Assessing Ecogeographic Rules in Two Sigmodontine Rodents along an Elevational Gradient in Central Chile
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Study Area
2.2. Body and Cranial Size Measurements
2.3. Data Analysis
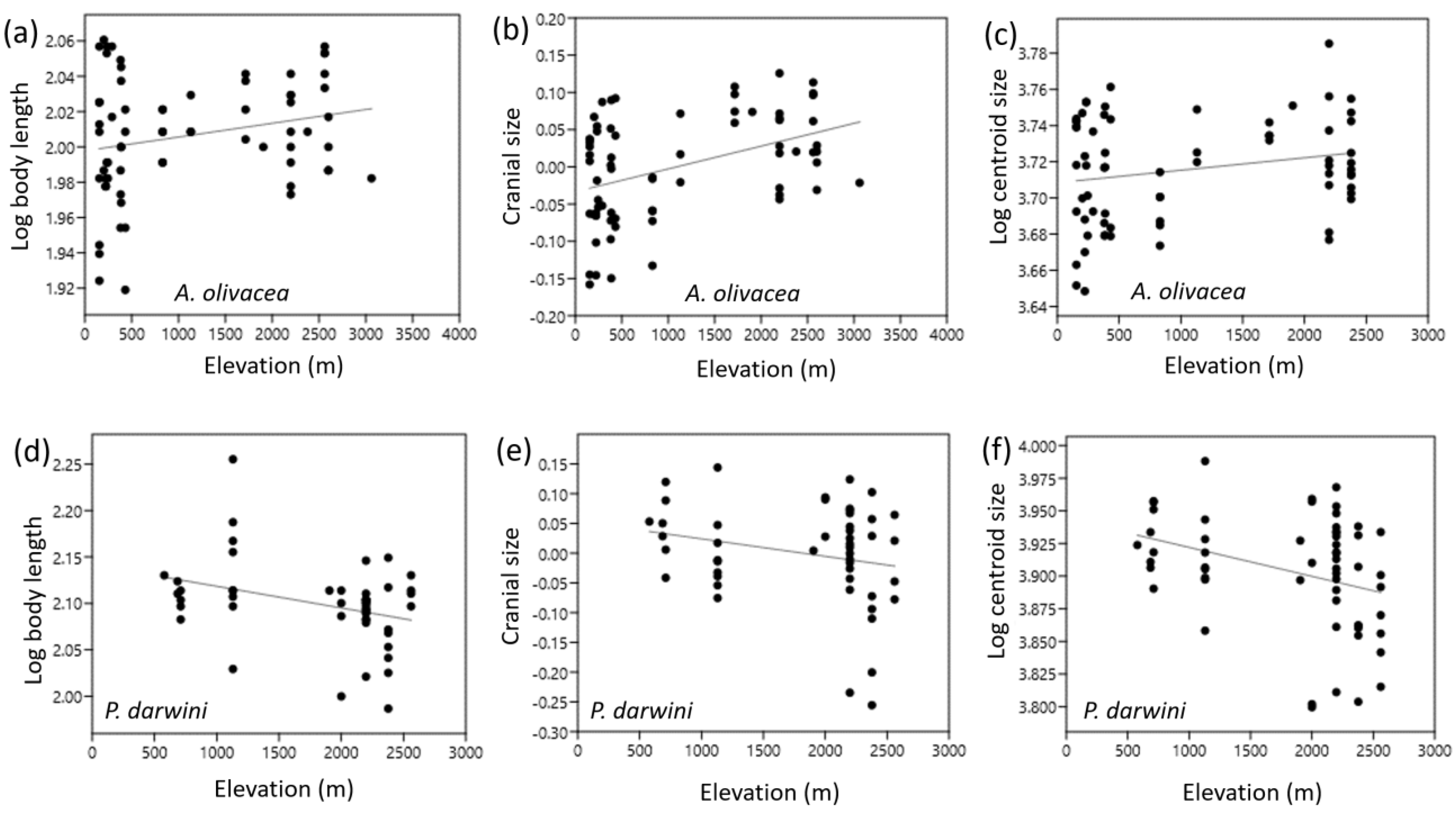
3. Results
3.1. A. olivacea
3.2. P. darwini
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Brown, J.H. Mammals on mountaintops: Nonequilibrium insular biogeography. Am. Nat. 1971, 105, 467–478. [Google Scholar] [CrossRef]
- Dickman, C.R. Body size, prey size, and community structure in insectivorous mammals. Ecology 1988, 69, 569–580. [Google Scholar] [CrossRef]
- White, C.R.; Seymour, R.S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl. Acad. Sci. USA 2003, 100, 4046–4049. [Google Scholar] [CrossRef]
- Sibly, R.M.; Brown, J.H. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl. Acad. Sci. USA 2007, 104, 17707–17712. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.A.; Lyons, S.K. How big should a mammal be? A macroecological look at mammalian body size over space and time. Phil. Trans. R. Soc. B 2011, 366, 2364–2378. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Perault, D.R. Body size variation of mammals in a fragmented, temperate rainforest. Conserv. Biol. 2007, 21, 1059–1069. [Google Scholar] [CrossRef]
- Hantak, M.M.; McLean, B.S.; Li, D.; Guralnick, R.P. Mammalian body size is determined by interactions between climate, urbanization, and ecological traits. Commun. Biol. 2021, 4, 972. [Google Scholar] [CrossRef]
- Withers, P.C.; Cooper, C.E.; Maloney, S.K.; Bozinovic, F.; Cruz-Neto, A.P. Ecological and Environmental Physiology of Mammals, 1st ed.; Oxford University Press: Oxford, UK, 2016; pp. 198–201. [Google Scholar]
- Bergmann, C. Ueber die verhältnisse der wärmeökonomie der thiere zu ihrer grösse. Göttinger Stud. 1847, 1, 595–708. [Google Scholar]
- Allen, J.A. The influence of physical conditions in the genesis of species. Radic. Rev. 1877, 1, 108–140. [Google Scholar]
- Ballinger, M.A.; Nachman, M.W. The contribution of genetic and environmental effects to Bergmann’s Rule and Allen’s Rule in house mice. Am. Nat. 2022, 199, 691–704. [Google Scholar] [CrossRef]
- Rundel, P.W. Tropical alpine climates. In Tropical Alpine Environments: Plant Form and Function; Rundel, P.W., Smith, A.P., Meinzer, F.C., Eds.; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Rezende, E.L.; Bozinovic, F.; Garland, T. Climatic adaptation and the evolution of basal and maximum rates of metabolism in rodents. Evolution 2004, 58, 1361–1374. [Google Scholar] [PubMed]
- Taylor, J.M.; Smith, S.C.; Calaby, J.H. Altitudinal distribution and body size among New Guinean Rattus (Rodentia: Muridae). J. Mammal. 1985, 66, 353–358. [Google Scholar] [CrossRef]
- Martínez, J.J.; DiCola, V. Geographic distribution and phenetic skull variation in two close species of Graomys (Rodentia, Cricetidae, Sigmodontinae). Zool. Anz. 2011, 250, 175–194. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Amshokova, A.; Balčiauskiené, L.; Benedek, A.M.; Cichocki, J.; Csanády, A.; Gil de Mendonça, P.; Nistreanu, V. Geographical clines in the size of the herb field mouse (Apodemus uralensis). Integr. Zool. 2020, 15, 55–68. [Google Scholar] [CrossRef]
- Gutiérrez-Pinto, N.; McCracken, K.G.; Alza, L.; Tubaro, P.; Kopuchian, C.; Astie, A.; Cadena, C.D. The validity of ecogeographical rules is context-dependent: Testing Bergmann’s and Allen’s rules by latitude and elevation in a widespread Andean duck. Biol. J. Linn. Soc. 2014, 111, 850–862. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Hodgson, D.J.; Tregenza, T. The evolution of body size under environmental gradients in ectotherms: Why should Bergmann’s rule apply to lizards? BMC Evol. Biol. 2008, 8, 68. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Z.; Liu, N. Altitudinal variation of skull size in Daurian pika (Ochotona daurica Pallas, 1868). Acta Zool. Acad. Sci. Hung. 2006, 52, 319–329. [Google Scholar]
- Cui, J.; Lei, B.; Newman, C.; Ji, S.; Su, H.; Buesching, C.D.; Macdonald, D.W.; Zhou, Y. Functional adaptation rather than ecogeographical rules determine body-size metrics along a thermal cline with elevation in the Chinese pygmy dormouse (Typhlomys cinereus). J. Therm. Biol. 2020, 88, 102510. [Google Scholar] [CrossRef]
- Hinckley, A.; Sanchez-Donoso, I.; Comas, M.; Camacho-Sanchez, M.; Hawkins, M.T.R.; Hasan, N.H.; Leonard, J.A. Challenging ecogeographical rules: Phenotypic variation in the Mountain Treeshrew (Tupaia montana) along tropical elevational gradients. PLoS ONE 2022, 17, e0268213. [Google Scholar] [CrossRef]
- Osgood, W.H. The Mammals of Chile; Field Museum of Natural History: Chicago, IL, USA, 1943; Volume 30, pp. 1–268. [Google Scholar]
- Mann, G. Los pequeños mamíferos de Chile (marsupiales, quirópteros, edentados y roedores). Gayana Zool. 1978, 40, 1–342. [Google Scholar]
- Iriarte, A. Guía de Los Mamíferos de Chile, 2nd ed.; Flora y Fauna Editorial: Santiago, Chile, 2021; p. 236. [Google Scholar]
- Rodríguez-Serrano, E.; Cancino, R.A.; Palma, R.E. Molecular phylogeography of Abrothrix olivaceus (Rodentia: Sigmodontinae) in Chile. J. Mammal. 2006, 87, 971–980. [Google Scholar] [CrossRef]
- Quiroga-Carmona, M.; Abud, C.; Lessa, E.P.; D’Elía, G. The mitochondrial genetic diversity of the olive field mouse Abrothrix olivacea (Cricetidae; Abrotrichini) is latitudinally structured across its geographical distribution. J. Mammal. Evol. 2022, 29, 413–430. [Google Scholar] [CrossRef]
- Smith, M.F.; Kelt, D.A.; Patton, J.L. Testing models of diversification in mice in the Abrothrix olivaceus/xanthorhinus complex in Chile and Argentina. Mol. Ecol. 2001, 10, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, P.S.; Rodríguez-Serrano, E.; Torres-Pérez, F.; Celis-Diez, J.L.; Palma, R.E. Genetic variability and structure of the Olive Field Mouse: A sigmodontine rodent in a biodiversity hotspot of southern Chile. PeerJ 2019, 7, e6955. [Google Scholar] [CrossRef]
- Meserve, P.L. Trophic relationships among small mammals in a Chilean semiarid thorn scrub community. J. Mammal. 1981, 62, 304–314. [Google Scholar] [CrossRef]
- Muñoz-Pedreros, A.; Gil, C. Orden Rodentia. In Mamíferos de Chile, 2nd ed.; Muñoz-Pedreros, A., Yáñez-Valenzuela, J., Eds.; CEA Ediciones: Valdivia, Chile, 2009; pp. 93–175. [Google Scholar]
- Murua, R.; González, L.A.; Meserve, P.L. Population ecology of Oryzomys longicaudatus philippii (Rodentia-Cricetidae) in Southern Chile. J. Anim. Ecol. 1986, 55, 281–293. [Google Scholar] [CrossRef]
- Simonetti, J.A.; Aguero, T. Assessing independance of animal movements: Spatio-temporal variability in Oryzomys longicaudatus. Mammalia 1990, 54, 316–320. [Google Scholar] [CrossRef]
- Gutiérrez-Tapia, P.; Palma, R.E. Integrating phylogeography and species distribution models: Cryptic distributional responses to past climate change in an endemic rodent from the central Chile hotspot. Divers. Distrib. 2016, 22, 638–650. [Google Scholar] [CrossRef]
- Palma, R.E.; Gutiérrez-Tapia, P.; González, J.F.; Boric-Bargetto, D.; Torres-Pérez, F. Mountaintops phylogeography: A case study using small mammals from the Andes and the coast of central Chile. PLoS ONE 2017, 12, e0186031. [Google Scholar] [CrossRef]
- Martínez, J.J.; Sandoval, L.; Carrizo, L.V. Taxonomic status of large- and middle-sized Calomys (Cricetidae: Sigmodontinae) from the southern central Andes inferred through geometric morphometrics of the skull. J. Mammal. 2016, 97, 1589–1601. [Google Scholar] [CrossRef]
- Di Castri, F.; Hajek, E.R. Bioclimatología de Chile; Vicerrectoría Académica de la Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Armesto, J.J.; Arroyo, M.T.K.; Hinojosa, L.F. The Mediterranean environment of central Chile. In The Physical Geography of South America; Veblen, T.T., Young, K.R., Orme, A.R., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 184–199. [Google Scholar]
- Schulz, J.J.; Cayuela, L.; Rey-Benayas, J.M.; Schröder, B. Factors influencing vegetation cover change in Mediterranean Central Chile (1975–2008). Appl. Veg. Sci. 2011, 14, 571–582. [Google Scholar] [CrossRef]
- Gallardo, M.H.; Palma, E. Systematics of Oryzomys longicaudatus (Rodentia: Muridae) in Chile. J. Mammal. 1990, 71, 333–342. [Google Scholar] [CrossRef]
- Teta, P.; Pardiñas, U.F.J. Variación morfológica cualitativa y cuantitativa en Abrothrix longipilis (Cricetidae, Sigmodontinae). Mastozool. Neotrop. 2014, 21, 291–309. [Google Scholar]
- Lebrun, R. MorphoDig, Version 1.6.7; Morphomuseum: Montpellier, France, 2018; Available online: http://morphomuseum.com/morphodig (accessed on 5 November 2023).
- Valladares-Gómez, A.; Huenumilla-Linares, M.; Rodríguez-Serrano, E.; Hernández, C.E.; Palma, R.E. Morphological variation in two sigmodontine rodents along the mainland and the Fuegian archipelago in Chilean southern Patagonia. Rev. Chil. Hist. Nat. 2020, 93, 6. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology, 1st ed.; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologist: A Primer, 1st ed.; Elsevier: New York, NY, USA, 2012. [Google Scholar]
- Rowe-Rowe, D.T.; Meester, J. Altitudinal variation in external measurements of two small-mammal species in the Natal Drakensberg. Ann. Transvaal. Mus. 1985, 34, 49–53. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Lima, M.; Bozinovic, F.; Jaksic, F.M. Body mass dynamics and growth patterns of leaf-eared mice Phyllotis darwini in a semi-arid region of the Neotropics. Acta Theriol. 1997, 42, 15–24. [Google Scholar] [CrossRef]
- Breno, M.; Leirs, H.; Van Dongen, S. Traditional and geometric morphometrics for studying skull morphology during growth in Mastomys natalensis (Rodentia: Muridae). J. Mammal. 2011, 92, 1395–1406. [Google Scholar] [CrossRef]
- Alhajeri, B.H. A phylogenetic test of the relationship between saltation and habitat openness in gerbils (Gerbillinae, Rodentia). Mamm. Res. 2016, 61, 231–241. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Z.; Liu, N. Effects of altitudinal change on the auditory bulla in Ochotona daurica (Mammalia, Lagomorpha). J. Zoolog. Syst. Evol. Res. 2007, 45, 151–154. [Google Scholar] [CrossRef]
- Quiroga-Carmona, M.; Teta, P.; D’Elía, G. The skull variation of the olive field mouse Abrothrix olivacea (Cricetidae: Abrotrichini) is localized and correlated to the ecogeographic features of its geographic distribution. PeerJ 2023, 11, e15200. [Google Scholar] [CrossRef]
- Bozinovic, F.; Rojas, J.M.; Gallardo, P.A.; Palma, R.E.; Gianoli, E. Body mass and water economy in the South American olivaceous field mouse along a latitudinal gradient: Implications for climate change. J. Arid Environ. 2011, 75, 411–415. [Google Scholar] [CrossRef]
- Jiménez, J.E.; Yáñez, J.L.; Tabilo, E.L.; Jaksic, F.M. Body size of Chilean foxes: A new pattern in light of new data. Acta Theriol. 1995, 40, 321–326. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Liu, N.; Liao, J. Effects of change in altitude on the auditory bulla of Midday Gerbil, Meriones meridianus. Pak. J. Zool. 2013, 45, 581–588. [Google Scholar]
- Wasserman, D.; Nash, D.J. Variation in body size, hair length, and hair density in the Deer Mouse Peromyscus maniculatus along an altitudinal gradient. Holartic Ecol. 1979, 2, 115–118. [Google Scholar] [CrossRef]
- Bozinovic, F.; Rosenmann, M.; Veloso, C. Termorregulación conductual en Phyllotis darwini (Rodentia: Cricetidae): Efecto de la temperatura ambiente, uso de nidos y agrupamiento social sobre el gasto de energía. Rev. Chil. Hist. Nat. 1988, 61, 81–86. [Google Scholar]
- Bozinovic, F.; Rosenmann, M. Maximum metabolic rate of rodents: Physiological and ecological consequences on distributional limits. Funct. Ecol. 1989, 3, 173–181. [Google Scholar] [CrossRef]
- Santibañez-Quezada, F. Atlas Agroclimático de Chile. Estado Actual y Tendencias del Clima. Tomo III: Regiones de Valparaíso, Metropolitana, O’Higgins y Maule; Universidad de Chile, Facultad de Ciencias Agronómicas: Santiago, Chile; FIA: La Reina, Chile, 2017. [Google Scholar]
- Cavieres, L.A.; Peñaloza, A.; Arroyo, M.K. Altitudinal vegetation belts in the high-Andes of central Chile (33° S). Rev. Chil. Hist. Nat. 2000, 73, 331–344. [Google Scholar] [CrossRef]
- Becerra, P.I. Relationship between climate and geographical variation of local woody species richness within the Mediterranean type region of Chile. Rev. Chil. Hist. Nat. 2016, 89, 12. [Google Scholar] [CrossRef]
- Fuentes, E.R.; Jaksic, F.M. Lizards and rodents: An explanation for their relative species diversity in Chile. Arch. Biol. Med. Exper. 1979, 12, 179–190. [Google Scholar]


| Locality | Latitude S | Longitude W | Elevation m | n (Ao) | n (Pd) |
|---|---|---|---|---|---|
| Campos Ahumada | 32°40′27.11″ | 70°31′58.07″ | 1715 | 4 | |
| Cerro El Roble | 32°58′34″ | 71°00′50″ | 2198 | 9 | 18 |
| Villa Alemana | 33°04′13.5″ | 71°21′24.4″ | 233 | 5 | |
| Tiltil | 33°05′ | 70°55′ | 578 | 1 | |
| Altos de Chicauma | 33°16′59″ | 70°58′14″ | 1905 | 1 | 2 |
| Farellones | 33°21′36″ | 70°17′28″ | 2377 | 12 | 8 |
| Valle Nevado | 33°21′43.4″ | 70°15′30.5″ | 2560 | 7 | |
| SC Apoquindo | 33°24′13″ | 70°29′01″ | 1130 | 3 | 9 |
| Los Dominicos | 33°24′ | 70°32′ | 710 | 5 | |
| Quebrada de La Plata | 33°29′ | 70°54′ | 685 | 3 | |
| La Florida Alto | 33°33′48.96″ | 70°31′54.48″ | 830 | 6 | |
| Melipilla (Pomaire) | 33°38′59.7″ | 71°10′45.2″ | 202 | 2 | |
| Talagante (Chiñihue) | 33°39.767′ | 71°06.141′ | 222 | 4 | |
| Talagante (El Oliveto) | 33°40′38.6″ | 70°52′33.8″ | 386 | 6 | |
| Talagante (Lonquén) | 33°40.612′ | 70°50.702′ | 431 | 4 | |
| Talagante (Isla de Maipo) | 33°43.628′ | 71°02.100′ | 289 | 2 | |
| Melipilla (Chocalán) | 33°44′5.5″ | 71°13′2.4″ | 154 | 9 | |
| Paine | 33°52′5.3″ | 70°50′12.1″ | 380 | 3 | |
| Altos de Cantillana | 33°55′40.95″ | 70°57′49.9″ | 2000 | 5 |
| Landmark | Anatomical Description |
|---|---|
| 1 | Rostralmost point of the nasal bones |
| 2 | Intersection of the nasofrontal suture in the midline |
| 3 | Intersection of the coronal and sagittal sutures |
| 4 | Intersection of the sagittal and parietal-interparietal sutures |
| 5 | Caudal end of the curvature of the occipital bone |
| 6 and 7 | Rostralmost point of the rostral palatine fissure, left and right |
| 8 and 9 | Caudalmost point of the palatine fissure, left and right |
| 10 | Caudalmost point of the suture between palatine bones and the rostral border of the mesopterygoid fossa |
| 11 | Rostralmost point of the foramen magnum |
| 12 and 13 | Rostralmost point of the molar row, left and right |
| 14 and 15 | Caudalmost point of the molar row, left and right |
| 16 and 17 | Rostralmost point of auditory bullae, left and right |
| 18 and 19 | Caudalmost point of auditory bullae, left and right |
| 20 and 21 | Lateralmost point of the foramen magnum, left and right |
| 22 and 23 | Rostralmost point of the zygomatic arch, left and right |
| 24 and 25 | Caudalmost point of the zygomatic arch, left and right |
| Effect | SS | MS | df | F | p |
|---|---|---|---|---|---|
| Individual | 0.93738487 | 0.0001499096 | 6253 | 5.99 | <0.0001 |
| Side | 0.02130345 | 0.0006872080 | 31 | 27.45 | <0.0001 |
| Ind*Side | 0.13117657 | 0.0000250385 | 5239 | 2.91 | <0.0001 |
| Error | 0.09944768 | 0.0000086027 | 11560 |
| Species/Measurement | r | p | n |
|---|---|---|---|
| Ao | |||
| Body length | 0.23 | 0.0598 | 69 |
| Tail length | −0.11 | 0.3701 | 70 |
| Hindfoot length | 0.07 | 0.5885 | 69 |
| Ear Length | −0.59 | 0.0001 | 70 |
| Cranial size | 0.41 | 0.0004 | 69 |
| Centroid size | 0.21 | 0.08 | 69 |
| Pd | |||
| Body length | −0.34 | 0.0175 | 49 |
| Tail length | 0.28 | 0.0532 | 50 |
| Hindfoot length | −0.08 | 0.6055 | 49 |
| Ear Length | −0.3 | 0.0371 | 50 |
| Cranial size | −0.23 | 0.1192 | 48 |
| Centroid size | −0.33 | 0.0104 | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valladares-Gómez, A.; Torres-Pérez, F.; Palma, R.E. Assessing Ecogeographic Rules in Two Sigmodontine Rodents along an Elevational Gradient in Central Chile. Animals 2024, 14, 830. https://doi.org/10.3390/ani14060830
Valladares-Gómez A, Torres-Pérez F, Palma RE. Assessing Ecogeographic Rules in Two Sigmodontine Rodents along an Elevational Gradient in Central Chile. Animals. 2024; 14(6):830. https://doi.org/10.3390/ani14060830
Chicago/Turabian StyleValladares-Gómez, Alejandro, Fernando Torres-Pérez, and R. Eduardo Palma. 2024. "Assessing Ecogeographic Rules in Two Sigmodontine Rodents along an Elevational Gradient in Central Chile" Animals 14, no. 6: 830. https://doi.org/10.3390/ani14060830





