The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows
Simple Summary
Abstract
1. Introduction
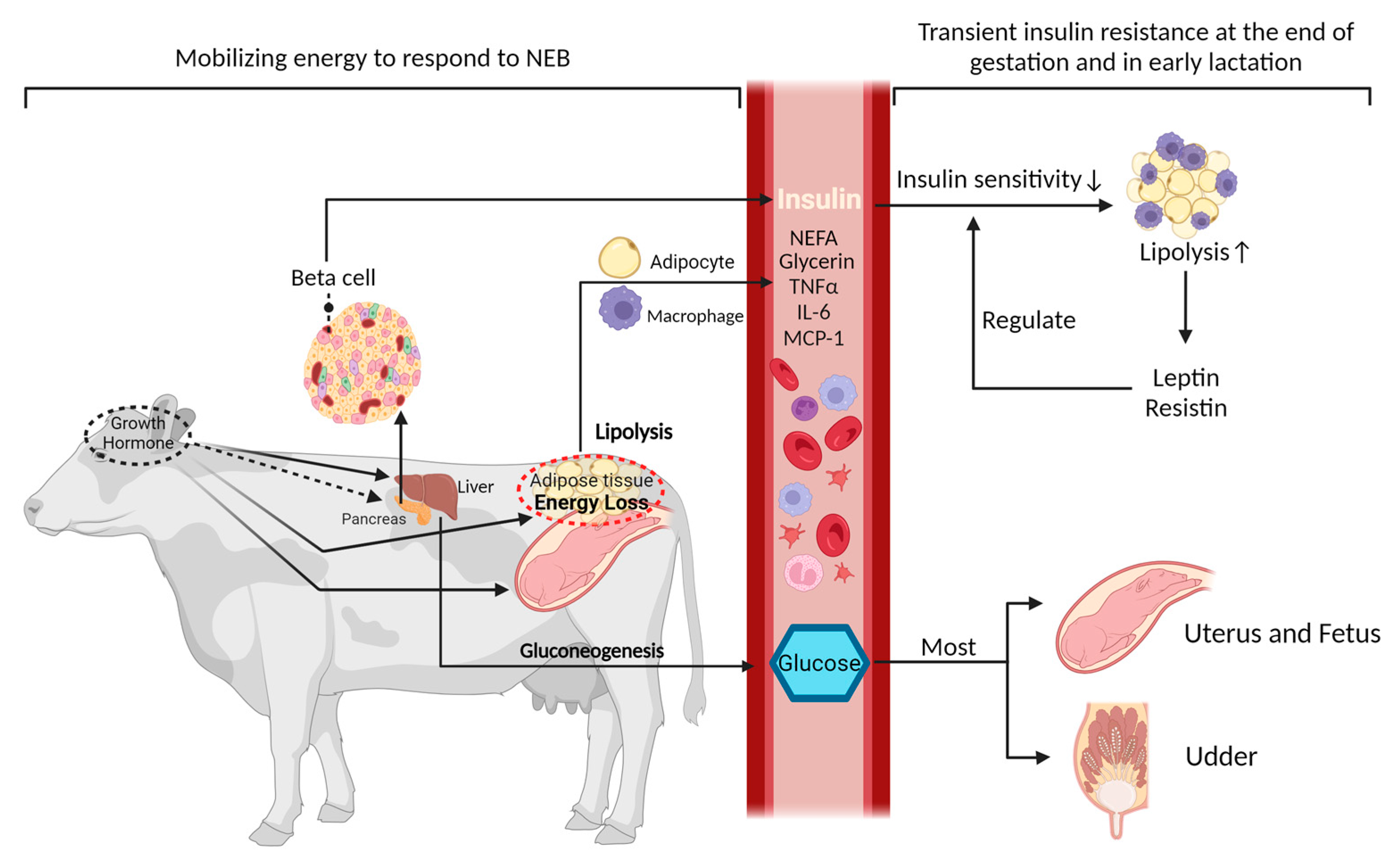
2. Regulation of Metabolic Function during the Peripartum Period
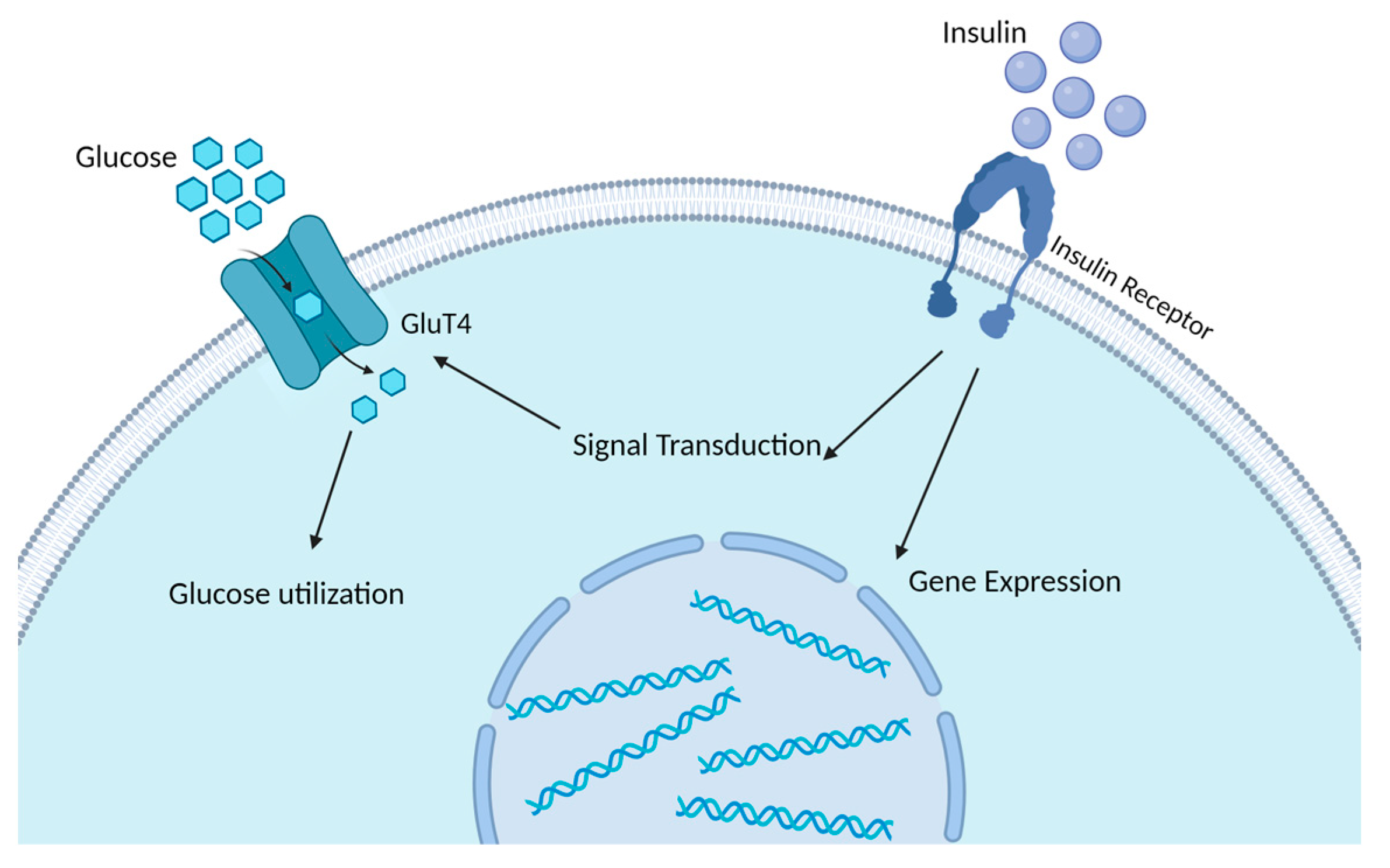
2.1. The Regulatory Role of Insulin in Glucose and Lipid Metabolism and the Development of Insulin Resistance
2.2. Metabolic Inflammation in Transition Dairy Cows
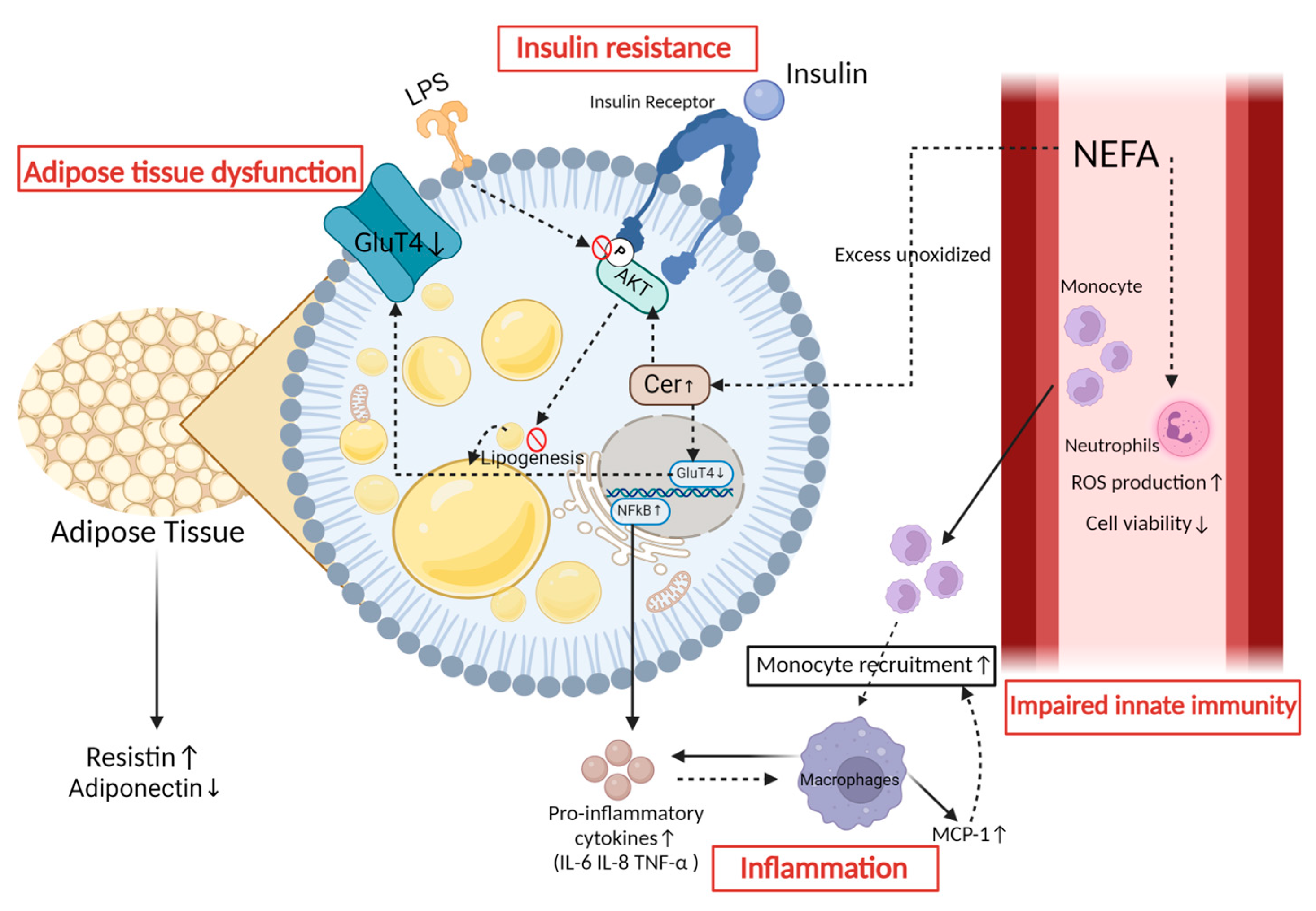
3. The Mechanism of Insulin Resistance in Peripartum Dairy Cows
4. The Impact of Metabolic Inflammation on Dairy Cows’ Health during the Periparturient Period
5. The Relationship between Insulin Resistance and Metabolic Inflammation
5.1. Insulin Resistance Elicits an Inflammatory Response
5.2. Inflammatory Mediators Contribute to the Development of Insulin Resistance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, J.E.; Bisinotto, R.S.; Ribeiro, E.S.; Lima, F.S.; Greco, L.F.; Staples, C.R.; Thatcher, W.W. Applying nutrition and physiology to improve reproduction in dairy cattle. Soc. Reprod. Fertil. Suppl. 2010, 67, 387–403. [Google Scholar] [CrossRef]
- Sinclair, K. Declining fertility, insulin resistance and fatty acid metabolism in dairy cows: Developmental consequences for the oocyte and pre-implantation embryo. Acta Sci. Vet. 2010, 38, s545–s557. [Google Scholar]
- Leiva, T.; Cooke, R.F.; Aboin, A.C.; Drago, F.L.; Gennari, R.; Vasconcelos, J.L. Effects of excessive energy intake and supplementation with chromium propionate on insulin resistance parameters in nonlactating dairy cows. J. Anim. Sci. 2014, 92, 775–782. [Google Scholar] [CrossRef]
- Youssef, M.; El-Ashker, M. Significance of insulin resistance and oxidative stress in dairy cattle with subclinical ketosis during the transition period. Trop. Anim. Health Prod. 2017, 49, 239–244. [Google Scholar] [CrossRef]
- Bradford, B.J.; Swartz, T.H. Review: Following the smoke signals: Inflammatory signaling in metabolic homeostasis and homeorhesis in dairy cattle. Animal 2020, 14, s144–s154. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Matthew, L. Mechanisms linking the somatotropic axis with insulin: Lessons from the postpartum dairy cow. N. Z. Soc. Anim. Prod. 2004, 64, 19–23. [Google Scholar]
- Ingvartsen, K.L.; Boisclair, Y.R. Leptin and the regulation of food intake, energy homeostasis and immunity with special focus on periparturient ruminants. Domest. Anim. Endocrinol. 2001, 21, 215–250. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.W. Review: Lipid biology in the periparturient dairy cow: Contemporary perspectives. Animal 2020, 14, s165–s175. [Google Scholar] [CrossRef]
- Zachut, M.; Honig, H.; Striem, S.; Zick, Y.; Boura-Halfon, S.; Moallem, U. Periparturient dairy cows do not exhibit hepatic insulin resistance, yet adipose-specific insulin resistance occurs in cows prone to high weight loss. J. Dairy Sci. 2013, 96, 5656–5669. [Google Scholar] [CrossRef]
- Reverchon, M.; Ramé, C.; Cognié, J.; Briant, E.; Elis, S.; Guillaume, D.; Dupont, J. Resistin in dairy cows: Plasma concentrations during early lactation, expression and potential role in adipose tissue. PLoS ONE 2014, 9, e93198. [Google Scholar] [CrossRef] [PubMed]
- Salin, S.; Vanhatalo, A.; Elo, K.; Taponen, J.; Boston, R.C.; Kokkonen, T. Effects of dietary energy allowance and decline in dry matter intake during the dry period on responses to glucose and insulin in transition dairy cows. J. Dairy Sci. 2017, 100, 5266–5280. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.; Nelli, R.K.; Strieder-Barboza, C.; de Souza, J.; Lock, A.L.; Contreras, G.A. The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows. Sci. Rep. 2018, 8, 13378. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflug. Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Kovács, K.; Kulcsár, M.; Gáspárdy, A.; Fébel, H.; Zsolnai, A.; Fésüs, L.; Delavaud, C.; Chilliard, Y.; Gilbert, R.O.; et al. Interrelationship of growth hormone AluI polymorphism and hyperketonemia with plasma hormones and metabolites in the beginning of lactation in dairy cows. Livest. Sci. 2009, 123, 180–186. [Google Scholar] [CrossRef]
- Herbein, J.H.; Aiello, R.J.; Eckler, L.I.; Pearson, R.E.; Akers, R.M. Glucagon, insulin, growth hormone, and glucose concentrations in blood plasma of lactating dairy cows. J. Dairy Sci. 1985, 68, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhu, Y.; Peng, Z.; Cui, Y.; Zhang, Q.; Shi, Z.; Guan, Y.; Sha, X.; Shen, T.; Yang, Y.; et al. High concentrations of fatty acids and β-hydroxybutyrate impair the growth hormone-mediated hepatic JAK2-STAT5 pathway in clinically ketotic cows. J. Dairy Sci. 2018, 101, 3476–3487. [Google Scholar] [CrossRef]
- Kim, J.W. Modulation of the somatotropic axis in periparturient dairy cows. Asian-Australas J. Anim. Sci. 2014, 27, 147–154. [Google Scholar] [CrossRef]
- Martens, H. The lipidosis of the liver of dairy cows: Part 1—Role of insulin and the Growth Hormone-IGF-1 axis. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2023, 51, 97–108. [Google Scholar] [CrossRef]
- Wathes, D.C.; Becker, F.; Buggiotti, L.; Crowe, M.A.; Ferris, C.; Foldager, L.; Grelet, C.; Hostens, M.; Ingvartsen, K.L.; Marchitelli, C.; et al. Associations between Circulating IGF-1 Concentrations, Disease Status and the Leukocyte Transcriptome in Early Lactation Dairy Cows. Ruminants 2021, 1, 147–177. [Google Scholar] [CrossRef]
- Witters, L.A.; Watts, T.D.; Daniels, D.L.; Evans, J.L. Insulin stimulates the dephosphorylation and activation of acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 1988, 85, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.B.; Cox, A.R.; Hartig, S.M. Acetyl-CoA and Metabolite Fluxes Regulate White Adipose Tissue Expansion. Trends Endocrinol. Metab. 2021, 32, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Elkins, D.A.; Spurlock, D.M. Phosphorylation of perilipin is associated with indicators of lipolysis in Holstein cows. Horm. Metab. Res. 2009, 41, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, C.L. Maternal and fetal influences on uterine and conceptus development in the cow: I. Growth of tissues of the gravid uterus. J. Anim. Sci. 1991, 69, 1945–1953. [Google Scholar] [CrossRef]
- Balogh, O.; Szepes, O.; Kovacs, K.; Kulcsár-Huszenicza, M.; Reiczigel, J.; Alcazar, J.; Keresztes, M.; Fébel, H.; Bartyik, J.; Gy, S.; et al. Interrelationships of growth hormone AluI polymorphism, insulinresistance, milk production and reproductive performance in Holstein-Friesian cos. Vet. Med. 2008, 53, 604–616. [Google Scholar] [CrossRef]
- Gross, J.J.; Wellnitz, O.; Bruckmaier, R.M. Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows. J. Anim. Sci. 2015, 93, 3395–3401. [Google Scholar] [CrossRef] [PubMed]
- Buttchereit, N.; Stamer, E.; Junge, W.; Thaller, G. Short communication: Genetic relationships among daily energy balance, feed intake, body condition score, and fat to protein ratio of milk in dairy cows. J. Dairy Sci. 2011, 94, 1586–1591. [Google Scholar] [CrossRef]
- Martens, H. Invited Review: Increasing Milk Yield and Negative Energy Balance: A Gordian Knot for Dairy Cows? Animals 2023, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Sundrum, A. Metabolic Disorders in the Transition Period Indicate that the Dairy Cows’ Ability to Adapt is Overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Horst, E.A.; Kvidera, S.K.; Baumgard, L.H. Invited review: The influence of immune activation on transition cow health and performance—A critical evaluation of traditional dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef]
- Arshad, U.; Santos, J.E.P. Hepatic triacylglycerol associations with production and health in dairy cows. J. Dairy Sci. 2022, 105, 5393–5409. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.T.; Obara, Y.; Itoh, F.; Hashimoto, H.; Takahashi, Y. Non-insulin- and insulin-mediated glucose uptake in dairy cows. J. Dairy Res. 1997, 64, 341–353. [Google Scholar] [CrossRef]
- Kronfeld, D.S. Major metabolic determinants of milk volume, mammary efficiency, and spontaneous ketosis in dairy cows. J. Dairy Sci. 1982, 65, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Strieder-Barboza, C.; De Koster, J. Symposium review: Modulating adipose tissue lipolysis and remodeling to improve immune function during the transition period and early lactation of dairy cows. J. Dairy Sci. 2018, 101, 2737–2752. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Wang, D. Effects of body condition on the insulin resistance, lipid metabolism and oxidative stress of lactating dairy cows. Lipids Health Dis. 2020, 19, 56. [Google Scholar] [CrossRef]
- Sadri, H.; Ghaffari, M.H.; Trakooljul, N.; Ceciliani, F.; Sauerwein, H. MicroRNA profiling of subcutaneous adipose tissue in periparturient dairy cows at high or moderate body condition. Sci. Rep. 2022, 12, 14748. [Google Scholar] [CrossRef]
- French, P.D. Dry Matter Intake and Blood Parameters of Nonlactating Holstein and Jersey Cows in Late Gestation. J. Dairy Sci. 2006, 89, 1057–1061. [Google Scholar] [CrossRef]
- Hayirli, A. The role of exogenous insulin in the complex of hepatic lipidosis and ketosis associated with insulin resistance phenomenon in postpartum dairy cattle. Vet. Res. Commun. 2006, 30, 749–774. [Google Scholar] [CrossRef]
- Cuiyu, Z.; Chang, Z.; Jiang, Z.; Cheng, X.; Hong You, Z. The Relationship between Insulin Resistance and Type II Ketosis in Dairy Cows. Acta Sci. Vet. 2019, 47. [Google Scholar] [CrossRef]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Daradics, Z.; Crecan, C.M.; Rus, M.A.; Morar, I.A.; Mircean, M.V.; Cătoi, A.F.; Cecan, A.D.; Cătoi, C. Obesity-Related Metabolic Dysfunction in Dairy Cows and Horses: Comparison to Human Metabolic Syndrome. Life 2021, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Contreras, G.A. Symposium review: Mechanistic insights into adipose tissue inflammation and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2022, 105, 3670–3686. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Leroy, J.; Opsomer, G. Metabolic Stress in the Transition Period of Dairy Cows: Focusing on the Prepartum Period. Animals 2020, 10, 1419. [Google Scholar] [CrossRef]
- Kerwin, A.L.; Burhans, W.S.; Mann, S.; Nydam, D.V.; Wall, S.K.; Schoenberg, K.M.; Perfield, K.L.; Overton, T.R. Transition cow nutrition and management strategies of dairy herds in the northeastern United States: Part II-Associations of metabolic- and inflammation-related analytes with health, milk yield, and reproduction. J. Dairy Sci. 2022, 105, 5349–5369. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Contreras, G.A.; Aitken, S.L. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev. 2009, 10, 53–63. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zhang, H.; Zhao, Z.; Peng, Z.; Wang, Z.; Liu, G.; Li, X. Non-Esterified Fatty Acids Over-Activate the TLR2/4-NF-Κb Signaling Pathway to Increase Inflammatory Cytokine Synthesis in Neutrophils from Ketotic Cows. Cell. Physiol. Biochem. 2018, 48, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, H.; Zhou, X.; Lian, S.; Wang, J.; Wu, R. Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows. Animals 2022, 12, 1190. [Google Scholar] [CrossRef]
- Scalia, D.; Lacetera, N.; Bernabucci, U.; Demeyere, K.; Duchateau, L.; Burvenich, C. In vitro effects of nonesterified fatty acids on bovine neutrophils oxidative burst and viability. J. Dairy Sci. 2006, 89, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, N.; Hooper, H.B.; Blouin, R.; Lacasse, P. Effect of intravenous lipid infusion on biomarkers of insulin resistance and immune functions of dry and nonpregnant dairy cows. J. Dairy Sci. 2023, 106, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Raphael, W.; Mattmiller, S.A.; Gandy, J.; Sordillo, L.M. Nonesterified fatty acids modify inflammatory response and eicosanoid biosynthesis in bovine endothelial cells. J. Dairy Sci. 2012, 95, 5011–5023. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, L.; Zhang, C.; Cheng, X.; Zhang, Y.; Guo, Y.; Long, M.; Yang, S.; He, J. Palmitic Acid and β-Hydroxybutyrate Induce Inflammatory Responses in Bovine Endometrial Cells by Activating Oxidative Stress-Mediated NF-κB Signaling. Molecules 2019, 24, 2421. [Google Scholar] [CrossRef]
- Mukesh, M.; Bionaz, M.; Graugnard, D.E.; Drackley, J.K.; Loor, J.J. Adipose tissue depots of Holstein cows are immune responsive: Inflammatory gene expression in vitro. Domest. Anim. Endocrinol. 2010, 38, 168–178. [Google Scholar] [CrossRef]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef]
- Chalmeh, A.; Pourjafar, M.; Badiei, K.; Mirzaei, A.; Jalali, M.; Mazrouei Sebdani, M. Effects of dietary antioxidants on glucose and insulin responses to glucose tolerance test in transition dairy cows. Domest. Anim. Endocrinol. 2021, 75, 106602. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Association of oxidative status and insulin sensitivity in periparturient dairy cattle: An observational study. J. Anim. Physiol. Anim. Nutr. 2016, 100, 279–286. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Liu, L.; Song, Y.; Du, X.; Feng, S.; Wang, X.; Li, X.; Wang, Z.; Li, X.; et al. Non-esterified Fatty Acid Induce Dairy Cow Hepatocytes Apoptosis via the Mitochondria-Mediated ROS-JNK/ERK Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 245. [Google Scholar] [CrossRef]
- Chang, R.; Jia, H.; Dong, Z.; Xu, Q.; Liu, L.; Majigsuren, Z.; Batbaatar, T.; Xu, C.; Yang, Q.; Sun, X. Free Fatty Acids Induce Apoptosis of Mammary Epithelial Cells of Ketotic Dairy Cows via the Mito-ROS/NLRP3 Signaling Pathway. J. Agric. Food Chem. 2023, 71, 12645–12656. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Y.; Jiang, C.; Luo, S.; Jia, H.; Xu, Q.; Zhao, C.; Liang, Y.; Cao, Z.; Shao, G.; et al. Oxidative stress, NF-κB signaling, NLRP3 inflammasome, and caspase apoptotic pathways are activated in mammary gland of ketotic Holstein cows. J. Dairy Sci. 2021, 104, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Karacaören, B.; Jaffrézic, F.; Kadarmideen, H.N. Genetic Parameters for Functional Traits in Dairy Cattle from Daily Random Regression Models. J. Dairy Sci. 2006, 89, 791–798. [Google Scholar] [CrossRef]
- Krattenmacher, N.; Thaller, G.; Tetens, J. Analysis of the genetic architecture of energy balance and its major determinants dry matter intake and energy-corrected milk yield in primiparous Holstein cows. J. Dairy Sci. 2019, 102, 3241–3253. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, C.; Basang, W.; Zhu, Y.; Wang, X.; Li, C.; Chen, L.; Zhou, X. Mechanisms by which mastitis affects reproduction in dairy cow: A review. Reprod. Domest. Anim. 2021, 56, 1165–1175. [Google Scholar] [CrossRef]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Chirivi, M.; Rendon, C.J.; Myers, M.N.; Prom, C.M.; Roy, S.; Sen, A.; Lock, A.L.; Contreras, G.A. Lipopolysaccharide induces lipolysis and insulin resistance in adipose tissue from dairy cows. J. Dairy Sci. 2022, 105, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, W.; Zushin, P.H.; Liu, B.; Jedrychowski, M.P.; Mina, A.I.; Deng, Z.; Cabarkapa, D.; Hall, J.A.; Palmer, C.J.; et al. Phosphorylation of Beta-3 adrenergic receptor at serine 247 by ERK MAP kinase drives lipolysis in obese adipocytes. Mol. Metab. 2018, 12, 25–38. [Google Scholar] [CrossRef]
- Casteilla, L.; Muzzin, P.; Revelli, J.P.; Ricquier, D.; Giacobino, J.P. Expression of beta 1- and beta 3-adrenergic-receptor messages and adenylate cyclase beta-adrenergic response in bovine perirenal adipose tissue during its transformation from brown into white fat. Biochem. J. 1994, 297 Pt 1, 93–97. [Google Scholar] [CrossRef]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef]
- Horst, E.A.; van den Brink, L.M.; Mayorga, E.J.; Al-Qaisi, M.; Rodriguez-Jimenez, S.; Goetz, B.M.; Abeyta, M.A.; Kvidera, S.K.; Caixeta, L.S.; Rhoads, R.P.; et al. Evaluating acute inflammation’s effects on hepatic triglyceride content in experimentally induced hyperlipidemic dairy cows in late lactation. J. Dairy Sci. 2020, 103, 9620–9633. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Ridgeway, S.D.; Kim, J.A. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Rico, J.E.; Myers, W.A.; Laub, D.J.; Davis, A.N.; Zeng, Q.; McFadden, J.W. Hot topic: Ceramide inhibits insulin sensitivity in primary bovine adipocytes. J. Dairy Sci. 2018, 101, 3428–3432. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Becattini, B.; Molinaro, A.; Henricsson, M.; Borén, J.; Solinas, G. Adipocyte PI3K Links Adipostasis with Basal Insulin Secretion through an Adipoincretin Effect. bioRxiv 2024, 2023.04.24.538076. [Google Scholar] [CrossRef]
- Mann, S.; Nydam, D.V.; Abuelo, A.; Leal Yepes, F.A.; Overton, T.R.; Wakshlag, J.J. Insulin signaling, inflammation, and lipolysis in subcutaneous adipose tissue of transition dairy cows either overfed energy during the prepartum period or fed a controlled-energy diet. J. Dairy Sci. 2016, 99, 6737–6752. [Google Scholar] [CrossRef] [PubMed]
- Angeli, E.; Barcarolo, D.; Durante, L.; Santiago, G.; Matiller, V.; Rey, F.; Ortega, H.H.; Hein, G.J. Effect of precalving body condition score on insulin signaling and hepatic inflammatory state in grazing dairy cattle. Domest. Anim. Endocrinol. 2021, 76, 106621. [Google Scholar] [CrossRef]
- Bossaert, P.; Leroy, J.L.; De Vliegher, S.; Opsomer, G. Interrelations between glucose-induced insulin response, metabolic indicators, and time of first ovulation in high-yielding dairy cows. J. Dairy Sci. 2008, 91, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.A.; Souza, A.H.; Grummer, R.R. Induction of hyperlipidemia by intravenous infusion of tallow emulsion causes insulin resistance in Holstein cows. J. Dairy Sci. 2007, 90, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Gao, W.; Jiang, Q.; Loor, J.J.; Zhao, C.; Du, X.; Zhang, M.; Song, Y.; Wang, Z.; Liu, G.; et al. Targeting IRE1α and PERK in the endoplasmic reticulum stress pathway attenuates fatty acid-induced insulin resistance in bovine hepatocytes. J. Dairy Sci. 2022, 105, 6895–6908. [Google Scholar] [CrossRef]
- Deng, Q.; Du, L.; Zhang, Y.; Liu, G. NEFAs Influence the Inflammatory and Insulin Signaling Pathways Through TLR4 in Primary Calf Hepatocytes in vitro. Front. Vet. Sci. 2021, 8, 755505. [Google Scholar] [CrossRef]
- Libby, P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef]
- Rico, J.E.; Zang, Y.; Haughey, N.J.; Rius, A.G.; McFadden, J.W. Short communication: Circulating fatty acylcarnitines are elevated in overweight periparturient dairy cows in association with sphingolipid biomarkers of insulin resistance. J. Dairy Sci. 2018, 101, 812–819. [Google Scholar] [CrossRef]
- Rico, J.E.; Saed Samii, S.; Mathews, A.T.; Lovett, J.; Haughey, N.J.; McFadden, J.W. Temporal changes in sphingolipids and systemic insulin sensitivity during the transition from gestation to lactation. PLoS ONE 2017, 12, e0176787. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F., 3rd; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef]
- Timmons, B.C.; Fairhurst, A.M.; Mahendroo, M.S. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J. Immunol. 2009, 182, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Chawla, A. Leukocyte set points in metabolic disease. F1000 Biol. Rep. 2012, 4, 13. [Google Scholar] [CrossRef]
- Kushibiki, S.; Hodate, K.; Shingu, H.; Obara, Y.; Touno, E.; Shinoda, M.; Yokomizo, Y. Metabolic and lactational responses during recombinant bovine tumor necrosis factor-alpha treatment in lactating cows. J. Dairy Sci. 2003, 86, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Mamedova, L.K.; Minton, J.E.; Drouillard, J.S.; Johnson, B.J. Daily injection of tumor necrosis factor-{alpha} increases hepatic triglycerides and alters transcript abundance of metabolic genes in lactating dairy cattle. J. Nutr. 2009, 139, 1451–1456. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Koiwa, M.; Hatsugaya, A.; Kudo, K.; Hoshi, F.; Itoh, N.; Yokota, H.; Okada, H.; Kawamura, S. Relationship between serum TNF activity and insulin resistance in dairy cows affected with naturally occurring fatty liver. J. Vet. Med. Sci. 2001, 63, 1021–1025. [Google Scholar] [CrossRef]
- Feinstein, R.; Kanety, H.; Papa, M.Z.; Lunenfeld, B.; Karasik, A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 1993, 268, 26055–26058. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.; Zhou, Z.; Lopreiato, V.; Trevisi, E.; Loor, J.J. Body condition score prior to parturition is associated with plasma and adipose tissue biomarkers of lipid metabolism and inflammation in Holstein cows. J. Anim. Sci. Biotechnol. 2018, 9, 12. [Google Scholar] [CrossRef]
- Dirandeh, E.; Ghorbanalinia, M.; Rezaei-Roodbari, A.; Colazo, M.G. Relationship between body condition score loss and mRNA of genes related to fatty acid metabolism and the endocannabinoid system in adipose tissue of periparturient cows. Animal 2020, 14, 1724–1732. [Google Scholar] [CrossRef]
- Grant, R.W.; Stephens, J.M. Fat in flames: Influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E205–E213. [Google Scholar] [CrossRef]
- Martel, C.A.; Mamedova, L.K.; Minton, J.E.; Jones, M.L.; Carroll, J.A.; Bradford, B.J. Continuous low-dose infusion of tumor necrosis factor alpha in adipose tissue elevates adipose tissue interleukin 10 abundance and fails to alter metabolism in lactating dairy cows. J. Dairy Sci. 2014, 97, 4897–4906. [Google Scholar] [CrossRef]
- de Sousa, O.A.; Cappellozza, B.I.; Fonseca, V.G.L.; Cooke, R.F. Insulin resistance increases as days on feed advance in feedlot Bos indicus beef cattle offered a high-concentrate finishing diet. J. Anim. Sci. 2022, 100, skac182. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, J.; Schaefer, K.; Kolodgie, F.D.; Savion, N.; Kotev-Emeth, S.; Dardik, R.; Simon, A.J.; Halak, M.; Pariente, C.; Engelberg, I.; et al. Leptin locally synthesized in carotid atherosclerotic plaques could be associated with lesion instability and cerebral emboli. J. Am. Heart Assoc. 2012, 1, e001727. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Cooke, R.F.; Marques, R.S.; Cappellozza, B.I.; Arispe, S.A.; Keisler, D.H.; Bohnert, D.W. Effects of vaccination against respiratory pathogens on feed intake, metabolic, and inflammatory responses in beef heifers. J. Anim. Sci. 2015, 93, 4443–4452. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.W.; Miller, A.; Leal Yepes, F.A.; Bitsko, E.; Nydam, D.; Mann, S. The effect of the transition period and postpartum body weight loss on macrophage infiltrates in bovine subcutaneous adipose tissue. J. Dairy Sci. 2019, 102, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Wen, Y.; Zhang, H.; Zhao, G.N.; Gao, Q. Signaling pathways in macrophages: Molecular mechanisms and therapeutic targets. MedComm 2023, 4, e349. [Google Scholar] [CrossRef]
- Tian, L.; Zuoqin, D.; Jiaqi, W.; Xiaomeng, J.; Xin, D.; Yan, Y.; Youkun, Z.; Jianbo, W. Obesity phenotype induced by high-fat diet promotes diethylnitrosamine (DEN)-induced precancerous lesions by M1 macrophage polarization in mice liver. J. Nutr. Biochem. 2024, 125, 109566. [Google Scholar] [CrossRef]
- Taylor, E. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Contreras, G.A.; Kabara, E.; Brester, J.; Neuder, L.; Kiupel, M. Macrophage infiltration in the omental and subcutaneous adipose tissues of dairy cows with displaced abomasum. J. Dairy Sci. 2015, 98, 6176–6187. [Google Scholar] [CrossRef] [PubMed]
- Jaakson, H.; Ling, K.; Samarütel, J.; Ilves, A.; Kaart, T.; Kärt, O.; Ots, M. Blood glucose and insulin responses during the glucose tolerance test in relation to dairy cow body condition and milk yield. Vet. Zootech. 2013, 62, 28–35. [Google Scholar]
- Monteiro, L.; Pereira, J.; Palhinha, L.; Moraes-Vieira, P.M.M. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J. Leukoc. Biol. 2019, 106, 703–716. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- Liefers, S.C.; Veerkamp, R.F.; Te Pas, M.F.W.; Chilliard, Y.; Van der Lende, T. Genetics and physiology of leptin in periparturient dairy cows. Domest. Anim. Endocrinol. 2005, 29, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Laishram, M.; Aggarwal, A.; Kumar, S. Changes in plasma concentration of leptin, adiponectin and resistin in Karan-Fries cows of high and medium body conditions during winter and summer seasons. Agric. Sci. Dig. 2020, 40, 203–206. [Google Scholar]
- Schoenberg, K.M.; Giesy, S.L.; Harvatine, K.J.; Waldron, M.R.; Cheng, C.; Kharitonenkov, A.; Boisclair, Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 2011, 152, 4652–4661. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Gessner, D.K.; Ringseis, R. Fibroblast growth factor 21 in dairy cows: Current knowledge and potential relevance. J. Anim. Sci. Biotechnol. 2021, 12, 97. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Giesy, S.L.; Krumm, C.S.; Perfield, J.W., 2nd; Butterfield, A.; Boisclair, Y.R. Fibroblast growth factor-21 (FGF21) administration to early-lactating dairy cows. II. Pharmacokinetics, whole-animal performance, and lipid metabolism. J. Dairy Sci. 2019, 102, 11597–11608. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 272. [Google Scholar] [CrossRef]
- Krumm, C.S.; Giesy, S.L.; Caixeta, L.S.; Perfield, J.W., 2nd; Sauerwein, H.; Moore, B.L.; Boisclair, Y.R. Fibroblast growth factor-21 (FGF21) administration to early-lactating dairy cows. I. Effects on signaling and indices of insulin action. J. Dairy Sci. 2019, 102, 11586–11596. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, M.; Tai, W.; Yu, H.; Hao, X.; Loor, J.J.; Jiang, Q.; Fang, Z.; Gao, X.; Fan, M.; et al. Tumor necrosis factor-α promotes lipolysis and reduces insulin sensitivity by activating nuclear factor kappa B and c-Jun N-terminal kinase in primary bovine adipocytes. J. Dairy Sci. 2022, 105, 8426–8438. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, J.C.; Luís, A.; Gorman, A.M.; Samali, A.; Kaltenecker, D.; Moriggl, R.; Kozlov, A.V. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine 2019, 124, 154577. [Google Scholar] [CrossRef] [PubMed]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Wang, C.H. ER stress in adipocytes and insulin resistance: Mechanisms and significance (Review). Mol. Med. Rep. 2014, 10, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Schlegel, G.; Ringseis, R.; Schwarz, F.J.; Eder, K. Up-regulation of endoplasmic reticulum stress induced genes of the unfolded protein response in the liver of periparturient dairy cows. BMC Vet. Res. 2014, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Gao, W.; Loor, J.J.; Aboragah, A.; Fang, Z.; Du, X.; Zhang, M.; Song, Y.; Liu, G.; Li, X. Reducing hepatic endoplasmic reticulum stress ameliorates the impairment in insulin signaling induced by high levels of β-hydroxybutyrate in bovine hepatocytes. J. Dairy Sci. 2021, 104, 12845–12858. [Google Scholar] [CrossRef]
- Yang, M.; Zuo, Z.; Li, B.; Liang, S.; Gou, L.; Ren, Z.; Ma, X. Resistin promotes the production of inflammatory factors in cultured bovine alveolar macrophages and its mechanism. Chin. J. Cell. Mol. Immunol. 2018, 34, 673–677. [Google Scholar]
- Barnes, K.M.; Miner, J.L. Role of resistin in insulin sensitivity in rodents and humans. Curr. Protein Pept. Sci. 2009, 10, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.S.; Weakley, S.M.; Yao, Q.; Chen, C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012, 165, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, K.; Jiang, R.; Contreras, G.A.; Xie, L.; Pascottini, O.B.; Opsomer, G.; Dong, Q. The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows. Animals 2024, 14, 832. https://doi.org/10.3390/ani14060832
Qiao K, Jiang R, Contreras GA, Xie L, Pascottini OB, Opsomer G, Dong Q. The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows. Animals. 2024; 14(6):832. https://doi.org/10.3390/ani14060832
Chicago/Turabian StyleQiao, Kaixi, Renjiao Jiang, Genaro Andres Contreras, Lei Xie, Osvaldo Bogado Pascottini, Geert Opsomer, and Qiang Dong. 2024. "The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows" Animals 14, no. 6: 832. https://doi.org/10.3390/ani14060832
APA StyleQiao, K., Jiang, R., Contreras, G. A., Xie, L., Pascottini, O. B., Opsomer, G., & Dong, Q. (2024). The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows. Animals, 14(6), 832. https://doi.org/10.3390/ani14060832






