Bovine Colostrum Supplementation in Rabbit Diet Modulates Gene Expression of Cytokines, Gut–Vascular Barrier, and Red-Ox-Related Molecules in the Gut Wall
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. RNA Extraction, Reverse Transcription, and Real-Time PCR from Rabbit Tissues
2.3. Gram Micro-Organism Staining
2.4. Microbiota Analysis
2.5. Vaccination Efficacy Evaluation
2.6. Statistical Analysis
3. Results
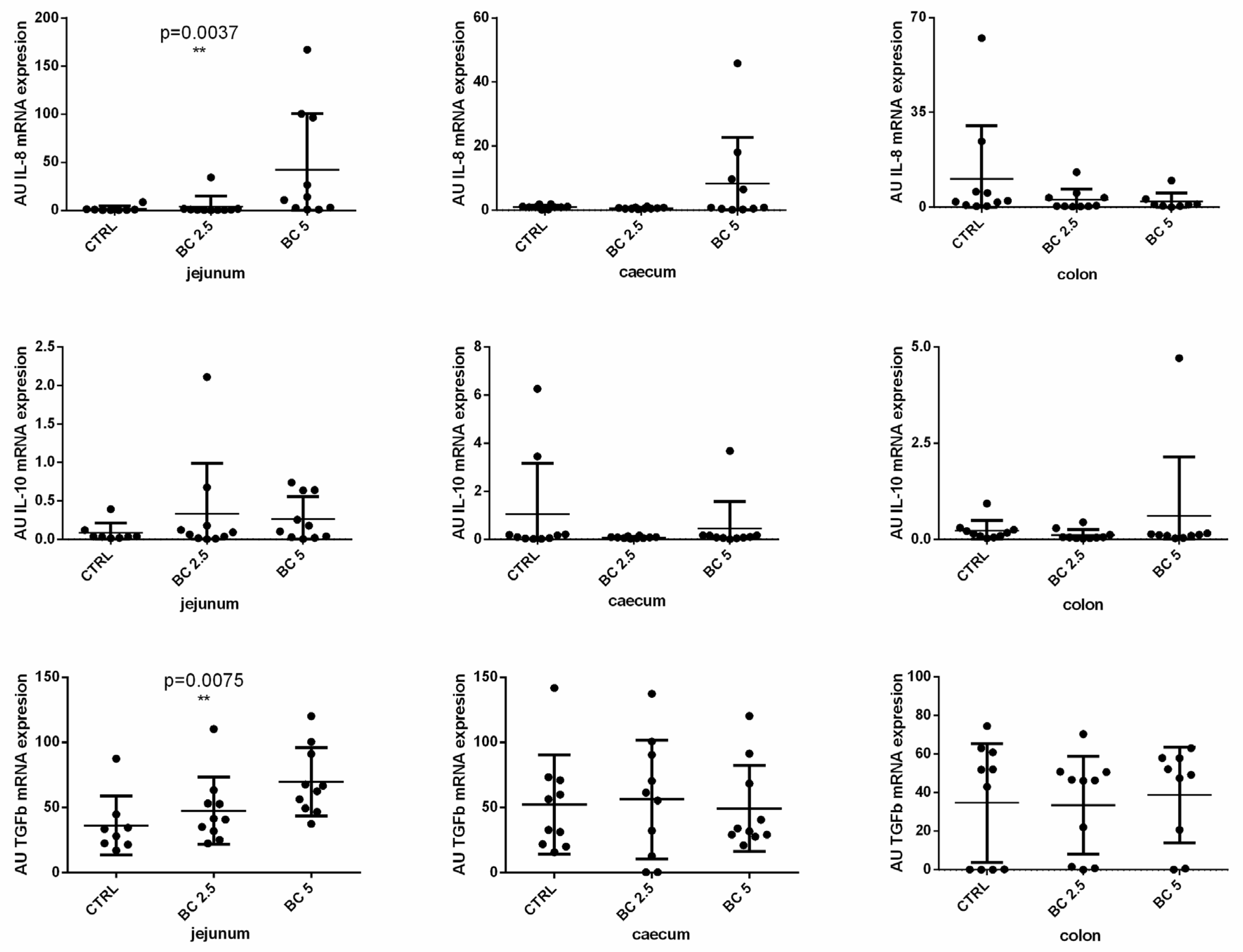
3.1. BC Supplementation Modulates Immune-Related Gene Expression in the Small Intestine
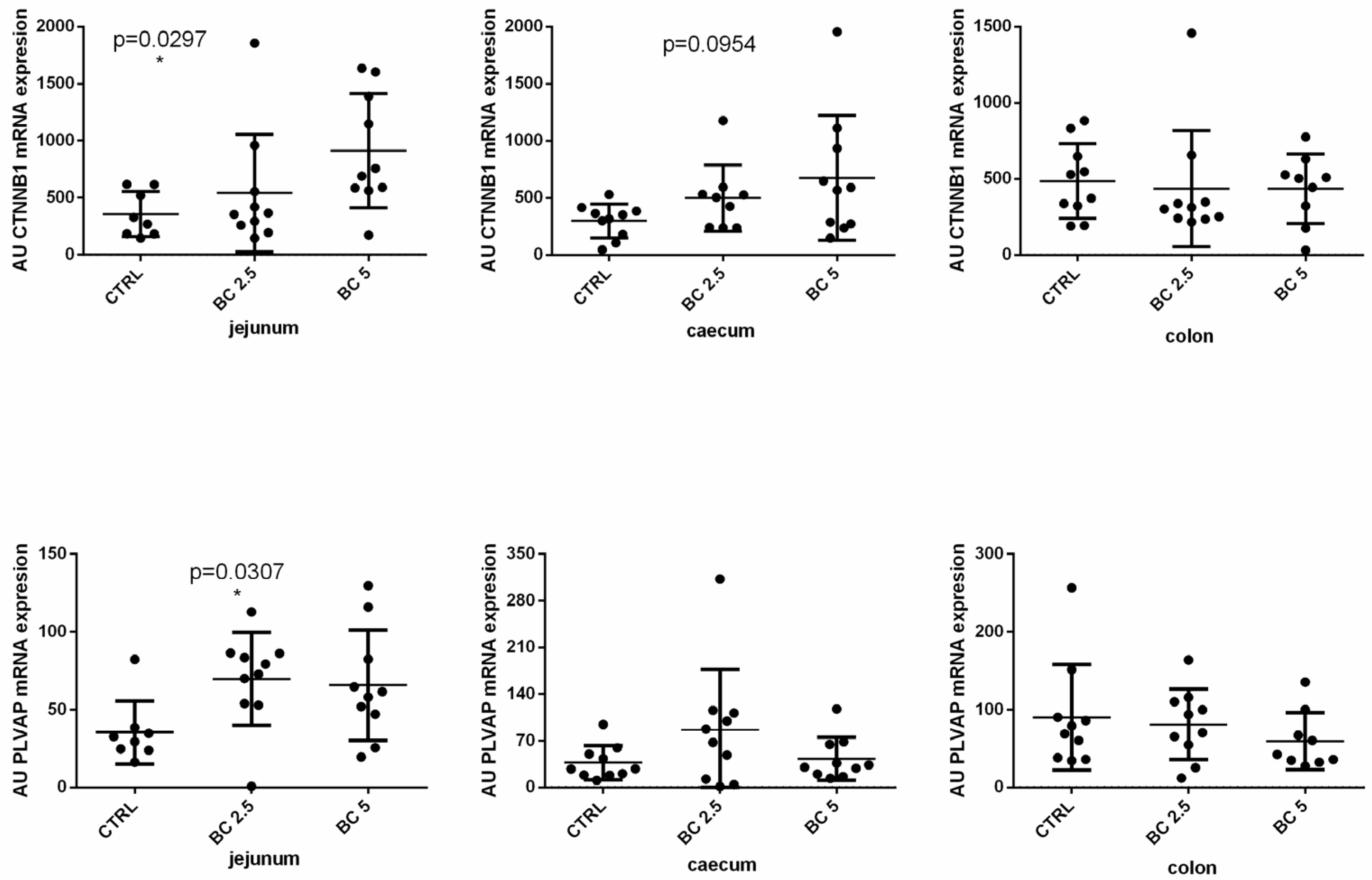
3.2. BC Supplementation Modulates Gut–Vascular Barrier-Related Gene Expression in the Gut
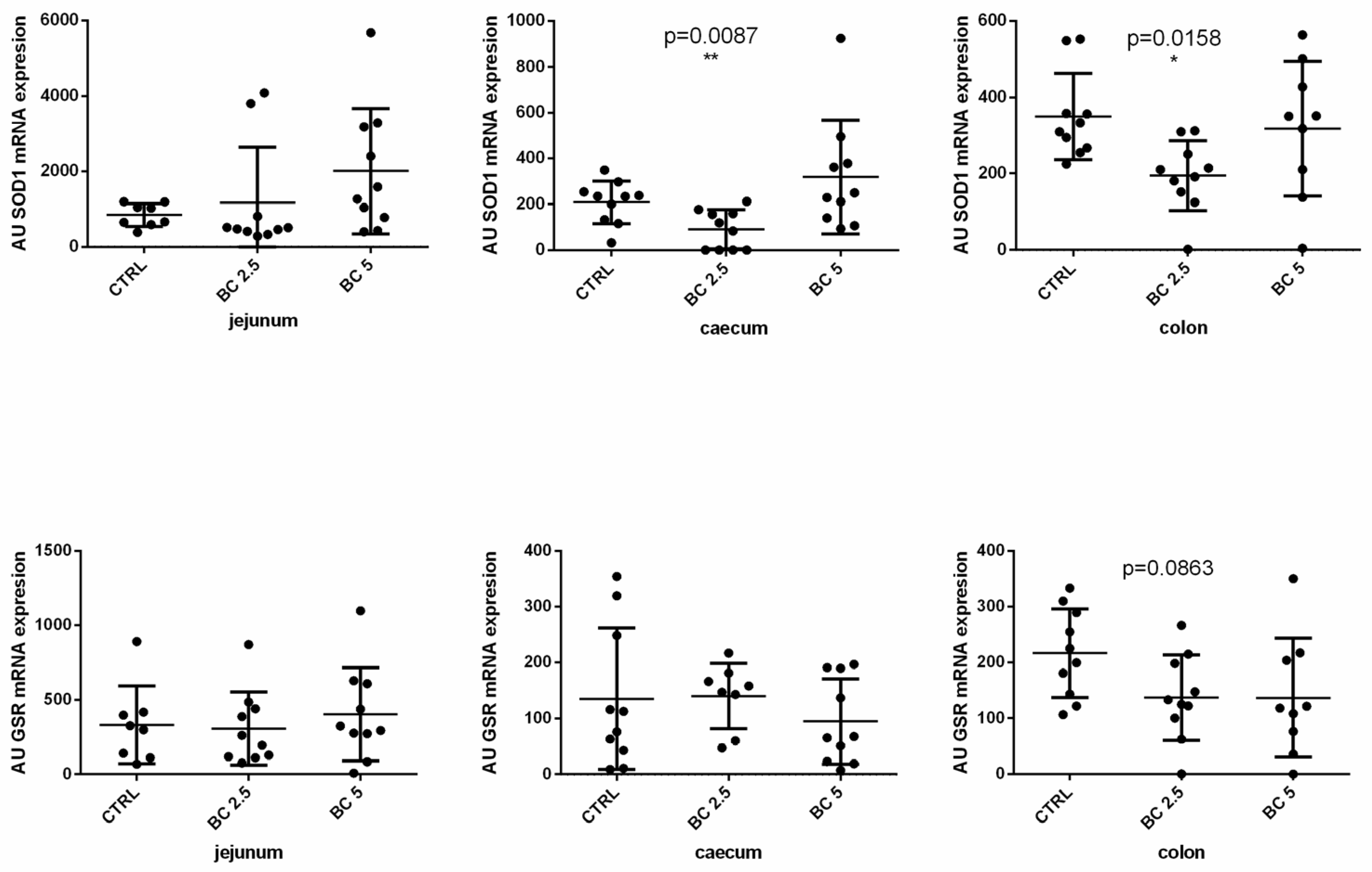
3.3. BC Supplementation Modulates Antioxidant-Related Gene Expression in the Gut
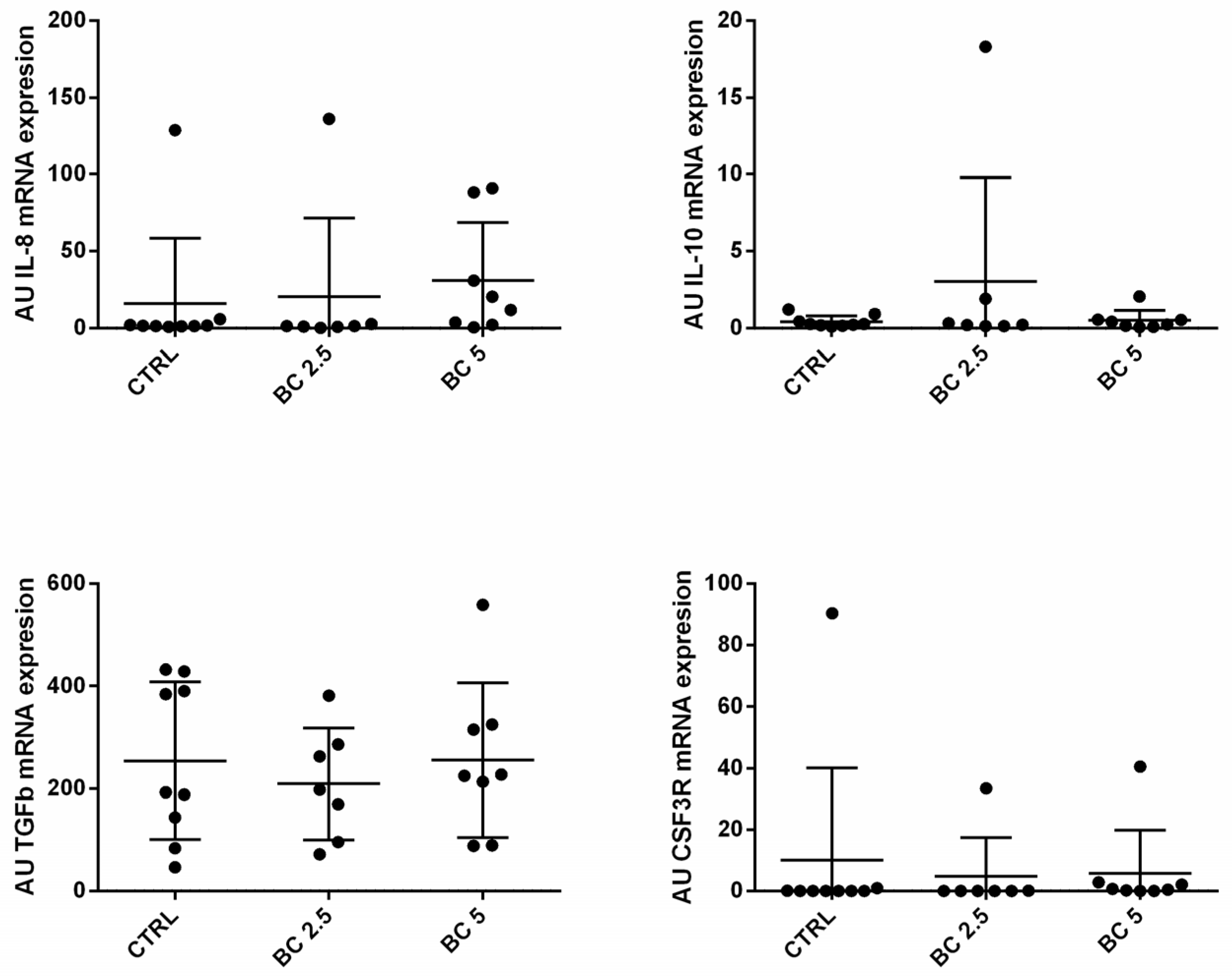
3.4. BC Supplementation Does Not Modulate Immune-Related Genes in Lymph Nodes
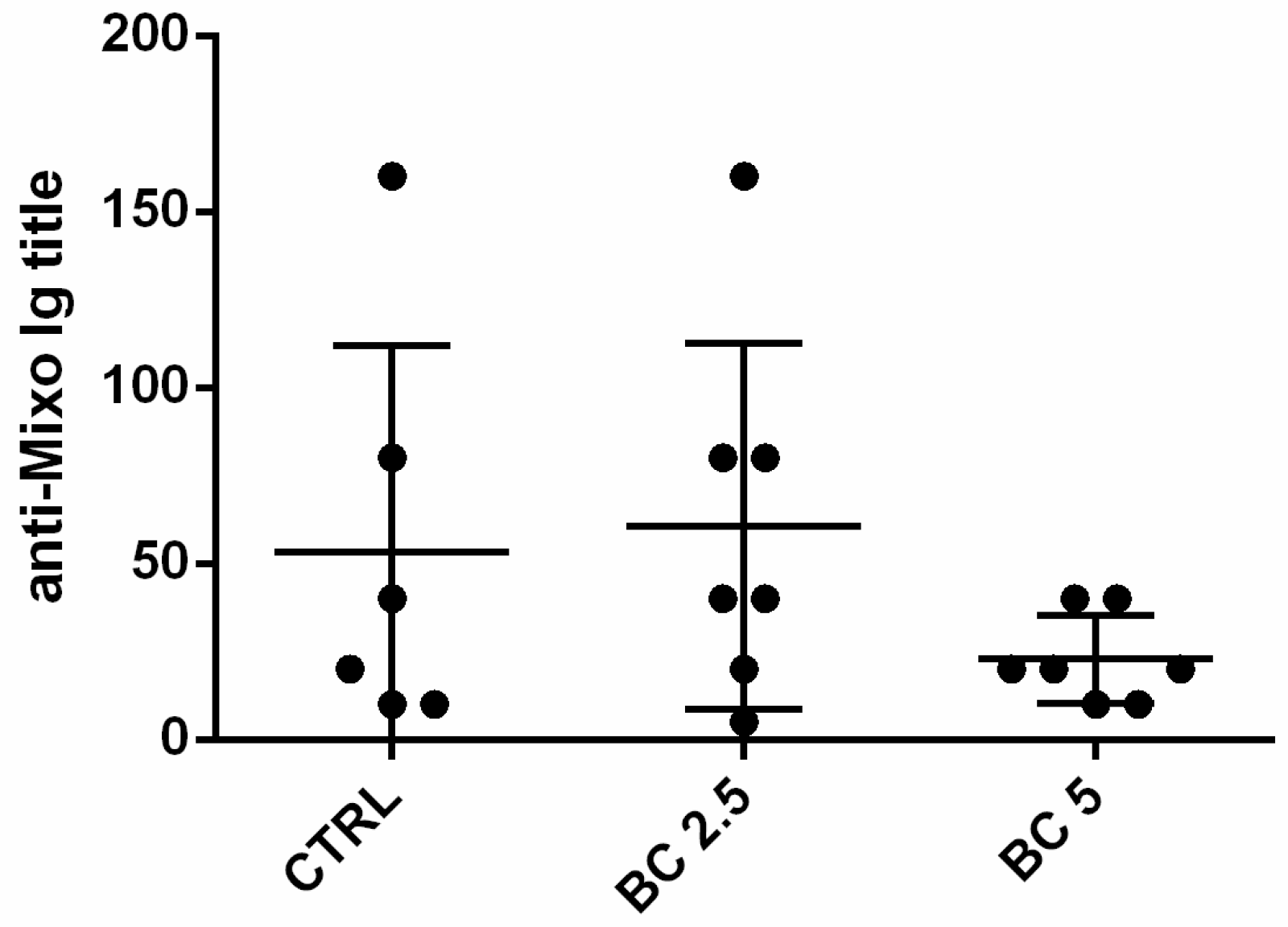
3.5. Response to Vaccination
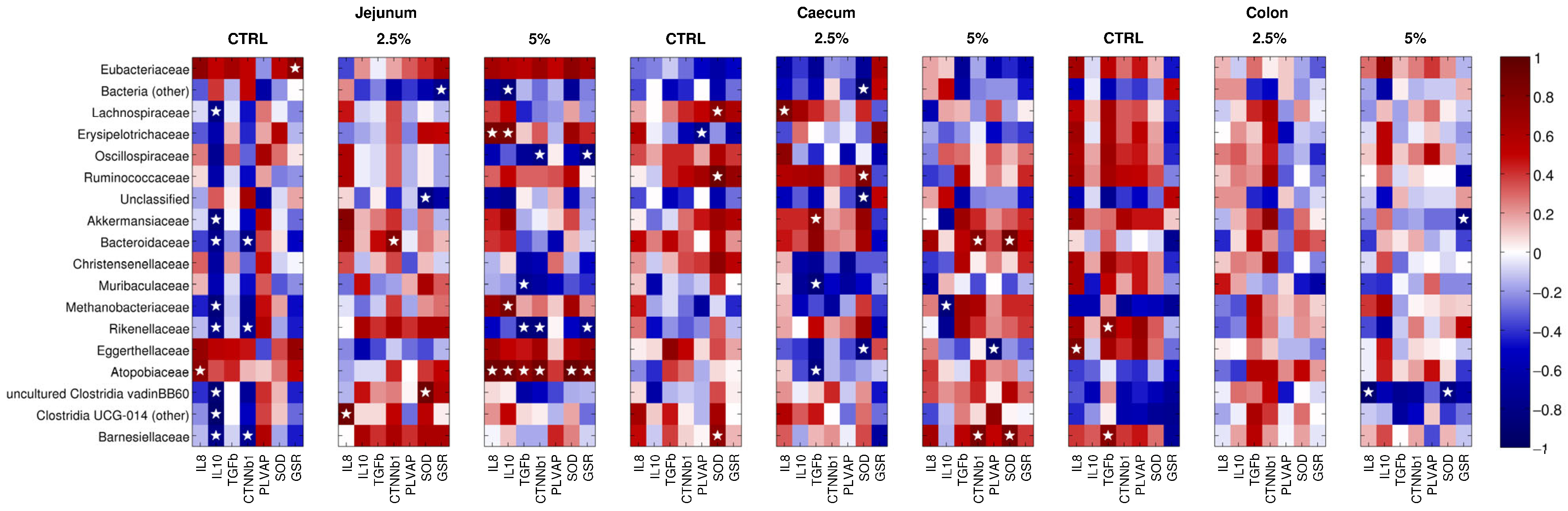
3.6. The Gene Expression of the Molecules under Study Correlates with the Abundance of Microbial Families
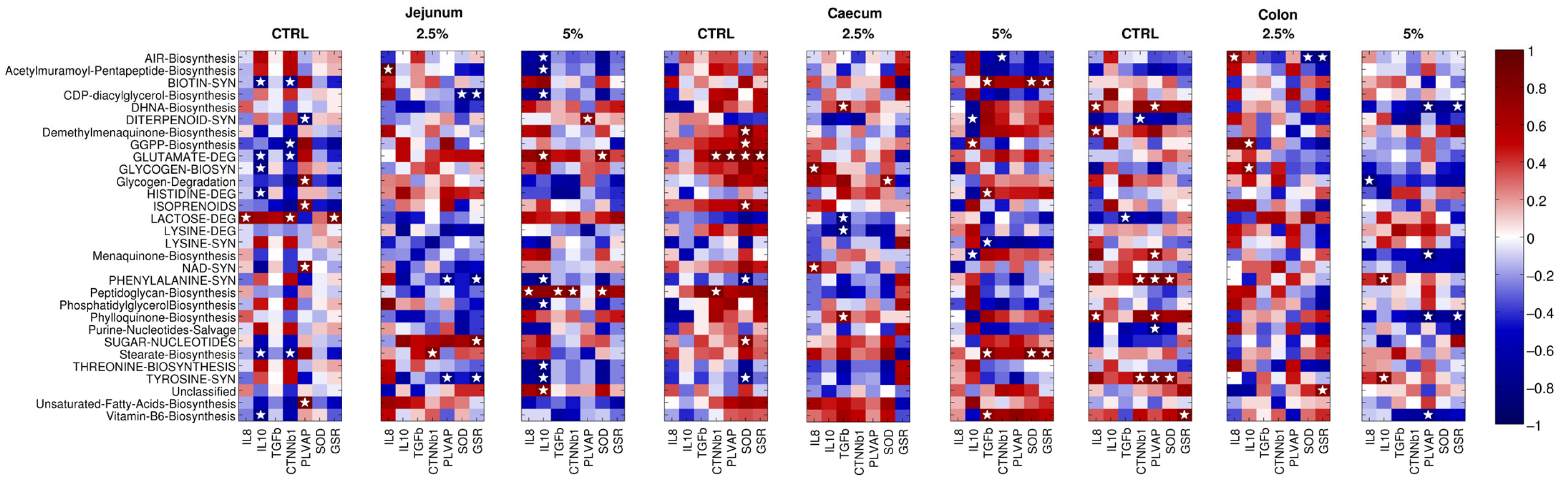
3.7. The Gene Expression of Immune, Gut–Vascular Barrier, and Antioxidant Molecule Correlates with Specific Bacterial Pathways
3.8. Body Weight Did Not Differ among the Three Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission, Directorate-General for Health and Food Safety. Commercial Rabbit Farming in the European Union—Overview Report; Publications Office of the European Union: Luxembourg, 2017; Available online: https://data.europa.eu/doi/10.2772/62174 (accessed on 25 February 2024).
- Crovato, S.; Pinto, A.; Martino, G.D.; Mascarello, G.; Rizzoli, V.; Marcolin, S.; Ravarotto, L. Purchasing Habits, Sustainability Perceptions, and Welfare Concerns of Italian Consumers Regarding Rabbit Meat. Foods 2022, 11, 1205. [Google Scholar] [CrossRef]
- Petracci, M.; Soglia, F.; Leroy, F. Rabbit Meat in Need of a Hat-Trick: From Tradition to Innovation (and Back). Meat Sci. 2018, 146, 93–100. [Google Scholar] [CrossRef]
- Trocino, A.; Cotozzolo, E.; Zomeño, C.; Petracci, M.; Xiccato, G.; Castellini, C. Rabbit Production and Science: The World and Italian Scenarios from 1998 to 2018. Ital. J. Anim. Sci. 2019, 18, 1361–1371. [Google Scholar] [CrossRef]
- Zerani, M.; Boiti, C.; Dall’Aglio, C.; Pascucci, L.; Maranesi, M.; Brecchia, G.; Mariottini, C.; Guelfi, G.; Zampini, D.; Gobbetti, A. Leptin Receptor Expression and in Vitro Leptin Actions on Prostaglandin Release and Nitric Oxide Synthase Activity in the Rabbit Oviduct. J. Endocrinol. 2005, 185, 319–325. [Google Scholar] [CrossRef]
- Meineri, G.; Giacobini, M.; Forneris, G. Evaluation of Physiological Parameters of the Plasma Oxidative Status in Rabbits. J. Appl. Anim. Res. 2017, 45, 315–319. [Google Scholar] [CrossRef]
- Cooper, T.K.; Meyerholz, D.K.; Beck, A.P.; Delaney, M.A.; Piersigilli, A.; Southard, T.L.; Brayton, C.F. Research-Relevant Conditions and Pathology of Laboratory Mice, Rats, Gerbils, Guinea Pigs, Hamsters, Naked Mole Rats, and Rabbits. ILAR J. 2021, 62, 77–132. [Google Scholar] [CrossRef]
- Attia, Y.A.; Hamed, R.S.; Bovera, F.; Abd El-Hamid, A.E.H.E.; Al-Harthi, M.A.; Shahba, H.A. Semen Quality, Antioxidant Status and Reproductive Performance of Rabbits Bucks Fed Milk Thistle Seeds and Rosemary Leaves. Anim. Reprod. Sci. 2017, 184, 178–186. [Google Scholar] [CrossRef]
- Bovera, F.; Marono, S.; Di Meo, C.; Piccolo, G.; Iannaccone, F.; Nizza, A. Effect of Mannanoligosaccharides Supplementation on Caecal Microbial Activity of Rabbits. Animal 2010, 4, 1522–1527. [Google Scholar] [CrossRef]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential Benefits of Colostrum in Gastrointestinal Diseases. Front. Biosci. 2016, 8, 331–351. [Google Scholar] [CrossRef]
- El Mashad, G.; Abd El Naby, S.; Mahmoud Abd El Bary, M. Bovine Colostrum versus Prebiotics in Children with Acute Gastroenteritis. Menoufia Med. J. 2016, 29, 95. [Google Scholar] [CrossRef]
- Menchetti, L.; Curone, G.; Filipescu, I.E.; Barbato, O.; Leonardi, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Riva, F.; Casano, A.B.; et al. The Prophylactic Use of Bovine Colostrum in a Murine Model of TNBS-Induced Colitis. Animals 2020, 10, 492. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Szymańska, P.; Fichna, J. Supplementation of Bovine Colostrum in Inflammatory Bowel Disease: Benefits and Contraindications. Adv. Nutr. 2021, 12, 533–545. [Google Scholar] [CrossRef]
- Chandwe, K.; Kelly, P. Colostrum Therapy for Human Gastrointestinal Health and Disease. Nutrients 2021, 13, 1956. [Google Scholar] [CrossRef]
- Langer, P. Differences in the Composition of Colostrum and Milk in Eutherians Reflect Differences in Immunoglobulin Transfer. J. Mammal. 2009, 90, 332–339. [Google Scholar] [CrossRef]
- Playford, R.J. Peptide Therapy and the Gastroenterologist: Colostrum and Milk-Derived Growth Factors. Clin. Nutr. 2001, 20, 101–106. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine Milk-Derived Exosomes from Colostrum Are Enriched with Proteins Implicated in Immune Response and Growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Mann, S.; Curone, G.; Chandler, T.L.; Sipka, A.; Cha, J.; Bhawal, R.; Zhang, S. Heat Treatment of Bovine Colostrum: II. Effects on Calf Serum Immunoglobulin, Insulin, and IGF-I Concentrations, and the Serum Proteome. J. Dairy Sci. 2020, 103, 9384–9406. [Google Scholar] [CrossRef]
- Shen, R.L.; Thymann, T.; Østergaard, M.V.; Støy, A.C.F.; Krych, Ł.; Nielsen, D.S.; Lauridsen, C.; Hartmann, B.; Holst, J.J.; Burrin, D.G.; et al. Early Gradual Feeding with Bovine Colostrum Improves Gut Function and NEC Resistance Relative to Infant Formula in Preterm Pigs. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 309, G310–G323. [Google Scholar] [CrossRef]
- Kehoe, S.I.; Jayarao, B.M.; Heinrichs, A.J. A Survey of Bovine Colostrum Composition and Colostrum Management Practices on Pennsylvania Dairy Farms. J. Dairy Sci. 2007, 90, 4108–4116. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y.; Maubois, J.L. Growth Factors from Bovine Milk and Colostrum: Composition, Extraction and Biological Activities. Lait 2006, 86, 99–125. [Google Scholar] [CrossRef]
- De Jesus Ferrari, D.V.; Polettini, J.; de Moraes, L.L.; de Campos, L.A.; da Silva, M.G.; Saeki, E.K.; Morceli, G. Profile of Pro-Inflammatory Cytokines in Colostrum of Nursing Mothers at the Extremes of Reproductive Age. PLoS ONE 2020, 15, e0231882. [Google Scholar] [CrossRef]
- Šuligoj, T.; Vigsnæs, L.K.; Van den Abbeele, P.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2017, 69, 42–51. [Google Scholar] [CrossRef]
- Xu, W.; Mann, S.; Curone, G.; Kenéz, Á. Heat Treatment of Bovine Colostrum: Effects on Colostrum Metabolome and Serum Metabolome of Calves. Animal 2021, 15, 100180. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Ohde, D.; Wirthgen, E.; Hoeflich, A. Cytokines in Milk and the Role of TGF-Beta. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 47–56. [Google Scholar] [CrossRef]
- Dallas, D.C.; Weinborn, V.; de Moura Bell, J.M.L.N.; Wang, M.; Parker, E.A.; Guerrero, A.; Hettinga, K.A.; Lebrilla, C.B.; German, J.B.; Barile, D. Comprehensive Peptidomic and Glycomic Evaluation Reveals That Sweet Whey Permeate from Colostrum Is a Source of Milk Protein-Derived Peptides and Oligosaccharides. Food Res. Int. 2014, 63, 203–209. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J.; Ward, R.E. Bioactive Components in Milk. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 653–658. [Google Scholar] [CrossRef]
- Brescia, P.; Rescigno, M. The Gut Vascular Barrier: A New Player in the Gut–Liver–Brain Axis. Trends Mol. Med. 2021, 27, 844–855. [Google Scholar] [CrossRef]
- Trocino, A.; Menegon, F.; Zomeño, C.; Pasqualin, D.; Cunial, G.; Xiccato, G.; Pirrone, F.; Bertotto, D.; Bortoletti, M.; Dorigo, F.; et al. A Pilot Study about On-Farm Assessment of Health and Welfare in Rabbits Kept in Different Housing Systems. Front. Vet. Sci. 2022, 9, 936643. [Google Scholar] [CrossRef]
- Serra, V.; Castrica, M.; Agradi, S.; Curone, G.; Vigo, D.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; Balzaretti, C.M.; De Bellis, R.; et al. Antioxidant Activity of Different Tissues from Rabbits Fed Dietary Bovine Colostrum Supplementation. Animals 2023, 13, 850. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Martino, M.; Mattioli, S.; Marconi, O.; Sileoni, V.; Ruggeri, S.; Tei, F.; Benincasa, P. The Effect of Dietary Alfalfa and Flax Sprouts on Rabbit Meat Antioxidant Content, Lipid Oxidation and Fatty Acid Composition. Meat Sci. 2015, 106, 31–37. [Google Scholar] [CrossRef]
- Agradi, S.; Cremonesi, P.; Menchetti, L.; Balzaretti, C.; Severgnini, M.; Riva, F.; Castiglioni, B.; Draghi, S.; Di Giancamillo, A.; Castrica, M.; et al. Bovine Colostrum Supplementation Modulates the Intestinal Microbial Community in Rabbits. Animals 2023, 13, 976. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Ali, Q.; Zhu, X.; Li, D.; Ma, S.; Wang, Z.; Wang, C.; Shi, Y. Gut Microbiota Modulate Rabbit Meat Quality in Response to Dietary Fiber. Front. Nutr. 2022, 9, 849429. [Google Scholar] [CrossRef]
- Peng, X.X.; Zhao, R.L.; Song, W.; Chu, H.R.; Li, M.; Song, S.Y.; Li, G.Z.; Liang, D.C. Selection of Suitable Reference Genes for Normalization of Quantitative Real-Time PCR in Cartilage Tissue Injury and Repair in Rabbits. Int. J. Mol. Sci. 2012, 13, 14344. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Tripathi, N.; Sapra, A. Gram Staining; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Botti, G.; Lavazza, A.; Cristoni, S.; Brocchi, E.; Capucci, L. Sviluppo e Standardizzazione di Un Test Elisa per la Sierologia della Mixomatosi. Atti Giornate Coniglicoltura ASIC 2007, 139. [Google Scholar]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef]
- Cremonesi, P.; Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Riva, F.; Marongiu, M.L.; Castiglioni, B.; Barbato, O.; Munga, A.; et al. Dietary Supplementation with Goji Berries (Lycium barbarum) Modulates the Microbiota of Digestive Tract and Caecal Metabolites in Rabbits. Animals 2022, 12, 121. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside Protects Pancreatic β-Cells against ER-Stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Torchinsky, M.B.; Garaude, J.; Martin, A.P.; Blander, J.M. Innate Immune Recognition of Infected Apoptotic Cells Directs T(H)17 Cell Differentiation. Nature 2009, 458, 78–82. [Google Scholar] [CrossRef]
- Kashiwagi, I.; Hayashi, A.; Kanai, T.; Yoshimura, A. ID: 93: Smad2 and Smad3 Inversely Regulate TGF-b Autoinduction in Clostridium butyricum-Activated Dendritic Cells. Cytokine 2015, 76, 82. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.D.; Son, H.W.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate Producers, “The Sentinel of Gut”: Their Intestinal Significance with and beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg Induction by a Rationally Selected Mixture of Clostridia Strains from the Human Microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Burke, F. The Cytokine Network. Immunol. Today 1989, 10, 299–304. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An Evolving Chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent Advances in Underlying Pathologies Provide Insight into Interleukin-8 Expression-Mediated Inflammation and Angiogenesis. Int. J. Inflam. 2011, 2011, 908468. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-α Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-ΚB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 519. [Google Scholar] [CrossRef]
- Przybylska, J.; Albera, E.; Kankofer, M. Antioxidants in Bovine Colostrum. Reprod. Domest. Anim. 2007, 42, 402–409. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Wnt/Beta-Catenin Is Essential for Intestinal Homeostasis and Maintenance of Intestinal Stem Cells. Mol. Cell Biol. 2007, 27, 7551–7559. [Google Scholar] [CrossRef]
- Aidos, L.; Pallaoro, M.; Mirra, G.; Serra, V.; Castrica, M.; Agradi, S.; Curone, G.; Vigo, D.; Riva, F.; Balzaretti, C.M.; et al. Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum. Vet. Sci. 2023, 10, 657. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Rossi, R.; Martino, P.A.; Aidos, L.; Maghin, F.; Domeneghini, C.; Corino, C. Copper Sulphate Forms in Piglet Diets: Microbiota, Intestinal Morphology and Enteric Nervous System Glial Cells. Anim. Sci. J. 2018, 89, 616–624. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, J.S.; Choi, H.S.; Hong, H.P.; Jang, K.H.; Paek, J.H.; Ah Kan, S.; Ko, Y.G. Antioxidant and Anticytokine Effects of Bovine Colostrum in Intestinal Ischemia/Reperfusion Injured Rat Model. Food Sci. Biotechnol. 2010, 19, 1295–1301. [Google Scholar] [CrossRef]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal Permeability in the Pathogenesis of Liver Damage: From Non-Alcoholic Fatty Liver Disease to Liver Transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of Superoxide Dismutase Genes: Implications in Disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Elsasser, T.H.; Ma, B.; Ravel, J.; Kahl, S.; Gajer, P.; Cross, A. Short-Term Feeding of Defatted Bovine Colostrum Mitigates Inflammation in the Gut via Changes in Metabolites and Microbiota in a Chicken Animal Model. Anim. Microbiome 2023, 5, 6. [Google Scholar] [CrossRef]
- Morinaga, K.; Kusada, H.; Tamaki, H. Bile Salt Hydrolases with Extended Substrate Specificity Confer a High Level of Resistance to Bile Toxicity on Atopobiaceae Bacteria. Int. J. Mol. Sci. 2022, 23, 10980. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Terveer, E.M.; Nooij, S.; Bloem, M.N.; Vendrik, K.E.W.; Caljouw, M.A.A.; Sanders, I.M.J.G.; van Dorp, S.M.; Wong, M.C.; Zwittink, R.D.; et al. Microbiota-Associated Risk Factors for Asymptomatic Gut Colonisation with Multi-Drug-Resistant Organisms in a Dutch Nursing Home. Genome Med. 2021, 13, 54. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, S.; Yan, R.; Gong, C.; Gui, Q.; Zhang, Q.; Xiang, L.; Chen, L.; Wang, P.; Li, S.; et al. Parishin from Gastrodia Elata Ameliorates Aging Phenotype in Mice in a Gut Microbiota-Related Manner. Front. Microbiol. 2022, 13, 877099. [Google Scholar] [CrossRef]

| Gene | Protein | Sequence | Gene Bank GI Number |
|---|---|---|---|
| ACT-β | β-actin | F: ACATGGAGAAGATCTGGCAC | GI:100009272 |
| R: GCGTGTTGAACGTCTCGAAC | |||
| IL-8 | interleukin-8 | F: CGGAAGAAACCACTTCGCCT | GI:100009129 |
| R: ATCCGTGTCAGAACTGCAGC | |||
| IL-10 | interleukin-10 | F: CCTTTGGCAGGGTGAAGACT | GI:100008701 |
| R: TCATCTCCGACAAGGCTTGG | |||
| TGF-β | transforming growth factor beta-1 proprotein | F: GCAGAGTGGTTGTCCTTCGA | GI:100008645 |
| R: CCGGAACTGATCCCGTTGAT | |||
| PLVAP | plasmalemma vesicle-associated protein | F: AAGGACGCCATCATGCAGAT | GI:100343789 |
| R: TCGAGATGATGGTGGCCATG | |||
| CTNN-β1 | β-catenin | F: AGCAGGGCTTTTCTCAGTCC | GI:100125985 |
| R: ACCCTCTGAGCTCGAGTCAT | |||
| SOD1 | CuZn superoxide dismutase | F: CACTTCGAGCAGAAGGGAAC | GI:100009313 |
| R: CGTGCCTCTCTTCATCCTTC | |||
| GSR | glutathione-disulfide reductase | F: ACGTGAGTCGCCTGAATACC | GI:100337794 |
| R: GATCTGGCTCTCGTGAGGAC | |||
| CSF3R | granulocyte colony-stimulating factor 3 receptor | F: CGGCCAGTGTGTATCATGTC | GI:100342746 |
| R: GGTCCCACAGAGGCATAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, F.; Draghi, S.; Inglesi, A.; Filipe, J.; Cremonesi, P.; Lavazza, A.; Cavadini, P.; Vigo, D.; Agradi, S.; Menchetti, L.; et al. Bovine Colostrum Supplementation in Rabbit Diet Modulates Gene Expression of Cytokines, Gut–Vascular Barrier, and Red-Ox-Related Molecules in the Gut Wall. Animals 2024, 14, 800. https://doi.org/10.3390/ani14050800
Riva F, Draghi S, Inglesi A, Filipe J, Cremonesi P, Lavazza A, Cavadini P, Vigo D, Agradi S, Menchetti L, et al. Bovine Colostrum Supplementation in Rabbit Diet Modulates Gene Expression of Cytokines, Gut–Vascular Barrier, and Red-Ox-Related Molecules in the Gut Wall. Animals. 2024; 14(5):800. https://doi.org/10.3390/ani14050800
Chicago/Turabian StyleRiva, Federica, Susanna Draghi, Alessia Inglesi, Joel Filipe, Paola Cremonesi, Antonio Lavazza, Patrizia Cavadini, Daniele Vigo, Stella Agradi, Laura Menchetti, and et al. 2024. "Bovine Colostrum Supplementation in Rabbit Diet Modulates Gene Expression of Cytokines, Gut–Vascular Barrier, and Red-Ox-Related Molecules in the Gut Wall" Animals 14, no. 5: 800. https://doi.org/10.3390/ani14050800











