Supplementation with Astragalus Root Powder Promotes Rumen Microbiota Density and Metabolome Interactions in Lambs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Animals and Materials
2.2. Sample Collection
2.3. Rumen Microbiota Density Measurement
2.4. LS-MS/MS Metabolic Profile Determination
2.5. Data Analysis
3. Results
3.1. Rumen Microbiota Density
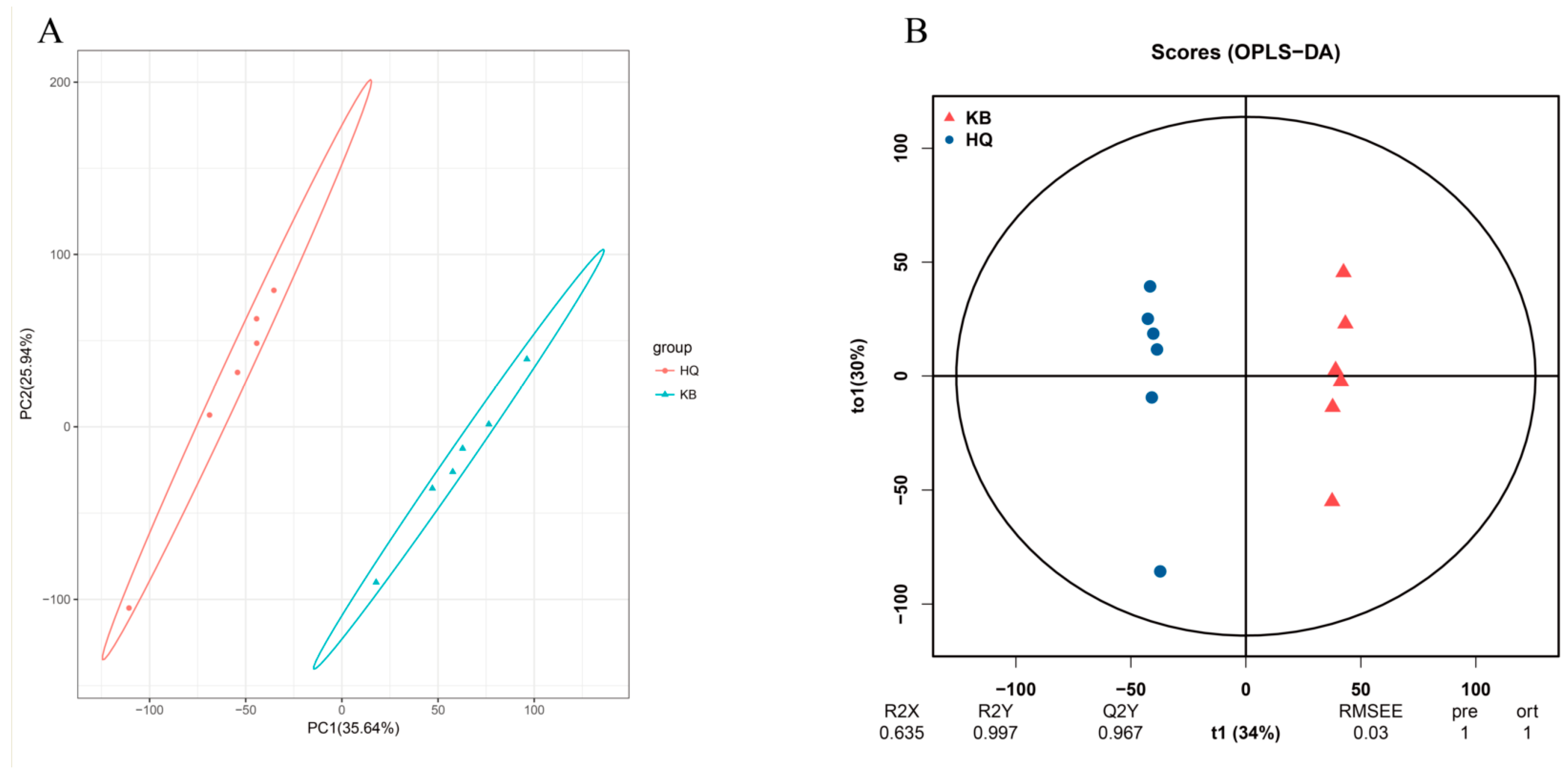
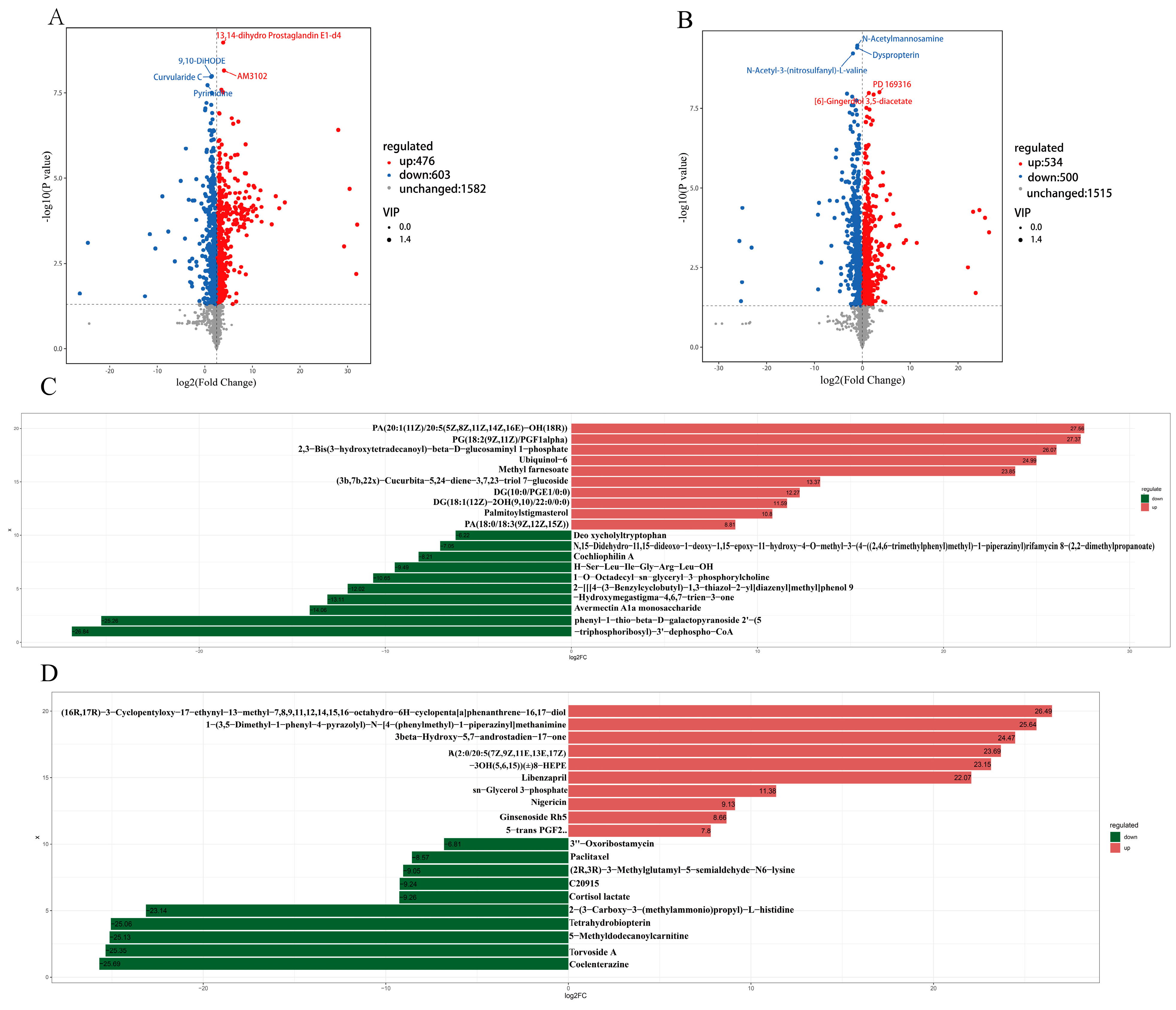
3.2. Metabolic Profiling of the Rumen Microbiota
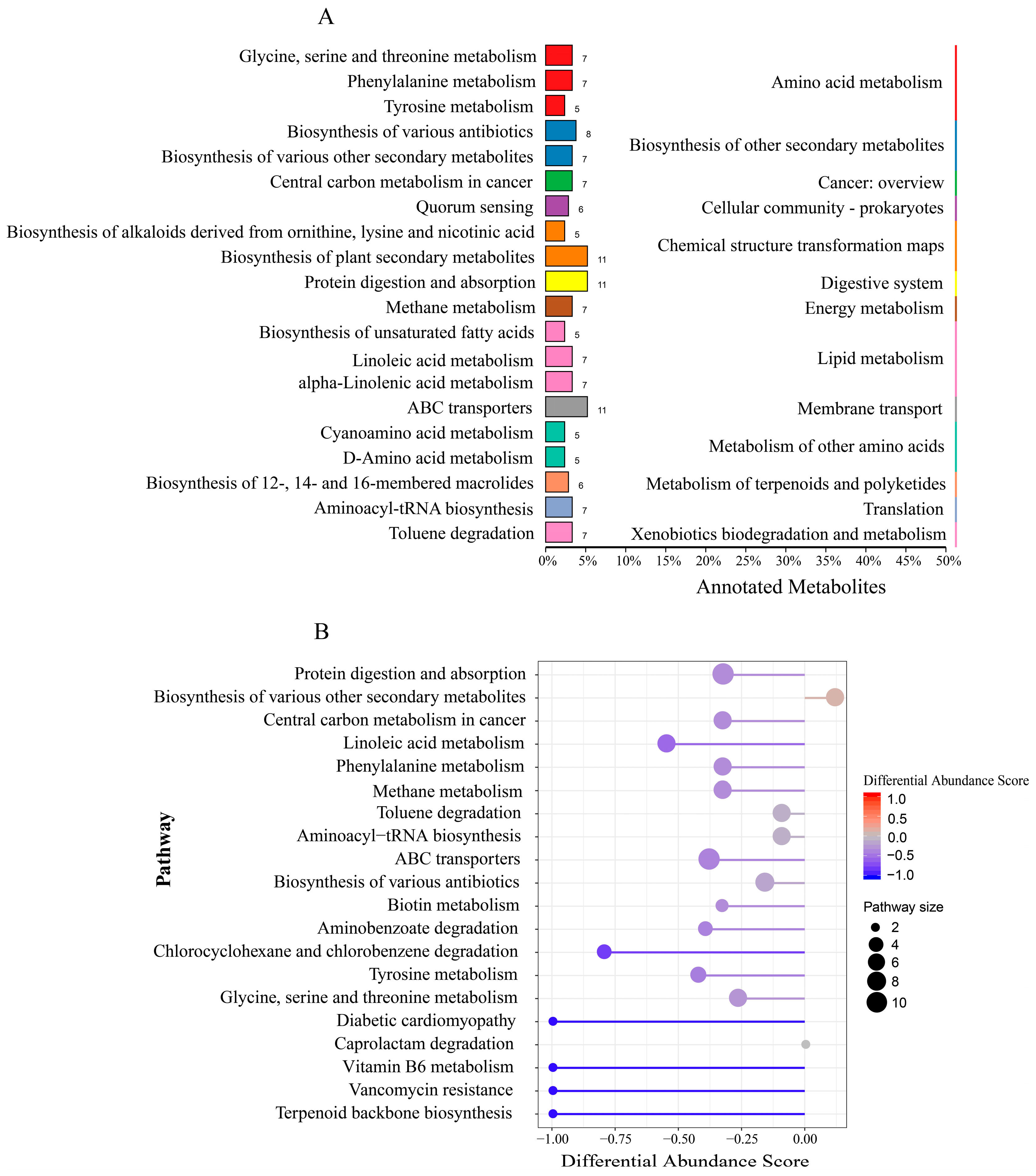
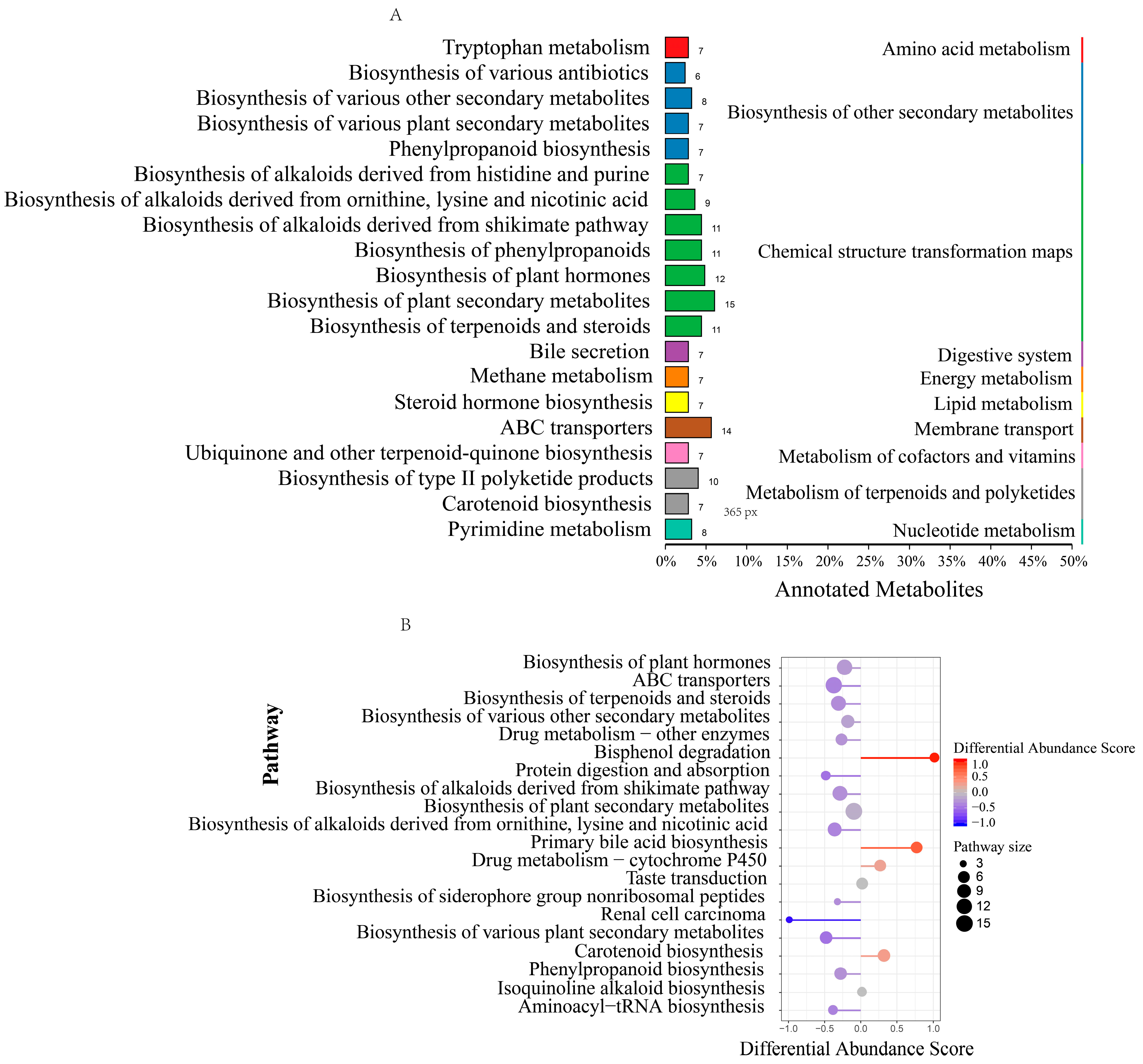
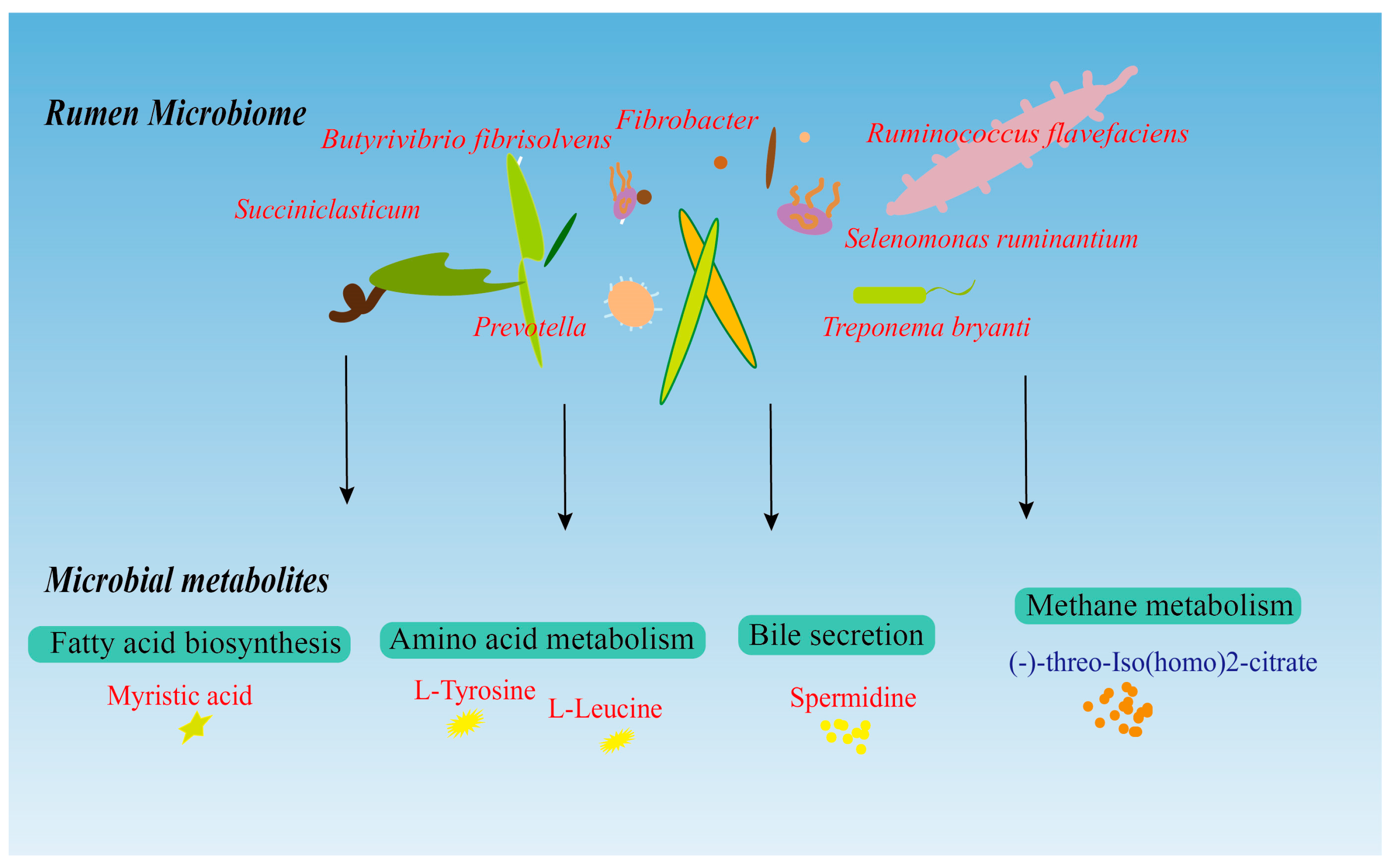
3.3. Rumen Microbiome–Metabolome Interaction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat. Foods 2020, 9, 1178. [Google Scholar] [CrossRef]
- Chai, J.; Diao, Q.; Wang, H.; Tu, Y.; Tao, X.; Zhang, N. Effects of weaning age on growth, nutrient digestibility and metabolism, and serum parameters in Hu lambs. Anim. Nutr. 2015, 1, 344–348. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Liu, T.; Zhang, Q.; Wang, G.; Li, F.; Li, F.; Yue, X.; Li, T. Effect of Early Weaning on the Intestinal Microbiota and Expression of Genes Related to Barrier Function in Lambs. Front. Microbiol. 2018, 9, 1431. [Google Scholar] [CrossRef]
- Guo, G.; Yang, W.; Fan, C.; Lan, R.; Gao, Z.; Gan, S.; Yu, H.; Yin, F.; Wang, Z. The effects of fucoidan as a dairy substitute on diarrhea rate and intestinal barrier function of the large intestine in weaned lambs. Front. Vet. Sci. 2022, 9, 1007346. [Google Scholar] [CrossRef]
- Khan, M.Z.H. Recent Biosensors for Detection of Antibiotics in Animal Derived Food. Crit. Rev. Anal. Chem. 2022, 52, 780–790. [Google Scholar] [CrossRef]
- Xu, Q.; Cheng, M.; Jiang, R.; Zhao, X.; Zhu, J.; Liu, M.; Chao, X.; Zhang, C.; Zhou, B. Effects of dietary supplement with a Chinese herbal mixture on growth performance, antioxidant capacity, and gut microbiota in weaned pigs. Front. Vet. Sci. 2022, 9, 971647. [Google Scholar] [CrossRef]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.; Tsim, K.W. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.W.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K.; Chan, K. Evaluation of the Pharmaceutical Properties and Value of Astragali Radix. Medicines 2018, 5, 46. [Google Scholar] [CrossRef]
- Hao, X.; Wang, P.; Ren, Y.; Liu, G.; Zhang, J.; Leury, B.; Zhang, C. Effects of Astragalus membranaceus roots supplementation on growth performance, serum antioxidant and immune response in finishing lambs. Asian-Australas. J. Anim. Sci. 2020, 33, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Adams, S.; Cai, W.; Qin, G.-X.; Atiba, E.M.; Jiang, H. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiologyopen 2019, 8, e00712. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xie, F.; Sun, D.; Liu, J.; Zhu, W.; Mao, S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome 2019, 7, 83. [Google Scholar] [CrossRef]
- Edwards, J.E.; McEwan, N.R.; Travis, A.J.; John Wallace, R. 16S rDNA library-based analysis of ruminal bacterial diversity. Antonie Van Leeuwenhoek 2004, 86, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; Wallace, R.J.; Morais, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Sha, Y.; Liu, X.; He, Y.; Guo, X.; Hu, J.; Wang, J.; Li, S.; Zhu, C.; Chen, G.; et al. Astragalus additive in feed improved serum immune function, rumen fermentation and the microbiota structure of early-weaned lambs. J. Appl. Microbiol. 2023, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Hales, K.E. Relationships between digestible energy and metabolizable energy in current feedlot diets. Transl. Anim. Sci. 2019, 3, 945–952. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Muyzer, G.; Waal, E.C.d.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- McHardy, I.H.; Goudarzi, M.; Tong, M.; Ruegger, P.M.; Schwager, E.; Weger, J.R.; Graeber, T.G.; Sonnenburg, J.L.; Horvath, S.; Huttenhower, C.; et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome 2013, 1, 17. [Google Scholar] [CrossRef]
- Palevich, N.; Kelly, W.J.; Leahy, S.C.; Denman, S.; Altermann, E.; Rakonjac, J.; Attwood, G.T. Comparative Genomics of Rumen Butyrivibrio spp. Uncovers a Continuum of Polysaccharide-Degrading Capabilities. Appl. Environ. Microbiol. 2019, 86, e01993-19. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Martin, S.A. Factors affecting lactate and malate utilization by Selenomonas ruminantium. Appl. Environ. Microbiol. 1997, 63, 4853–4858. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, J.; Martin, C.; Morgavi, D.P. The use of direct-fed microbials for mitigation of ruminant methane emissions: A review. Animal 2014, 8, 250–261. [Google Scholar] [CrossRef] [PubMed]
- SAWANON, S.; KOBAYASHI, Y. Synergistic fibrolysis in the rumen by cellulolytic Ruminococcus flavefaciens and non-cellulolytic Selenomonas ruminantium: Evidence in defined cultures. Anim. Sci. J. 2006, 77, 208–214. [Google Scholar] [CrossRef]
- Stanton, T.B.; Canale-Parola, E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch. Microbiol. 1980, 127, 145–156. [Google Scholar] [CrossRef]
- van Gylswyk, N.O. Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 1995, 45, 297–300. [Google Scholar] [CrossRef]
- Emerson, E.L.; Weimer, P.J. Fermentation of model hemicelluloses by Prevotella strains and Butyrivibrio fibrisolvens in pure culture and in ruminal enrichment cultures. Appl. Microbiol. Biotechnol. 2017, 101, 4269–4278. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Bjorck, I.; Backhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Y.; Dingkao, R.; Zhang, W.; Lv, W.; Wei, H.; Shi, H.; Hu, J.; Wang, J.; Li, S.; et al. Interactions Between Rumen Microbes, VFAs, and Host Genes Regulate Nutrient Absorption and Epithelial Barrier Function During Cold Season Nutritional Stress in Tibetan Sheep. Front. Microbiol. 2020, 11, 593062. [Google Scholar] [CrossRef]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Li, T.; Wei, H.; et al. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Y.; Sun, H.; Chang, H.; Zhao, H.; Zhang, S.; Shan, C. Decreased SLC27A5 Suppresses Lipid Synthesis and Tyrosine Metabolism to Activate the Cell Cycle in Hepatocellular Carcinoma. Biomedicines 2022, 10, 234. [Google Scholar] [CrossRef]
- Hase, A.; Jung, S.E.; aan het Rot, M. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol. Biochem. Behav. 2015, 133, 1–6. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S, discussion 1548S. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.C.; Craig, R. Cysteamine effects on monoamines, dopamine-beta-hydroxylase and the hypothalamic-pituitary axis. Neuroendocrinology 1985, 41, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zuo, B.; Wang, W.; Wang, S.; Wang, J. Dietary Supplementation of Leucine in Premating Diet Improves the Within-Litter Birth Weight Uniformity, Antioxidative Capability, and Immune Function of Primiparous SD Rats. BioMed Res. Int. 2018, 2018, 1523147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Zhong, Y.; Yang, Y.; Yin, Y. Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis. Nutrients 2020, 12, 1299. [Google Scholar] [CrossRef]
- Holman, R.T. The slow discovery of the importance of omega 3 essential fatty acids in human health. J. Nutr. 1998, 128, 427S–433S. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X. The Effects of a Low Linoleic Acid/alpha-Linolenic Acid Ratio on Lipid Metabolism and Endogenous Fatty Acid Distribution in Obese Mice. Int. J. Mol. Sci. 2023, 24, 12117. [Google Scholar] [CrossRef]
- Huang, X.; Chen, F.; Guan, J.; Xu, C.; Li, Y.; Xie, D. Beneficial effects of re-feeding high alpha-linolenic acid diets on the muscle quality, cold temperature and disease resistance of tilapia. Fish Shellfish Immunol. 2022, 126, 303–310. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Mariz, L.D.S.; Amaral, P.M.; Valadares Filho, S.C.; Santos, S.A.; Detmann, E.; Marcondes, M.I.; Pereira, J.M.V.; Silva Junior, J.M.; Prados, L.F.; Faciola, A.P. Dietary protein reduction on microbial protein, amino acid digestibility, and body retention in beef cattle: 2. Amino acid intestinal absorption and their efficiency for whole-body deposition. J. Anim. Sci. 2018, 96, 670–683. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. Arginine: Clinical potential of a semi-essential amino acid. Altern. Med. Rev. 2002, 7, 512–522. [Google Scholar] [PubMed]
- Sha, Y.; Guo, X.; He, Y.; Li, W.; Liu, X.; Zhao, S.; Hu, J.; Wang, J.; Li, S.; Zhao, Z.; et al. Synergistic Responses of Tibetan Sheep Rumen Microbiota, Metabolites, and the Host to the Plateau Environment. Int. J. Mol. Sci. 2023, 24, 14856. [Google Scholar] [CrossRef]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [PubMed]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Ransom-Jones, E.; Jones, D.L.; McCarthy, A.J.; McDonald, J.E. The Fibrobacteres: An important phylum of cellulose-degrading bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef]
- Seshadri, R.; Leahy, S.C.; Attwood, G.T.; Teh, K.H.; Lambie, S.C.; Cookson, A.L.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Hadjithomas, M.; Varghese, N.J.; et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 2018, 36, 359–367. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8(+) T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Gabel, G.; Aschenbach, J.R.; Muller, F. Transfer of energy substrates across the ruminal epithelium: Implications and limitations. Anim. Health Res. Rev. 2002, 3, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]


| Ingredients | Content |
|---|---|
| Corn | 40.00 |
| Spray corn husks | 10.00 |
| Rice alcohol grain | 10.00 |
| A powder | 10.00 |
| Soybean meal | 8.50 |
| Corn germ meal | 8.00 |
| Rapeseed dregs | 3.00 |
| Cotton pulp | 6.00 |
| Limestone | 2.30 |
| Calcium bicarbonate | 0.80 |
| Premix 1 | 1.40 |
| Total | 100.00 |
| Nutrient levels 2 | |
| DE 3, MJ/kg | 12.95 |
| Crude protein 4 | ≥18.00 |
| Crude ash 4 | ≤12.00 |
| Ca | 0.60~1.30 |
| P | ≥0.50 |
| NaCl | 0.50~1.50 |
| Lysine | ≥0.60 |
| Gene | Primer (5′-3′) | Length | Annealing Temperature | Login ID |
|---|---|---|---|---|
| Bacterium | F: CCTACGGGAGGCAGCAG | 181 bp | 60 °C | * |
| R: TTACCGCGGCTGCTGG | ||||
| Bf | F: CCTGACTAAGAAGCACCGGC | 107 bp | 60 °C | U41167.1 |
| R: GTAAAACCGCCTACGCTCCC | ||||
| Rf | F: TATCTTAGTGGCGGACGGGT | 157 bp | 60 °C | MT356193.1 |
| R: TCTAATCAGACGCGAGCCCA | ||||
| Fi | F: AACTCCACGTGTGGGATGAA | 164 bp | 60 °C | NR_042150.1 |
| R: CCAGTGATTCCGAACAACGC | ||||
| Pr | F: GGATGGGGATGCGTCTGATT | 129 bp | 60 °C | NR_028866.1 |
| R: CTGCCTCCCGTAGGAGTTTG | ||||
| Su | F: CGTCCGATTAGCTGGTTGGT | 185 bp | 60 °C | NR_026205.1 |
| R: AAGAACTTCCTCACCCACGC | ||||
| Cb | F: GCAACGCGAAGAACCTTACC | 110 bp | 60 °C | NR-042144.1 |
| R: GCGGGACTTAACCCAACATC | ||||
| Sr | F: GAATCATTGGGCGTAAAGGG | 140 bp | 60 °C | AB198442.1 |
| R: CATTTCACCGCTACACTAGG | ||||
| Tb | F: CCTTATGTCCAGGGCTACAC | 115 bp | 60 °C | NR-118718.2 |
| R: CGGGTTTCAGACTCCTATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, P.; Sha, Y.; Liu, X.; He, Y.; Wang, F.; Hu, J.; Wang, J.; Li, S.; Chen, X.; Yang, W.; et al. Supplementation with Astragalus Root Powder Promotes Rumen Microbiota Density and Metabolome Interactions in Lambs. Animals 2024, 14, 788. https://doi.org/10.3390/ani14050788
Shao P, Sha Y, Liu X, He Y, Wang F, Hu J, Wang J, Li S, Chen X, Yang W, et al. Supplementation with Astragalus Root Powder Promotes Rumen Microbiota Density and Metabolome Interactions in Lambs. Animals. 2024; 14(5):788. https://doi.org/10.3390/ani14050788
Chicago/Turabian StyleShao, Pengyang, Yuzhu Sha, Xiu Liu, Yanyu He, Fanxiong Wang, Jiang Hu, Jiqing Wang, Shaobin Li, Xiaowei Chen, Wenxin Yang, and et al. 2024. "Supplementation with Astragalus Root Powder Promotes Rumen Microbiota Density and Metabolome Interactions in Lambs" Animals 14, no. 5: 788. https://doi.org/10.3390/ani14050788
APA StyleShao, P., Sha, Y., Liu, X., He, Y., Wang, F., Hu, J., Wang, J., Li, S., Chen, X., Yang, W., Chen, Q., & Gao, M. (2024). Supplementation with Astragalus Root Powder Promotes Rumen Microbiota Density and Metabolome Interactions in Lambs. Animals, 14(5), 788. https://doi.org/10.3390/ani14050788





