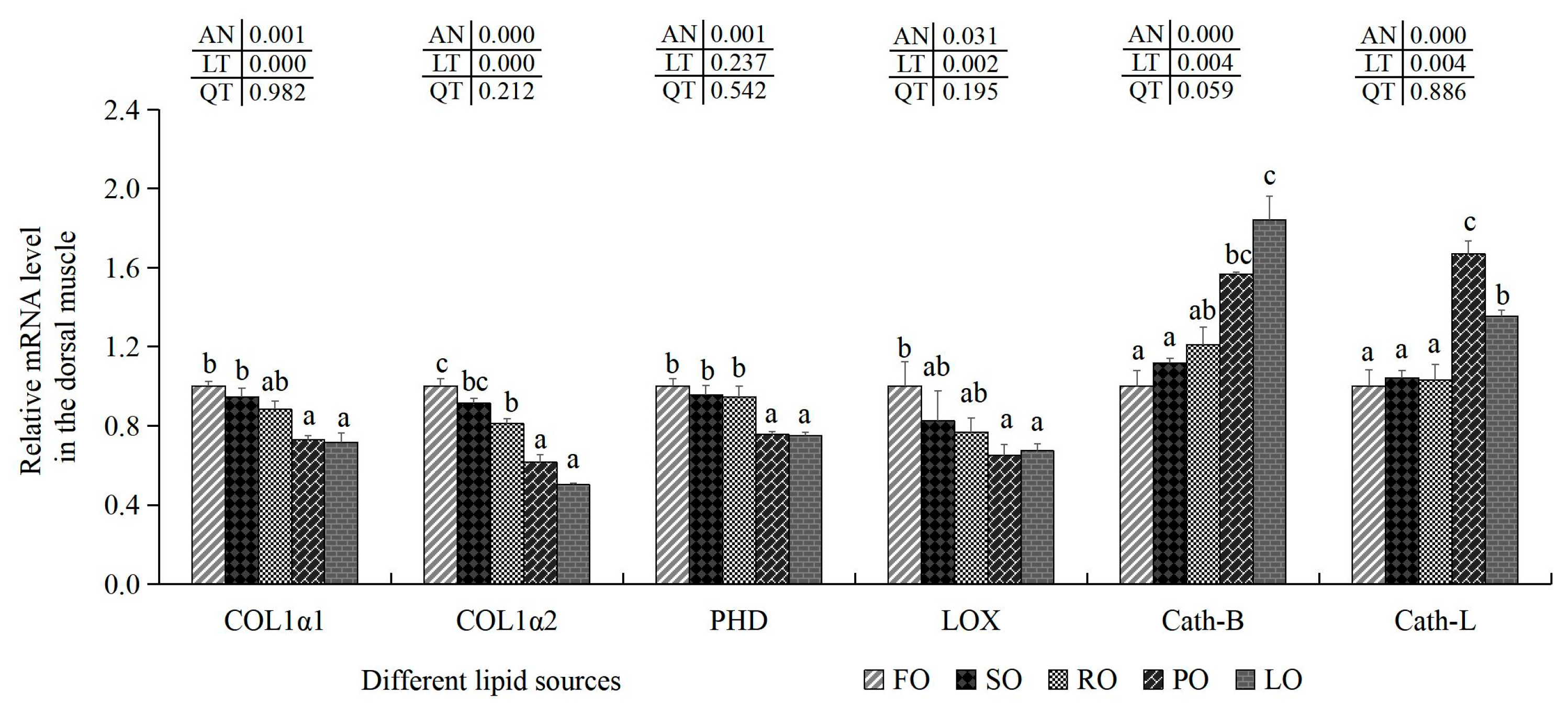
2.7.2. Collagen Synthesis-Related Indexes and Cathepsin Contents
2.8. Measurement and Analysis of Function Gene Expression Variations
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Proximate Composition
3.3. Hematological and Biochemical Parameters
3.4. Digestive Enzymes
3.5. Hepatic Immune and Inflammatory Indices
3.6. Muscle Quality
3.6.1. Muscle Texture, Odor and Histomorphometry
3.6.2. Collagen Synthesis-Related Indexes and Cathepsin Content in the Muscle
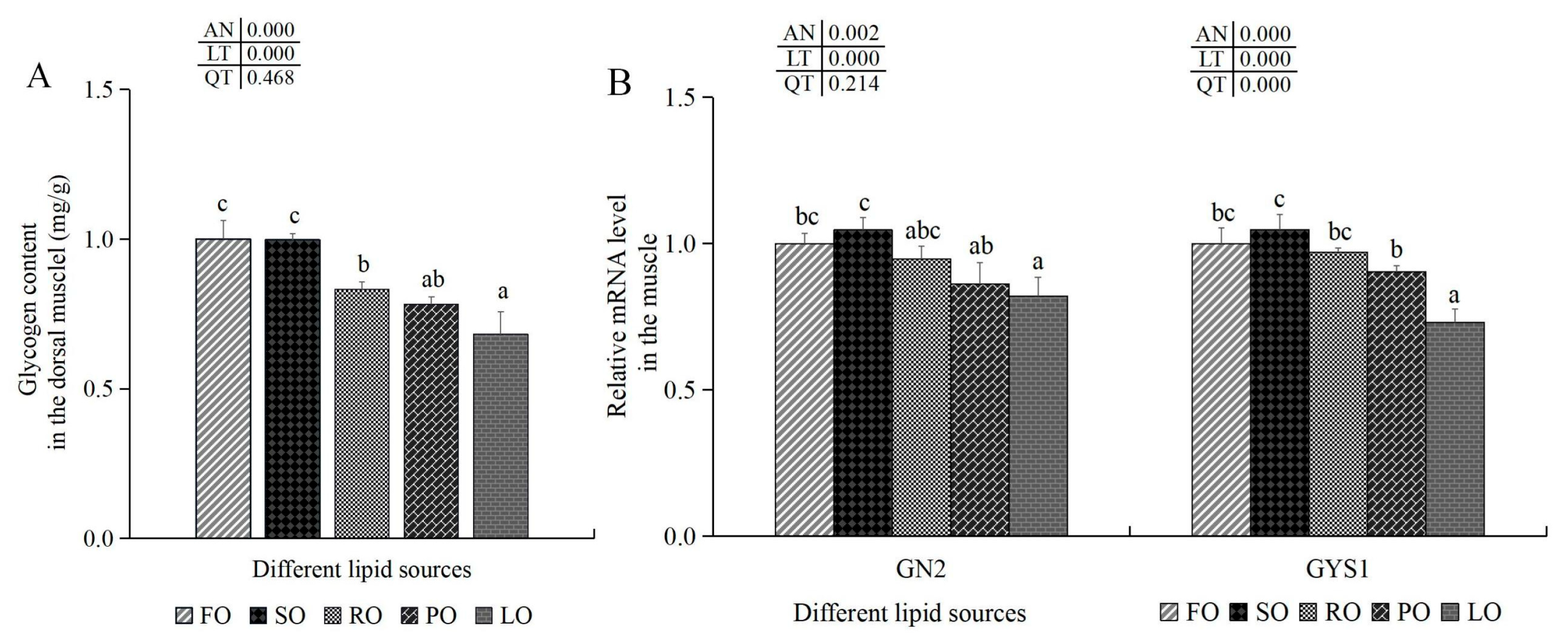
3.6.3. Glycogen Synthesis and Antioxidant-Related Indexes in the Muscle
4. Discussion
4.1. Growth Indices and Proximate Composition
4.2. Serum Biochemical Parameters
4.3. Digestive Enzymes
4.4. Immune and Inflammatory Indices
4.5. Muscle Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Q.; Chen, Q.; Shen, Z.; Li, D.; Han, T.; Qin, J.; Chen, L.; Du, Z. The metabolomics responses of Chinese mitten-hand crab (Eriocheir sinensis) to different dietary oils. Aquaculture 2017, 479, 188–199. [Google Scholar] [CrossRef]
- Monroig, O.; Tocher, D.R.; Castro, L.F.C. Chapter 3—Polyunsaturated fatty acid biosynthesis and metabolism in fish. In Polyunsaturated Fatty Acid Metabolism; Burdge, G.C., Ed.; AOCS Press: Urbana, IL, USA, 2018; pp. 31–60. [Google Scholar]
- Turchini, G.M.; Ng, W.K.; Tocher, D.R. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press Inc.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Zhang, C.; Huang, K.; Lu, K.; Wang, L.; Song, K.; Zhang, L.; Li, P. Effects of different lipid sources on growth performance, body composition and lipid metabolism of bullfrog Lithobates catesbeiana. Aquaculture 2016, 457, 104–108. [Google Scholar] [CrossRef]
- Apraku, A.; Liu, L.; Leng, X.J.; Rupia, E.J.; Ayisi, C.L. Evaluation of blended virgin coconut oil and fish oil on growth performance and resistance to Streptococcus iniae challenge of Nile tilapia (Oreochromis niloticus). Egypt. J. Basic Appl. Sci. 2017, 4, 175–184. [Google Scholar]
- Zhang, F.; Li, L.; Li, P.; Meng, X.; Cui, X.; Ma, Q.; Wei, Y.; Liang, M.; Xu, H. Fish oil replacement by beef tallow in juvenile turbot diets: Effects on growth performance, body composition and volatile flavor compounds in the muscle. Aquaculture 2023, 564, 739070. [Google Scholar] [CrossRef]
- Lazzari, R.; Emanuelli, T.; Maschio, D.; Ferreira, C.C.; Battisti, E.K.; Radunz Neto, J. The inclusion of soybean oil in the diets of silver catfish (Rhamdia quelen) in relation to growth quality and fillet acceptability. Lat. Am. J. Aquat. Res. 2016, 44, 39–45. [Google Scholar] [CrossRef]
- Yıldız, M.; Eroldoğan, T.O.; Ofori-Mensah, S.; Engin, K.; Baltacı, M.A. The effects of fish oil replacement by vegetable oils on growth performance and fatty acid profile of rainbow trout: Re-feeding with fish oil finishing diet improved the fatty acid composition. Aquaculture 2018, 488, 123–133. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Huang, Y.; Hao, T.; Wang, S.; Huang, B.; Sun, Y. Effects of replacing fish oil with wheat germ oil on growth, fat deposition, serum biochemical indices and lipid metabolic enzyme of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 505, 54–62. [Google Scholar]
- Dernekbaşı, S.; İsmihan, K.; Karataş, E.; Parlak Akyüz, A. Potential of using peanut oil as alternative to fish oil for European seabass diets (Dicentrarchus Labrax) in recirculated systems. Alinteri. J. Agr. Sci. 2021, 36, 109–121. [Google Scholar] [CrossRef]
- Castro, C.; Corraze, G.; Firmino-Diógenes, A.; Larroquet, L.; Panserat, S.; Oliva-Teles, A. Regulation of glucose and lipid metabolism by dietary carbohydrate levels and lipid sources in gilthead sea bream juveniles. Brit. J. Nutr. 2016, 116, 19–34. [Google Scholar] [CrossRef]
- Yang, W.; Wu, J.; Song, R.; Li, Z.; Jia, X.; Qian, P.; Zhang, H.; Zhang, P.; Xue, X.; Li, S.; et al. Effects of dietary soybean lecithin on growth performances, body composition, serum biochemical parameters, digestive and metabolic abilities in largemouth bass Micropterus salmoides. Aquac. Res. 2023, 29, 101528. [Google Scholar] [CrossRef]
- Wu, J.; Yang, W.; Song, R.; Li, Z.; Jia, X.; Zhang, H.; Zhang, P.; Xue, X.; Li, S.; Xie, Y.; et al. Dietary soybean lecithin improves growth, immunity, antioxidant capability and intestinal barrier functions in Largemouth Bass Micropterus salmoides juveniles. Metabolites 2023, 13, 512. [Google Scholar] [CrossRef]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Valet, P.; Castan-Laurell, I. Apelin and energy metabolism. Front. Physiol. 2015, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Lessan, N.; Ali, T. Energy metabolism and intermittent fasting: The ramadan perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhao, X.; Jiao, L.; Shen, Y.; Luo, J.; Zhu, T.; Zhao, W.; Gen, Z.; Zhou, Q.; Jin, M. Effects of different lipid sources on growth performance, fatty acids composition in tissue and expression of genes related to lipid metabolism in largemouth bass (Micropterus salmoides). Aquac. Res. 2022, 23, 101013. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, B.; Liu, K.; Dong, X.; Yang, Q.; Chi, S.; Liu, H.; Zhang, S.; Wang, H. Effects of different dietary lipids on growth, body composition and lipid metabolism-related enzymes and genes in juvenile largemouth bass, Micropterus salmoides. Aquac. Nutr. 2019, 25, 1318–1326. [Google Scholar] [CrossRef]
- EL-Deep, M.H.; Amber, K.A.; Eid, Y.Z.; Alrashood, S.T.; Khan, H.A.; Sakr, M.S.; Dawood, M.A.O. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against Escherichia coli in the growing rabbits’ cecum. Front. Vet. Sci. 2020, 7, 579576. [Google Scholar] [CrossRef]
- Fountoulaki, E.; Vasilaki, A.; Hurtado, R.; Grigorakis, K.; Karacostas, I.; Nengas, I.; Rigos, G.; Kotzamanis, Y.; Venou, B.; Alexis, M.N. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: Recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 2009, 289, 317–326. [Google Scholar]
- Mu, H.; Li, J.; Pan, X.; Liu, J.; Chen, J.; Pan, Y.; Zhang, W.; Mai, K. Alterations in fatty acid composition and volatile compounds in muscle of large yellow croaker Larimichthys crocea fed different dietary lipid sources. Aquac. Rep. 2021, 20, 100688. [Google Scholar] [CrossRef]
- Zhuang, J.; Abdullah; Wang, Y.; Shen, W.; Zheng, W.; Liu, T.; Wang, J.; Feng, F. Evaluating dynamic effects of dietary glycerol monolaurate on the productive performance and flesh quality of large yellow croaker (Larimichthys crocea). Food Chem. 2022, 387, 132833. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C.; Anderson, D.M. Full substitution of fish oil with camelina (Camelina sativa) oil, with partial substitution of fish meal with camelina meal, in diets for farmed Atlantic salmon (Salmo salar) and its effect on tissue lipids and sensory quality. Food Chem. 2014, 157, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, L.; Wu, P.; Liu, Y.; Ren, H.; Jin, X.; Jiang, J.; Kuang, S.; Li, S.; Tang, L.; et al. Modification of beneficial fatty acid composition and physicochemical qualities in the muscle of sub-adult grass carp (Ctenopharyngodon idella): The role of lipids. Aquaculture 2022, 561, 738656. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Li, X.; Tan, S.; Cheng, Z.; Leng, X. Influences of dietary Eucommia ulmoides extract on growth, flesh quality, antioxidant capacity and collagen-related genes expression in grass carp (Ctenopharyngodon idellus). Anim. Feed. Sci. Technol. 2021, 277, 114965. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Li, W.; Qiao, F.; Zhang, W.; Du, Z.; Zhang, M. Partial replacement of soybean meal by yellow mealworm (Tenebrio molitor) meal influences the flesh quality of Nile tilapia (Oreochromis niloticus). Anim. Nut. 2023, 12, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Harimana, Y.; Tang, X.; Xu, P.; Xu, G.; Karangwa, E.; Zhang, K.; Sun, Y.; Li, Y.; Ma, S.; Uriho, A.; et al. Effect of long-term moderate exercise on muscle cellularity and texture, antioxidant activities, tissue composition, freshness indicators and flavor characteristics in largemouth bass (Micropterus salmoides). Aquaculture 2019, 510, 100–108. [Google Scholar] [CrossRef]
- China Fishery Statistical Yearbook; Ministry of Agriculture and Rural Affairs of the People’s Republic of China; China Agriculture Press: Beijing, China, 2023.
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, H.; Zhai, X.; Chen, Y.; Lin, S. Linseed oil can decrease liver fat deposition and improve antioxidant ability of juvenile largemouth bass, Micropterus salmoides. Fish Physiol. Biochem. 2019, 45, 1513–1521. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, C.; Chen, Q.; Ouyang, Q.; Shi, J.; El-Seedi, H.R.; Zou, X. Classification for Penicillium expansum spoilage and defect in apples by electronic nose combined with chemometrics. Sensors 2020, 20, 2130. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Zhao, H.; Chen, W.; Chen, Y.; Lin, S. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 2019, 506, 394–400. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.O.; Lee, S.M. Total replacement of dietary fish oil with alternative lipid sources in a practical diet for mandarin fish, Siniperca scherzeri, juveniles. Aquat. Sci. 2019, 22, 8. [Google Scholar] [CrossRef]
- Yu, H.; Ren, Y.; Wei, H.; Xing, W.; Xu, G.; Li, T.; Xue, M.; Luo, L. Dietary oxidized fish oil negatively affected the feed utilization, health status and fillet quality of juvenile Amur sturgeon, A. schrenckii. Aquaculture 2022, 546, 737290. [Google Scholar] [CrossRef]
- Yu, H.; Li, L.; Yu, L.; Zhang, L.; Li, F.; Guo, M.; Zhang, J.; Hou, J.; Zhang, Y. Effect of supplemental dietary α-linolenic Acid (18:3n-3) on the growth performance, body composition, and fatty acid profile of Coho salmon (Oncorhynchus kisutch) alevins cultured in freshwater. Aquac. Res. 2023, 2023, 4869006. [Google Scholar] [CrossRef]
- Sun, C.; Liu, B.; Zhou, Q.; Xiong, Z.; Shan, F.; Zhang, H. Response of Macrobrachium rosenbergii to vegetable oils replacing dietary fish oil: Insights from antioxidant defense. Front. Physiol. 2020, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Jin, M.; Li, Y.; Lu, Y.; Hou, Y.; Zhou, Q. Dietary lipid sources influence fatty acid composition in tissue of large yellow croaker (Larmichthys crocea) by regulating triacylglycerol synthesis and catabolism at the transcriptional level. PLoS ONE 2017, 12, e0169985. [Google Scholar] [CrossRef] [PubMed]
- Piedecausa, M.A.; Mazón, M.J.; García García, B.; Hernández, M.D. Effects of total replacement of fish oil by vegetable oils in the diets of sharpsnout seabream (Diplodus puntazzo). Aquaculture 2007, 263, 211–219. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated fatty acids in obesity-related inflammation. Int. J. Mol. Sci. 2020, 22, 330. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Liang, Z.; Xie, Y.; Su, J.; Luo, Q.; Zhu, J.; Liu, Q.; Han, T.; Wang, A. Effects of dietary fish oil replacement by soybean oil and l-carnitine supplementation on growth performance, fatty acid composition, lipid metabolism and liver health of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2020, 516, 734596. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, W.; Chen, Y.; Hu, Y.; Zhou, Q.; Peng, M.; Kumar, V. Effect of oil source on growth performance, antioxidant capacity, fatty acid composition and fillet quality of juvenile grass carp (Ctenopharyngodon idella). Aquac. Nut. 2020, 26, 1186–1197. [Google Scholar] [CrossRef]
- Nayak, M.; Saha, A.; Pradhan, A.; Samanta, M.; Giri, S.S. Dietary fish oil replacement by linseed oil: Effect on growth, nutrient utilization, tissue fatty acid composition and desaturase gene expression in silver barb (Puntius gonionotus) fingerlings. Comp. Biochem. Physiol. B 2017, 205, 1–12. [Google Scholar] [CrossRef]
- Falahatkar, B.; Asheri, S.; Safarpour Amlashi, A.; Ershad Langroudi, H. Canola oil, as a good alternative dietary lipid source in sturgeon: Effects on growth, physiology and fatty acid profile in Beluga sturgeon Huso huso L. Aquac. Nut. 2018, 24, 1263–1273. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.M. Effect of dietary lipid sources on body fatty acid composition of Chinese longsnout catfish Leiocassis longirostris Günther. Fish. Aquatic. Sci. 2015, 18, 359–365. [Google Scholar]
- Kanani, H.G.; Nobahar, Z.; Kakoolaki, S.; Jafarian, H. Effect of ginger- and garlic-supplemented diet on growth performance, some hematological parameters and immune responses in juvenile Huso huso. Fish Physiol. Biochem. 2014, 40, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xing, W.; Li, T.; Xu, G.; Ma, Z.; Jiang, N.; Luo, L. Effects of alternative dietary lipid sources on growth performance, health status and fillet fatty acid composition of hybrid sturgeon (Acipenser baeri Brandt ♀ ×Acipenser schrenckii Brandt ♂). Aquac. Nutr. 2020, 26, 1419–1430. [Google Scholar] [CrossRef]
- Huo, W.; Li, M.; Wang, J.; Wang, Z.; Huang, Y.; Chen, W. Effects of dietary lipid sources on growth performance, nutrient digestibility, blood T lymphocyte subsets, and cardiac antioxidant status of broilers. Anim. Nutr. 2019, 5, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570127/ (accessed on 16 April 2021).
- Liu, H.; Zhu, H.; Xia, H.; Yang, X.; Yang, L.; Wang, S.; Wen, J.; Sun, G. Different effects of high-fat diets rich in different oils on lipids metabolism, oxidative stress and gut microbiota. Food Res. Int. 2021, 141, 110078. [Google Scholar] [CrossRef] [PubMed]
- Chaidate, I.; Somchai, C.; Jos, N.; Henk, H. A cow-level association of ruminal pH on body condition score, serum beta-hydroxybutyrate and postpartum disorders in Thai dairy cattle. Anim. Sci. J. 2014, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Pérez, E.; Bionaz, M.; Garrido-Sartore, M.; Cancino-Padilla, N.; Morales, M.S.; Romero, J.; Leskinen, H.; Garnsworthy, P.C.; Loor, J.J. Effect of soybean oil and fish oil on lipid-related transcripts in subcutaneous adipose tissue of dairy cows. Animals 2019, 10, 54. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and insulin Receptors in adipose tissue development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef]
- Zeigerer, A.; Sekar, R.; Kleinert, M.; Nason, S.; Habegger, K.M.; Müller, T.D. Glucagon’s metabolic action in health and disease. Compr. Physiol. 2021, 11, 1759–1783. [Google Scholar]
- De Godoy, M.R.C.; Conway, C.E.; McLeod, K.R.; Harmon, D.L. Influence of feeding a fish oil-containing diet to young, lean, adult dogs: Effects on lipid metabolites, postprandial glycaemia and body weight. Arch. Anim. Nutr. 2015, 69, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Komal, F.; Khan, M.K.; Imran, M.; Ahmad, M.H.; Anwar, H.; Ashfaq, U.A.; Ahmad, N.; Masroor, A.; Ahmad, R.S.; Nadeem, M.; et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in PCOS rat model. J. Transl. Med. 2020, 18, 349. [Google Scholar] [CrossRef] [PubMed]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish feed intake, feeding behavior, and the physiological response of apelin to fasting and refeeding. Front. Endocrinol. 2021, 12, 798903. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Yuan, D.; Wang, T.; Zhou, C.; Lin, F.; Chen, H.; Wu, H.; Yang, S.; Wang, Y.; Liu, J.; et al. Characterization, tissue distribution and regulation of agouti-related protein (AgRP) in a cyprinid fish (Schizothorax prenanti). Gene 2013, 527, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.C.; Chen, Q. Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus). Aquac. Rep. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Shang, X.; Wang, C.; Zhang, G.; Liu, Q.; Guo, G.; Huo, W.; Zhang, J.; Pei, C. Effects of soybean oil and dietary copper levels on nutrient digestion, ruminal fermentation, enzyme activity, microflora and microbial protein synthesis in dairy bulls. Arch. Anim. Nutr. 2020, 74, 257–270. [Google Scholar] [CrossRef]
- Douglas, S.E.; Mandla, S.; Gallant, J.W. Molecular analysis of the amylase gene and its expression during development in the winter flounder, Pleuronectes americanus. Aquaculture 2000, 190, 247–260. [Google Scholar] [CrossRef]
- Liao, W.; Lin, Z.; Liao, M.; Xue, Y.; Zhou, J.; Wang, Y.; Hou, D.; Sun, C. Effects of sodium humate and probiotics on growth performance enzyme activity and microbial environment of Litopenaeus vannamei in high-density zero-water exchange systems. Front. Mar. Sci. 2022, 9, 989325. [Google Scholar] [CrossRef]
- Wu, F.; Gu, Z.; Chen, X.; Yu, L.; Lu, X.; Zhang, L.; Wen, H.; Tian, J. Effect of lipid sources on growth performance, muscle composition, haemolymph biochemical indices and digestive enzyme activities of red swamp crayfish (Procambarus clarkii). Aquac. Nutr. 2021, 27, 1996–2006. [Google Scholar] [CrossRef]
- Menoyo, D.; Lopez-Bote, C.J.; Bautista, J.M.; Obach, A. Growth, digestibility and fatty acid utilization in large Atlantic salmon (Salmo salar) fed varying levels of n-3 and saturated fatty acids. Aquaculture 2003, 225, 295–307. [Google Scholar] [CrossRef]
- Lakwani, M.A.S.; Kenanoğlu, O.N.; Taştan, Y.; Bilen, S. Effects of black mustard (Brassica nigra) seed oil on growth performance, digestive enzyme activities and immune responses in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2022, 53, 300–313. [Google Scholar] [CrossRef]
- Mu, H.; Wei, C.; Xu, W.; Gao, W.; Zhang, W.; Mai, K. Effects of replacement of dietary fish oil by rapeseed oil on growth performance, anti-oxidative capacity and inflammatory response in large yellow croaker Larimichthys crocea. Aquac. Rep. 2020, 16, 100251. [Google Scholar] [CrossRef]
- Wu, C.; Lu, B.; Wang, Y.; Jin, C.; Zhang, Y.; Ye, J. Effects of dietary vitamin D3 on growth performance, antioxidant capacities and innate immune responses in juvenile black carp Mylopharyngodon piceus. Fish Physiol. Biochem. 2020, 46, 2243–2256. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, A.M.; Sosnowski, D.K.; Lee, T.Y.T.; Keshavarz-Bahaghighat, H.; Seubert, J.M. Insights into the cardioprotective properties of n-3 PUFAs against ischemic heart disease via modulation of the innate immune system. Chem. Biol. Interact. 2019, 308, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ye, J.; Gao, J.E.; Chen, L.; Lu, Z. The effects of dietary carbohydrate on the growth, antioxidant capacities, innate immune responses and pathogen resistance of juvenile black carp Mylopharyngodon piceus. Fish Shellfish Immunol. 2016, 49, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Sudiyono, S.; Dwi Widyawati, S.; Hanifa, A.; Hadi, R.; Pratitis, W.; Wati, A.; Pawestri, W. Effect of using protected linseed in rations on sheep blood hematology. Livest. Sci. 2023, 21, 118. [Google Scholar] [CrossRef]
- Soremekun, O.; Soremekun, C.; Machipisa, T.; Soliman, M.; Nashiru, O.; Chikowore, T.; Fatumo, S. Genome-wide association and mendelian randomization analysis reveal the causal relationship between white blood cell subtypes and asthma in Africans. Front. Genet 2021, 12, 749415. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Westerterp, M.; Murphy, A.J.; Subramanian, V.; Ferrante, A.W.; Tall, A.R.; Accili, D. Expanded granulocyte/monocyte compartment in myeloid-specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circ. Res. 2013, 112, 992–1003. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Jia, W.; Sun, D.; Zhao, L.; Zhang, C.; Wang, C.; Lyu, Q.; Chen, Y.; Chen, G.; et al. Association of the total white blood cell, neutrophils, and nonocytes count with the presence, severity, and types of carotid atherosclerotic plaque. Front. Med. 2020, 7, 313. [Google Scholar] [CrossRef]
- Marchix, J.; Choque, B.; Kouba, M.; Fautrel, A.; Catheline, D.; Legrand, P. Excessive dietary linoleic acid induces proinflammatory markers in rats. J. Nutr. Biochem. 2015, 26, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, D.; Guo, H.; Yuan, H.; Zhang, W.; Wang, B.; Lu, Y.; Chen, D.; Zhang, S. Comprehensive comparison of thirteen kinds of cytokine receptors from the endangered fish Chinese sturgeon (Acipenser sinensis). Dev. Comp. Immunol. 2021, 123, 104132. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Shen, H.; Liu, J.; Xie, F.; Zhang, W.; Mai, K. High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2018, 77, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jiang, F.; Yu, B.; Huang, Z.; Luo, Y.; Wu, A.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Effect of different dietary lipid sources on growth performance, nutrient digestibility, and intestinal health in weaned pigs. Animals 2023, 13, 3006. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, C.; Ma, Y.; Ye, R.; Chen, H.; Xie, D.; Zhang, G.; Zhang, M.; Wang, M.; You, C.; et al. Effects of dietary n-3 highly unsaturated fatty acids levels on growth, lipid metabolism and innate immunity in juvenile golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. 2020, 105, 177–185. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, W.; Wu, P.; Liu, Y.; Kuang, S.; Tang, L.; Tang, W.; Zhou, X.; Feng, L. Novel insight into nutritional regulation in enhancement of immune status and mediation of inflammation dynamics integrated study in vivo and in vitro of teleost grass carp (Ctenopharyngodon idella): Administration of threonine. Front. Immunol. 2022, 13, 770969. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Zhao, J.; Chen, D.; Zhou, X.; et al. Effects of dietary glutamate supplementation on flesh quality, antioxi dant defense and gene expression related to lipid metabolism and myogenic regulation in Jian carp (Cyprinus carpio var. Jian). Aquaculture 2019, 502, 212–222. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Zhang, P.; Xie, Y.; Yao, X.; Pan, X.; Fu, Y.; Wei, J.; Bai, H.; Shao, X.; et al. Effects of selenoprotein extracts from Cardamine hupingshanensis on growth, selenium metabolism, antioxidant capacity, immunity and intestinal health in largemouth bass Micropterus salmoides. Front. Immunol. 2024, 15, 1342210. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Z.; Liu, Y.; Zhang, W.; Gong, Y.; Tang, Y.; Chen, F.; Zhang, J.; Liu, G.; Zhang, H.; et al. Effects of supplemental octanoate on hepatic lipid metabolism, serum biochemical indexes, antioxidant capacity and inflammation-related genes expression of large yellow croaker (Larimichthys crocea) fed with high soybean oil diet. Front. Immunol. 2023, 14, 1162633. [Google Scholar] [CrossRef]
- Song, M.; Lee, D.; Chun, K.; Kim, E.H. The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W.J.A. Maternal selenium-enriched yeast supplementation in sows enhances offspring growth and antioxidant status through the Nrf2/Keap1 pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yan, M.; Zhao, Y.; Zhou, X.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Wang, Y.; et al. Dietary isoleucine improved flesh quality, muscle antioxidant capacity, and muscle growth associated with AKT/TOR/S6K1 and AKT/FOXO3a signaling in hybrid bagrid catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris♂). J. Anim. Sci. Biotechnol. 2021, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Jiang, W.; Wu, P.; Liu, Y.; Jiang, J.; Li, S.; Tang, L.; Kuang, S.; Feng, L.; Zhou, X. Flesh quality loss in response to dietary isoleucine deficiency and excess in fish: A link to impaired Nrf2-dependent antioxidant defense in muscle. PLoS ONE 2014, 9, e115129. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Gong, Y.; Weng, M.; Wang, X.; Zhang, W.; Wang, Z.; Sun, J.; Cao, X.; Zhang, J.; Zhao, M.; Zhang, Z.; et al. Effects of vegetable oil replacement on intramuscular fat deposition a nd flesh quality of large yellow croaker (Larimichthys crocea) juveniles. Aquaculture 2023, 575, 739731. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Kaneko, G.; Xie, J.; Wang, G.; Yu, D.; Li, Z.; Ma, L.; Qi, D.; Tian, J.; et al. Quantitative phosphoproteomic analysis of soft and firm grass carp muscle. Food Chem. 2020, 303, 125367. [Google Scholar] [CrossRef]
- Cai, W.; Fu, L.; Liu, C.; He, L.; Liu, H.; Han, D.; Zhu, X.; Yang, Y.; Jin, J.; Xie, S. Dietary ribose supplementation improves flesh quality through purine metabolism in gibel carp (Carassius auratus gibelio). Anim. Nutr. 2023, 13, 50–63. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, H.; Peng, Z.; Yu, J.; Yan, L.; Wang, W.; Ren, T.; Han, Y. Comparison of nutritional quality, flesh quality, muscle cellularity, and expression of muscle growth-related genes between wild and recirculating aquaculture system (RAS)-farmed black rockfish (Sebastes schlegelii). Aquac. Int. 2023, 31, 2263–2280. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Jia, S.; Li, Y.; Li, Q.; Li, K.; Hong, H.; Luo, Y. Stunning stress-induced textural softening in silver carp (Hypophthalmichthys molitrix) fillets and underlying mechanisms. Food Chem. 2019, 295, 520–529. [Google Scholar] [CrossRef]
- He, X.; Shu, H.; Xu, T.; Huang, Y.; Mo, J.; Ai, C. Effects of broad bean diet on the growth performance, muscle characteristics, antioxidant capacity, and intestinal health of Nile tilapia (Oreochromis niloticus). Animals 2023, 13, 3705. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Zhang, H.; Li, Z.; Wang, G.; Wu, H.; Xie, J.; Yu, D.; Xia, Y.; Zhang, K.; Gong, W. Proteomic signature of muscle fibre hyperplasia in response to faba bean intake in grass carp. Sci. Rep. 2017, 7, 45950. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.L.; Torrecillas, S.; Montero, D.; Izquierdo, M.S.; Ginés, R. Effect of combined fish meal and fish oil replacement on growth performance, flesh quality and shelf life of European sea bass (Dicentrarchus labrax). Aquaculture 2022, 560, 738452. [Google Scholar] [CrossRef]
- Matos, E.; Silva, T.S.; Wulff, T.; Valente, L.M.P.; Sousa, V.; Sampaio, E.; Gonçalves, A.; Silva, J.M.G.; Guedes de Pinho, P.; Dinis, M.T.; et al. Influence of supplemental maslinic acid (olive-derived triterpene) on the post-mortem muscle properties and quality traits of gilthead seabream. Aquaculture 2013, 396–399, 146–155. [Google Scholar] [CrossRef]
- Marr, L.; Biswas, D.; Daly, L.A.; Browning, C.; Vial, S.C.M.; Maskell, D.P.; Hudson, C.; Bertrand, J.A.; Pollard, J.; Ranson, N.A.; et al. Mechanism of glycogen synthase inactivation and interaction with glycogenin. Nat. Commun. 2022, 13, 3372. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.; Matos, E.; Cordeiro, O.D.; Colen, R.; Wulff, T.; Sampaio, E.; Sousa, V.; Valente, L.M.; Gonçalves, A.; Silva, J.M.; et al. Dietary tools to modulate glycogen storage in gilthead seabream muscle: Glycerol supplementation. J. Agric. Food Chem. 2012, 60, 10613–10624. [Google Scholar] [CrossRef]
- Shen, L.; Luo, J.; Lei, H.; Jiang, Y.; Bai, L.; Li, M.; Tang, G.; Li, X.; Zhang, S.; Zhu, L. Effects of muscle fiber type on glycolytic potential and meat quality traits in different Tibetan pig muscles and their association with glycolysis-related gene expression. Genet. Mol. Res. 2015, 14, 14366–14378. [Google Scholar] [CrossRef]
- Turchini, G.M.; Moretti, V.M.; Mentasti, T.; Orban, E.; Valfrè, F. Effects of dietary lipid source on fillet chemical composition, flavour volatile compounds and sensory characteristics in the freshwater fish tench (Tinca tinca L.). Food Chem. 2007, 102, 1144–1155. [Google Scholar] [CrossRef]
- Sérot, T.; Regost, C.; Arzel, J. Identification of odour-active compounds in muscle of brown trout (Salmo trutta) as affected by dietary lipid sources. J. Sci. Food Agric. 2002, 82, 636–643. [Google Scholar] [CrossRef]
- Sérot, T.; Regost, C.; Prost, C.; Robin, J.; Arzel, J. Effect of dietary lipid sources on odour-active compounds in muscle of turbot (Psetta maxima). J. Sci. Food Agric. 2001, 81, 1339–1346. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef]




| Ingredient (%) | Composition of Diets (%) | ||||
|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | |
| Casein a | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 |
| Defatted fish meal (DFM) b | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Gelatin c | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Lipid source d | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Dextrin c | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Mineral premix e | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 |
| Vitamin premix f | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Choline chloride g | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Microcrystalline cellulose h | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 |
| Proximate composition (%) | |||||
| Crude protein | 50.38 | 50.25 | 50.34 | 50.45 | 50.01 |
| Crude lipid | 9.30 | 9.32 | 9.38 | 9.29 | 9.35 |
| Ash | 4.94 | 4.80 | 4.87 | 4.79 | 4.89 |
| Items | Dietary Lipid Sources | |||||
|---|---|---|---|---|---|---|
| DFM | FO | SO | RO | PO | LO | |
| C6:0 | 0.14 | 0.13 | 0.12 | 0.46 | 0.11 | 0.12 |
| C8:0 | 0.14 | 0.09 | 0.08 | 0.34 | 0.07 | 0.09 |
| C10:0 | 0.14 | 0.16 | 0.15 | 0.34 | 0.14 | 0.20 |
| C11:0 | 0.15 | 0.03 | 0.03 | 0.06 | 0.03 | 0.03 |
| C12:0 | 0.27 | 0.17 | 0.16 | 0.35 | 0.14 | 0.21 |
| C13:0 | 0.25 | 0.05 | 0.04 | 0.10 | 0.04 | 0.04 |
| C14:0 | 12.30 | 7.03 | 1.00 | 2.04 | 0.89 | 2.01 |
| C15:0 | 0.97 | 0.67 | 0.16 | 0.35 | 0.15 | 0.18 |
| C16:0 | 32.13 | 22.39 | 13.72 | 9.19 | 12.34 | 25.21 |
| C17:0 | 1.37 | 0.74 | 0.23 | 0.40 | 0.20 | 0.33 |
| C18:0 | 6.09 | 5.36 | 4.35 | 2.34 | 5.63 | 14.20 |
| C20:0 | 0.80 | 0.82 | 0.36 | 1.15 | 1.84 | 0.30 |
| C21:0 | 0.53 | 0.12 | 0.11 | 0.22 | 0.10 | 0.10 |
| C22:0 | 0.59 | 0.36 | 0.33 | 0.68 | 2.54 | 0.12 |
| C23:0 | 0.53 | 0.13 | 0.11 | 0.23 | 0.11 | 0.10 |
| C24:0 | 0.68 | 0.20 | 0.16 | 0.42 | 0.84 | 0.14 |
| ∑SFA | 57.07 | 38.43 | 21.09 | 18.67 | 25.16 | 43.40 |
| C14:1 | 0.34 | 0.09 | 0.08 | 0.17 | 0.08 | 0.09 |
| C16:1 | 14.48 | 5.65 | 0.65 | 0.82 | 0.56 | 2.50 |
| C18:1n-9 (OA) | 9.76 | 23.23 | 21.89 | 47.51 | 36.22 | 36.34 |
| C20:1 | 1.35 | 5.28 | 0.27 | 1.30 | 0.76 | 0.75 |
| C24:1 | 0.95 | 1.64 | 0.12 | 0.64 | 0.12 | 0.14 |
| ∑MUFA | 26.88 | 35.99 | 23.02 | 50.43 | 37.74 | 39.82 |
| C18:2n-6 (LA) | 1.35 | 8.73 | 45.89 | 23.94 | 35.76 | 14.14 |
| C18:3n-6 | 0.66 | 0.11 | 0.10 | 0.20 | 0.10 | 0.13 |
| C20:3n-6 | 0.53 | 0.10 | 0.09 | 0.21 | 0.08 | 0.14 |
| C20:4n-6 (ARA) | 0.75 | 0.79 | 0.10 | 0.19 | 0.09 | 0.20 |
| n-6 PUFA | 3.28 | 9.72 | 46.18 | 24.53 | 36.04 | 14.61 |
| C18:3n-3 (ALA) | 0.78 | 2.16 | 8.84 | 5.44 | 0.20 | 0.63 |
| C20:3n-3 | 0.50 | 0.10 | 0.09 | 0.19 | 0.08 | 0.15 |
| C20:5n-3 (EPA) | 6.29 | 6.62 | 0.37 | 0.21 | 0.36 | 0.40 |
| C22:6n-3 (DHA) | 2.48 | 6.75 | 0.21 | 0.07 | 0.21 | 0.25 |
| n-3 PUFA | 10.05 | 15.63 | 9.50 | 5.91 | 0.85 | 1.43 |
| n-3 LC-PUFA | 9.27 | 13.47 | 0.66 | 0.47 | 0.65 | 0.80 |
| PUFA | 13.34 | 28.14 | 55.68 | 30.45 | 36.89 | 16.04 |
| DHA/EPA | 0.39 | 1.02 | 0.57 | 0.34 | 0.57 | 0.61 |
| n-3/n-6 | 3.06 | 1.61 | 0.21 | 0.24 | 0.02 | 0.10 |
| Gene | Primers | Primer Sequence (5′–3′) | Reference | Product Length |
|---|---|---|---|---|
| LZM | F | TCATTGCTGCCATCATCTC | XM_038713810.1 | 115 |
| R | TCAACCTGCATCAGTCCC | |||
| HEPC | F | GCTCTGCCGTCCCATTCA | XM_038710826.1 | 106 |
| R | CCACGATTCCATTGACATTTCTTGA | |||
| NRAMP | F | TCATTCCCATCCTCACTTTC | XM_038723474.1 | 143 |
| R | TGCAGTAACATACACCACGAC | |||
| TGFβ1 | F | TGCGGAACTGGCTCAAAG | XM_038693206.1 | 111 |
| R | TCCCAGAAATGCCGAAAC | |||
| IL1β | F | CAATGTCGCCAGACTGAA | XM_038733429.1 | 138 |
| R | GGGTGATGTGGTGGTTGA | |||
| IL8 | F | TTCTCCTGGCTGCTTTGG | XM_038704093.1 | 115 |
| R | TGGATGGCCCTCCTGTTA | |||
| IL12 | F | CCGCTGTTATTCAGTCTTACC | XM_038693841.1 | 117 |
| R | GCATCAGGGAGCAGTTCA | |||
| IL15 | F | TTCAGAAATCCGATGTGGC | XM_038693994.1 | 101 |
| R | GTCGATGGTGGGCGTGTA | |||
| Cu/Zn-SOD | F | TAAGGCTATCTGGAATATCATCAAC | XM_038708943.1 | 146 |
| R | AATCGCCCTCCTGCTCAA | |||
| Mn-SOD | F | CAGGGATCTACAGGTCTCATTC | XM_038727054.1 | 139 |
| R | GACGCTCGCTCACATTCTC | |||
| CAT | F | TGCTGTCCGCTTCTCCAC | XM_038704976.1 | 105 |
| R | TCCCAGTTGCCCTCCTCA | |||
| GPX3 | F | CCCTCCAGTTGGAAACGA | XM_038699914.1 | 139 |
| R | ACTTGGGTGCCACCTCAT | |||
| GR | F | CACGAGCAGGAAGAGTCAG | XM_038700350.1 | 144 |
| R | GCTTTGGTAGCACCCATTT | |||
| GST-omega | F | GGCTTTCACCACCTATGC | XM_038739072.1 | 124 |
| R | TTCAGACTTTCTGCCCACA | |||
| Nrf2 | F | AAGACAAGCGTAAGAAGCG | XM_038720536.1 | 107 |
| R | CAGGCAGATTGATAATCATAGA | |||
| Keap1a | F | AGGTGGTGGGAAGACTTATTG | XM_038728593.1 | 150 |
| R | GCTCCAGGTGCTTAGTGAGG | |||
| Keap1b | F | TGAACGAGCTGCGTCTGG | XM_038713667.1 | 139 |
| R | TTGGTGAACATAGCCCTAAAGA | |||
| COL1α1 | F | TCTGGTTCGGCGAGACAATG | XM_038692282.1 | 108 |
| R | TGGACATGAGACGCAGGAAAGT | |||
| COL1α2 | F | TTCTGCGACTTCACCACCCG | XM_038724497.1 | 108 |
| R | TCCGAACCAGACGTGCTTTT | |||
| PHD | F | GTTCTGTATTGGACGCTCTGT | XM_038711098.1 | 137 |
| R | CCGCCTTCTGCAACTTTT | |||
| LOX | F | TATTTGGCACGCCGCTTTG | XM_038733746.1 | 115 |
| R | GCCGCTCTTTGGTTATCTCCTT | |||
| Cath-B | F | GGCTTTGGATGTAATGGTGG | XM_038701777.1 | 116 |
| R | GGGATGGTGTAGGGACGA | |||
| Cath-L | F | CAGACTGGTGCTGGTGCA | XM_038729783.1 | 113 |
| R | GGGAAATCAGGCGTTTGTAC | |||
| GYS1 | F | AAGACGAACGCTATGACGAG | XM_038697432.1 | 140 |
| R | TTTCACGCTTGCGACACC | |||
| GN2 | F | CACGCAGCATTGTTGTCA | XM_038722837.1 | 126 |
| R | AGGCCAGATGTAGAGGGTC | |||
| β-action | F | GCGTGACATCAAGGAGAAGC | XM_038695351.1 | 149 |
| R | CTGGGCAACGGAACCTCT |
| Items | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| IBW (g) | 9.33 ± 0.01 | 9.33 ± 0.01 | 9.34 ± 0.01 | 9.32 ± 0.01 | 9.32 ± 0.01 | 0.149 | - | - |
| FBW (g) | 30.85 ± 0.45 c | 29.93 ± 0.58 c | 30.85 ± 0.93 c | 27.21 ± 0.37 b | 25.31 ± 0.20 a | 0.000 | 0.000 | 0.006 |
| WG (%) | 230.46 ± 5.07 c | 220.91 ± 6.00 c | 230.49 ± 9.64 c | 191.81 ± 3.77 b | 171.57 ± 2.42 a | 0.000 | 0.000 | 0.006 |
| SGR (%/d) | 2.44 ± 0.03 c | 2.38 ± 0.04 c | 2.44 ± 0.06 c | 2.19 ± 0.03 b | 2.04 ± 0.02 a | 0.000 | 0.000 | 0.003 |
| FCR | 1.03 ± 0.04 a | 1.08 ± 0.05 a | 1.06 ± 0.05 a | 1.15 ± 0.08 a | 1.47 ± 0.03 b | 0.000 | 0.000 | 0.000 |
| PER (%) | 1.92 ± 0.06 b | 1.85 ± 0.08 b | 1.88 ± 0.08 b | 1.72 ± 0.11 b | 1.36 ± 0.03 a | 0.000 | 0.000 | 0.001 |
| HSI (%) | 1.16 ± 0.04 a | 1.28 ± 0.08 ab | 1.35 ± 0.07 b | 1.16 ± 0.05 a | 1.23 ± 0.03 ab | 0.009 | 0.952 | 0.062 |
| VSI (%) | 8.55 ± 0.34 bc | 8.16 ± 0.04 ab | 8.71 ± 0.12 c | 8.33 ± 0.08 abc | 7.90 ± 0.18 a | 0.002 | 0.061 | 0.120 |
| IPF (%) | 2.33 ± 0.08 ab | 2.53 ± 0.08 b | 2.11 ± 0.07 a | 2.27 ± 0.17 ab | 2.43 ± 0.04 b | 0.004 | 0.886 | 0.196 |
| CF (g/cm3) | 2.15 ± 0.02 b | 2.06 ± 0.03 ab | 2.05 ± 0.05 ab | 1.96 ± 0.06 a | 2.15 ± 0.01 b | 0.001 | 0.560 | 0.002 |
| CR (%) | 40.79 ± 0.29 ab | 41.47 ± 1.42 b | 42.17 ± 1.68 b | 38.97 ± 1.31 ab | 37.60 ± 0.93 a | 0.006 | 0.010 | 0.014 |
| SR (%) | 98.89 ± 1.92 | 97.78 ± 1.92 | 100.00 ± 0.00 | 96.67 ± 3.34 | 94.44 ± 1.93 | 0.068 | - | - |
| Body Composition (%) | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| Whole body | ||||||||
| Moisture | 71.45 ± 0.20 c | 70.49 ± 0.05 a | 72.07 ± 0.17 d | 71.05 ± 0.12 b | 72.00 ± 0.13 d | 0.000 | 0.153 | 0.367 |
| Crude protein | 17.35 ± 0.37 | 17.45 ± 0.21 | 17.21 ± 0.18 | 17.02 ± 0.26 | 17.09 ± 0.16 | 0.262 | - | - |
| Crude lipid | 7.43 ± 0.20 c | 8.15 ± 0.09 d | 6.87 ± 0.16 ab | 6.82 ± 0.13 a | 7.23 ± 0.12 bc | 0.000 | 0.062 | 0.563 |
| Ash | 3.60 ± 0.16 | 3.73 ± 0.07 | 3.58 ± 0.15 | 3.88 ± 0.15 | 3.60 ± 0.16 | 0.116 | - | - |
| Dorsal muscle | ||||||||
| Moisture | 75.12 ± 0.47 b | 74.64 ± 0.48 b | 75.11 ± 0.58 b | 74.67 ± 0.22 b | 73.37 ± 0.48 a | 0.005 | 0.007 | 0.042 |
| Crude protein | 21.98 ± 0.23 | 21.80 ± 0.43 | 21.19 ± 0.29 | 21.51 ± 0.32 | 21.81 ± 0.34 | 0.093 | - | - |
| Crude lipid | 1.19 ± 0.10 a | 1.31 ± 0.09 a | 1.23 ± 0.10 a | 1.29 ± 0.11 a | 1.70 ± 0.09 b | 0.000 | 0.003 | 0.022 |
| Ash | 1.04 ± 0.07 | 1.02 ± 0.10 | 1.03 ± 0.04 | 1.04 ± 0.04 | 1.07 ± 0.04 | 0.869 | - | - |
| Items | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| C10:0 | 0.40 ± 0.05 b | 0.26 ± 0.03 a | 0.74 ± 0.02 c | 0.31 ± 0.01 a | 0.34 ± 0.04 ab | 0.000 | 0.838 | 0.369 |
| C11:0 | - | 0.25 ± 0.02 ab | 0.84 ± 0.03 d | 0.27 ± 0.01 b | 0.23 ± 0.02 a | 0.000 | 0.390 | 0.001 |
| C12:0 | 0.51 ± 0.05 b | 0.34 ± 0.04 a | 0.98 ± 0.01 c | 0.48 ± 0.04 b | 0.51 ± 0.04 b | 0.000 | 0.851 | 0.115 |
| C13:0 | 0.60 ± 0.07 b | 0.39 ± 0.01 a | 1.05 ± 0.05 c | 0.55 ± 0.02 b | 0.52 ± 0.03 b | 0.000 | 0.994 | 0.133 |
| C14:0 | 1.33 ± 0.07 ab | 1.19 ± 0.03 a | 1.91 ± 0.02 c | 1.21 ± 0.11 a | 1.52 ± 0.08 b | 0.000 | 0.471 | 0.422 |
| C15:0 | 0.89 ± 0.04 b | 0.69 ± 0.02 a | 1.81 ± 0.06 c | 0.95 ± 0.04 b | 0.88 ± 0.03 b | 0.000 | 0.762 | 0.057 |
| C16:0 | 14.08 ± 1.65 a | 17.07 ± 0.03 b | 12.21 ± 0.02 a | 17.11 ± 0.08 b | 21.03 ± 0.09 c | 0.000 | 0.926 | 0.114 |
| C17:0 | 1.36 ± 0.16 b | 0.92 ± 0.03 a | 2.36 ± 0.07 c | 1.24 ± 0.06 b | 1.15 ± 0.03 b | 0.000 | 0.048 | 0.003 |
| C18:0 | 6.01 ± 0.28 bc | 5.64 ± 0.03 ab | 5.29 ± 0.05 a | 6.35 ± 0.10 c d | 6.49 ± 0.08 d | 0.000 | 0.737 | 0.055 |
| C20:0 | 1.42 ± 0.04 b | 1.05 ± 0.02 a | 2.90 ± 0.05 d | 1.55 ± 0.05 c | 1.38 ± 0.04 b | 0.000 | 0.829 | 0.043 |
| C21:0 | - | - | - | 0.33 ± 0.02 b | 0.16 ± 0.01 a | 0.000 | 0.004 | 0.955 |
| C22:0 | 1.56 ± 0.09 d | 0.93 ± 0.12 b | 0.49 ± 0.02 a | 1.37 ± 0.04 cd | 1.26 ± 0.07 c | 0.000 | 0.838 | 0.003 |
| C23:0 | - | 0.98 ± 0.05 b | - | 0.34 ± 0.01 a | - | 0.000 | 0.394 | 0.130 |
| C24:0 | 2.16 ± 0.02 d | 1.25 ± 0.05 b | 0.64 ± 0.03 a | 1.79 ± 0.05 c | 0.75 ± 0.07 a | 0.000 | 0.034 | 0.204 |
| ∑SFA | 30.32 ± 1.00 a | 30.95 ± 0.21 a | 31.20 ± 0.09 a | 33.51 ± 0.12 b | 36.06 ± 0.18 c | 0.000 | 0.000 | 0.000 |
| C14:1 | 0.32 ± 0.02 a | 0.59 ± 0.02 c | 1.15 ± 0.03 d | 0.46 ± 0.04 b | 0.39 ± 0.02 a | 0.000 | 1.000 | 0.001 |
| C16:1 | 1.71 ± 0.11 a | 1.68 ± 0.03 a | 2.64 ± 0.07 b | 1.76 ± 0.02 a | 3.06 ± 0.03 c | 0.000 | 0.050 | 0.414 |
| C18:1n-9 | 17.08 ± 0.15 a | 18.57 ± 1.04 b | 24.89 ± 0.08 d | 24.27 ± 0.14 c d | 23.24 ± 0.09 c | 0.000 | 0.000 | 0.002 |
| C20:1 | 2.29 ± 0.15 b | 1.62 ± 0.07 a | 3.61 ± 0.06 c | 2.49 ± 0.07 b | 2.45 ± 0.08 b | 0.000 | 0.344 | 0.291 |
| C24:1 | - | 1.31 ± 0.05 b | - | 0.46 ± 0.02 a | - | 0.000 | 0.399 | 0.218 |
| ∑MUFA | 21.40 ± 0.30 a | 23.76 ± 0.97 b | 32.30 ± 0.10 d | 29.44 ± 0.09 c | 29.13 ± 0.01 c | 0.000 | 0.001 | 0.002 |
| C18:2n-6 | 22.13 ± 0.62 e | 26.50 ± 0.04 d | 9.69 ± 0.01 a | 19.31 ± 0.13 c | 17.42 ± 0.15 b | 0.000 | 0.118 | 0.251 |
| C18:3n-6 | 1.69 ± 0.12 c | 1.20 ± 0.02 a | 2.90 ± 0.08 d | 1.63 ± 0.10 bc | 1.42 ± 0.06 ab | 0.000 | 0.924 | 0.080 |
| C20:3n-6 | 2.32 ± 0.14 b | 1.72 ± 0.09 a | 2.85 ± 0.12 c | 2.21 ± 0.06 b | 1.80 ± 0.03 a | 0.000 | 0.501 | 0.143 |
| C20:4n-6 | 2.28 ± 0.12 b | 1.64 ± 0.19 a | 2.77 ± 0.05 c | 2.38 ± 0.05 b | 1.86 ± 0.07 a | 0.000 | 0.912 | 0.183 |
| n-6PUFA | 28.42 ± 0.96 d | 31.06 ± 0.26 e | 18.22 ± 0.13 a | 25.53 ± 0.07 c | 22.49 ± 0.26 b | 0.000 | 0.035 | 0.335 |
| C18:3n-3 | 2.35 ± 0.06 b | 2.31 ± 0.03 b | 3.73 ± 0.05 c | 1.53 ± 0.06 a | 1.51 ± 0.05 a | 0.000 | 0.111 | 0.027 |
| C20:3n-3 | 1.46 ± 0.12 b | 1.00 ± 0.11 a | 2.68 ± 0.08 c | - | 1.27 ± 0.04 b | 0.000 | 0.416 | 0.661 |
| C20:5n-3 | 3.37 ± 0.01 e | 1.21 ± 0.19 a | 3.11 ± 0.07 c | 1.56 ± 0.05 b | 1.61 ± 0.06 b | 0.000 | 0.055 | 0.060 |
| C22:6n-3 | 8.66 ± 0.14 c | 5.88 ± 0.05 b | 5.12 ± 0.04 a | 5.30 ± 0.09 a | 5.19 ± 0.09 a | 0.000 | 0.000 | 0.000 |
| n-3PUFA | 15.83 ± 0.04 e | 10.39 ± 0.33 c | 14.64 ± 0.14 d | 8.38 ± 0.10 a | 9.57 ± 0.07 b | 0.000 | 0.004 | 0.591 |
| n-3LC-PUFA | 13.49 ± 0.09 d | 8.08 ± 0.30 b | 10.91 ± 0.09 c | 6.85 ± 0.05 a | 8.06 ± 0.02 b | 0.000 | 0.003 | 0.110 |
| PUFA | 44.25 ± 0.96 d | 41.45 ± 0.55 c | 32.86 ± 0.14 ab | 33.92 ± 0.17 b | 32.07 ± 0.25 a | 0.000 | 0.000 | 0.012 |
| DHA/EPA | 2.56 ± 0.04 ab | 4.96 ± 0.86 c | 1.65 ± 0.03 a | 3.41 ± 0.16 b | 3.22 ± 0.17 b | 0.000 | 0.992 | 0.974 |
| n-3/n-6 | 0.56 ± 0.02 c | 0.33 ± 0.01 a | 0.80 ± 0.01 d | 0.33 ± 0.00 a | 0.43 ± 0.01 b | 0.000 | 0.442 | 0.483 |
| Items | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| WBC (109/L) | 165.57 ± 7.92 a | 191.04 ± 8.06 b | 166.27 ± 6.81 a | 160.81 ± 5.94 a | 156.23 ± 2.15 a | 0.001 | 0.045 | 0.120 |
| RBC (1012/L) | 2.39 ± 0.09 a | 2.77 ± 0.12 bc | 2.84 ± 0.19 c | 2.47 ± 0.10 ab | 2.43 ± 0.10 a | 0.003 | 0.593 | 0.003 |
| HGB (g/L) | 70.67 ± 5.51 a | 80.67 ± 7.02 ab | 86.33 ± 7.37 b | 71.33 ± 2.08 a | 73.67 ± 1.53 ab | 0.019 | 0.822 | 0.030 |
| PLT (109/L) | 84.50 ± 1.00 a | 124.33 ± 11.24 c | 90.00 ± 2.65 a | 119.00 ± 8.05 bc | 99.75 ± 8.47 ab | 0.000 | 0.449 | 0.158 |
| MCH (pg) | 28.40 ± 0.46 a | 29.40 ± 0.79 abc | 28.60 ± 0.66 ab | 30.13 ± 0.25 bc | 30.20 ± 0.56 c | 0.008 | 0.004 | 0.766 |
| MCV (fL) | 132.47 ± 1.30 a | 131.47 ± 1.36 a | 133.03 ± 1.75 a | 131.48 ± 4.79 a | 146.80 ± 3.21 b | 0.000 | 0.010 | 0.003 |
| MCHC (g/L) | 210.67 ± 3.79 ab | 255.33 ± 3.06 c | 214.33 ± 3.06 ab | 226.17 ± 14.34 b | 201.33 ± 3.51 a | 0.000 | 0.206 | 0.037 |
| NEU (109/L) | 12.94 ± 1.72 b | 34.77 ± 2.73 c | 11.80 ± 1.01 b | 4.83 ± 0.94 a | 7.33 ± 0.54 a | 0.000 | 0.036 | 0.296 |
| LYM (109/L) | 140.96 ± 3.69 b | 120.51 ± 4.39 a | 142.62 ± 2.24 b | 139.92 ± 3.23 b | 136.37 ± 1.54 b | 0.000 | 0.541 | 0.660 |
| MON (109/L) | 10.47 ± 2.00 a | 27.85 ± 1.48 c | 16.04 ± 1.78 b | 13.88 ± 0.68 ab | 12.40 ± 0.88 ab | 0.000 | 0.412 | 0.041 |
| Items | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| HDL (mmol/L) | 2.21 ± 0.40 | 2.29 ± 0.19 | 2.52 ± 0.38 | 2.11 ± 0.04 | 2.80 ± 0.09 | 0.054 | - | - |
| LDL (mmol/L) | 2.01 ± 0.10 a | 2.06 ± 0.04a b | 2.38 ± 0.11 bc | 2.01 ± 0.18 a | 2.61 ± 0.16 c | 0.000 | 0.014 | 0.406 |
| GLU (mmol/L) | 8.35 ± 0.96 a | 10.91 ± 0.45 b | 12.41 ± 0.27 c | 8.12 ± 0.23 a | 10.30 ± 0.40 b | 0.000 | 0.739 | 0.089 |
| TG (mmol/L) | 4.14 ± 0.69 a | 6.69 ± 0.36 b | 4.83 ± 0.51 a | 4.35 ± 0.23 a | 6.23 ± 0.47 b | 0.000 | 0.396 | 0.992 |
| TC (mmol/L) | 5.32 ± 0.16 a | 5.79 ± 0.11 b | 6.34 ± 0.09 c | 5.72 ± 0.20 b | 6.83 ± 0.09 d | 0.000 | 0.001 | 0.899 |
| BUN (mmol/L) | 2.45 ± 0.28 ab | 2.37 ± 0.19 ab | 2.34 ± 0.31 a | 2.70 ± 0.21 bc | 2.94 ± 0.10 c | 0.003 | 0.017 | 0.009 |
| ALB (g/L) | 6.85 ± 0.10 b | 6.21 ± 0.10 a | 6.38 ± 0.16 a | 6.57 ± 0.06 ab | 7.30 ± 0.26 c | 0.000 | 0.341 | 0.812 |
| ALP (U/L) | 70.80 ± 0.46 c | 53.03 ± 1.34 a | 81.83 ± 0.61 d | 61.43 ± 6.10 ab | 61.72 ± 3.53 b | 0.000 | 0.099 | 0.000 |
| AST (U/L) | 14.43 ± 1.28 a | 24.03 ± 0.15 c | 13.21 ± 0.92 a | 20.64 ± 0.51 b | 19.96 ± 0.41 b | 0.000 | 0.629 | 0.596 |
| ALT (U/L) | 2.25 ± 0.25 b | 2.18 ± 0.13 b | 1.58 ± 0.03 a | 2.08 ± 0.16 b | 2.50 ± 0.17 b | 0.001 | 0.548 | 0.002 |
| Items | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| INS/(mIU/L) | 120.95 ± 2.91 | 120.88 ± 0.74 | 118.88 ± 1.84 | 117.50 ± 2.15 | 119.86 ± 1.05 | 0.214 | - | - |
| GC (ng/L) | 347.73 ± 1.91 a | 374.46 ± 13.56 b | 375.55 ± 6.80 b | 351.38 ± 7.56 ab | 372.21 ± 11.96 ab | 0.008 | 0.357 | 0.264 |
| APLN (mg/L) | 1116.55 ± 33.16 b | 1035.43 ± 14.76 b | 1232.33 ± 55.87 c | 922.42 ± 37.28 a | 832.69 ± 19.85 a | 0.000 | 0.006 | 0.027 |
| ADPN (ng/L) | 19.20 ± 0.90 | 18.01 ± 0.85 | 18.62 ± 0.43 | 17.43 ± 1.11 | 18.92 ± 1.32 | 0.240 | - | - |
| AGRP (mg/L) | 131.04 ± 5.38 | 131.04 ± 1.79 | 134.93 ± 1.89 | 130.44 ± 7.23 | 138.46 ± 6.12 | 0.295 | - | - |
| Items | Dietary Lipid Sources | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| Liver | ||||||||
| AMS (U/mg prot) | 0.24 ± 0.01 c | 0.24 ± 0.01 c | 0.21 ± 0.01 c | 0.18 ± 0.01 b | 0.14 ± 0.01 a | 0.000 | 0.000 | 0.001 |
| TRY (U/mg prot) | 205.82 ± 11.08 bc | 212.25 ± 7.00 c | 209.66 ± 5.38 c | 186.36 ± 8.95 ab | 173.82 ± 8.21 a | 0.012 | 0.001 | 0.008 |
| LPS (U/mg prot) | 21.35 ± 2.19 b | 20.40 ± 1.36 ab | 18.73 ± 1.83 ab | 17.60 ± 2.97 ab | 15.80 ± 0.99 a | 0.043 | 0.001 | 0.701 |
| Intestines | ||||||||
| AMS (U/mg prot) | 1.44 ± 0.12 a | 2.02 ± 0.08 b | 1.60 ± 0.19 a | 1.71 ± 0.12 ab | 1.58 ± 0.04 a | 0.001 | 0.939 | 0.072 |
| TRY (U/mg prot) | 1621.45 ± 31.45 bc | 1635.07 ± 33.81 c | 2023.67 ± 19.41 d | 1322.17 ± 51.77 a | 1409.68 ± 46.55 ab | 0.000 | 0.114 | 0.063 |
| LPS (U/mg prot) | 41.28 ± 1.65 bc | 46.24 ± 4.37 c | 41.75 ± 1.03 bc | 39.37 ± 2.66 b | 29.79 ± 0.73 a | 0.487 | - | - |
| Items | Dietary Lipid Source | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| Hardness/(g/s) | 85.16 ± 1.15 b | 83.50 ± 0.78 b | 81.69 ± 1.48 ab | 79.56 ± 1.80 a | 78.99 ± 1.77 a | 0.002 | 0.000 | 0.537 |
| Toughness/mm | 1.44 ± 0.03 | 1.44 ± 0.02 | 1.43 ± 0.03 | 1.42 ± 0.03 | 1.36 ± 0.07 | 0.098 | - | - |
| Firmness/g | 150.72 ± 3.70 c | 150.46 ± 1.02 c | 146.13 ± 5.99 bc | 136.87 ± 3.37 ab | 129.97 ± 5.24 a | 0.000 | 0.000 | 0.058 |
| Chewiness/(g·s) | 81.32 ± 1.45 b | 80.78 ± 2.85 b | 80.33 ± 1.83 ab | 75.26 ± 1.36 a | 75.16 ± 2.09 a | 0.005 | 0.000 | 0.435 |
| Springiness/% | 34.45 ± 1.19 b | 35.59 ± 0.81 b | 33.76 ± 1.53 b | 33.42 ± 0.87 b | 28.53 ± 0.48 a | 0.000 | 0.001 | 0.001 |
| Myofiber density (fibers/mm2) | 189.51 ± 12.22 b | 187.46 ± 5.00 b | 184.42 ± 3.01 ab | 169.53 ± 12.58 ab | 163.01 ± 4.22 a | 0.011 | 0.000 | 0.258 |
| Myofiber diameter (µm) | 45.86 ± 3.39 a | 48.07 ± 1.36 ab | 50.45 ± 0.99 ab | 50.74 ± 2.77 ab | 53.07 ± 1.00 b | 0.018 | 0.000 | 0.682 |
| Items | Dietary Lipid Source | |||||||
|---|---|---|---|---|---|---|---|---|
| FO | SO | RO | PO | LO | AN | LT | QT | |
| HYP (μg/mg) | 0.34 ± 0.01 c | 0.34 ± 0.01 c | 0.32 ± 0.01 bc | 0.28 ± 0.02 a | 0.30 ± 0.01 ab | 0.000 | 0.000 | 0.662 |
| Collagen (μg/mg) | 2.79 ± 0.15 b | 2.71 ± 0.07 b | 2.56 ± 0.08 ab | 2.39 ± 0.14 a | 2.38 ± 0.04 a | 0.002 | 0.000 | 0.667 |
| PHD (pg/mL) | 63.00 ± 4.19 b | 55.96 ± 6.19 ab | 52.43 ± 4.35 ab | 50.41 ± 2.05 a | 48.65 ± 5.25 a | 0.024 | 0.001 | 0.213 |
| LOX (pg/mL) | 8.24 ± 0.35 c | 5.79 ± 0.54 ab | 7.25 ± 1.05 bc | 4.37 ± 0.24 a | 6.13 ± 1.18 ab | 0.001 | 0.035 | 0.156 |
| PYD (nmol/L) | 46.93 ± 2.43 b | 44.82 ± 1.57 ab | 46.62 ± 2.47 ab | 41.30 ± 1.09 a | 43.86 ± 2.22 ab | 0.039 | 0.047 | 0.689 |
| Cath-B (ng/mL) | 302.76 ± 11.75 a | 303.01 ± 12.56 a | 321.35 ± 5.09 bc | 331.60 ± 11.27 bc | 352.11 ± 6.93 c | 0.001 | 0.000 | 0.142 |
| Cath-L (ng/mL) | 153.70 ± 1.77 | 154.86 ± 2.22 | 150.59 ± 5.24 | 157.10 ± 5.48 | 160.97 ± 8.79 | 0.253 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Yao, X.; Jing, F.; Yang, W.; Wu, J.; Zhang, H.; Zhang, P.; Xie, Y.; Pan, X.; Zhao, L.; et al. Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides). Animals 2024, 14, 781. https://doi.org/10.3390/ani14050781
Song R, Yao X, Jing F, Yang W, Wu J, Zhang H, Zhang P, Xie Y, Pan X, Zhao L, et al. Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides). Animals. 2024; 14(5):781. https://doi.org/10.3390/ani14050781
Chicago/Turabian StyleSong, Rui, Xinfeng Yao, Futao Jing, Wenxue Yang, Jiaojiao Wu, Hao Zhang, Penghui Zhang, Yuanyuan Xie, Xuewen Pan, Long Zhao, and et al. 2024. "Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides)" Animals 14, no. 5: 781. https://doi.org/10.3390/ani14050781
APA StyleSong, R., Yao, X., Jing, F., Yang, W., Wu, J., Zhang, H., Zhang, P., Xie, Y., Pan, X., Zhao, L., & Wu, C. (2024). Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides). Animals, 14(5), 781. https://doi.org/10.3390/ani14050781







